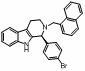
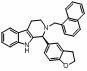
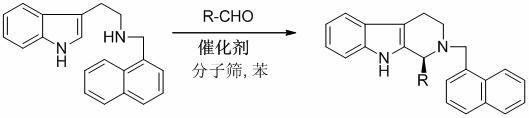
Method for synthesizing optically active tetrahydro-beta-carboline derivative through catalysis of chiral spirocyclic phosphoric acid
A technology of spirophosphoric acid catalysis and spirophosphoric acid catalyst, which is applied in the directions of organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems such as insufficient enantioselectivity, narrow use range of substrates, etc. Highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Under nitrogen protection, put 1 mmol N b -α-Naphthylmethyltryptamine, 0.02 mmol ( S )-O,O'-{7,7'-[6,6'-di-(1-naphthyl)-1,1'-spirobidihydroindane]} phosphoric acid catalyst and 1.5 grams of 4? Molecular sieve powder mixed In 5 ml of benzene solvent, then add 3 mmol of p-bromobenzaldehyde, at 30 oC After reacting for 24 hours, the optically active (S)-(4-bromophenyl)-2-(α-naphthylmethyl)-2,3,4,9-tetrahydro-β-carba phenoline, yield 96%. The optical purity of the product was 98% ee by HPLC. [Daicel Chiralpak AD-H, n -Hexane / i -propanol = 90 / 10, 1.0 mL / min, λ = 254 nm, t (minor) = 8.646 min, t (major) = 11.146 min]. [α] D 20 = +51.2°(c = 1.03, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 2.66-2.81 (m, 3H), 3.13-3.19 (m, 1H), 3.85 (d, J = 13.6 Hz, 1H), 4.20 (d, J = 13.6 Hz, 1H), 4.63 (s, 1H), 7.07-7.22 (m, 6H), 7.38-7.51 (m, 7H), 7.75 (d, J = 7.6 Hz, 1H), 7.82 (d, J = 7.6 Hz, 1H), 8.01 (d, J = 8.4 Hz, 1H); 13 C NMR (100 MHz, CDCl 3 ) Δ 20.5, 47.7, 56.4...
Embodiment 2~17
[0021] As in the feeding process of Example 1, where the type of aldehyde is changed, the experimental results in Table 1 can be obtained.
[0022]
[0023] Here the catalyst structural formula is:
[0024] Table 1: Experimental results of asymmetric reaction
[0025] Entry R time [h] product number Yield ee [%] 2 m -BrC 6 h 4 12 5h 94 95 3 m -ClC 6 h 4 12 5i 99 97 4 m -FC 6 h 4 12 5j 97 97 5 p -NO 2 C 6 h 4 12 5k 99 96 6 m -NO 2 C 6 h 4 12 5l 99 94 7 3,5-(CF 3 ) 2 C 6 h 3 16 5m 98 90 8 Ph 30 5n 90 97 9 p -MeOC 6 h 4 40 5o 94 93 10 piperonyl 44 5p 95 90 11 dihydrobenzofuryl 48 5q 91 92 12 furyl 10 5r 92 98 13 Et 4 5s 76 90 14 n -pentyl 5 5t 98 91 15 i -Pr 5 5u 96 97 16 Cy 6 5v 99 98 17 i -Pr 3 5w 96 95
[0026] T...
Embodiment 18
[0030] Example of deprotection of N-protected optically active tetrahydro-β-carboline derivatives: 0.2 mmol of compound (S)-isopropyl-2-(α-naphthylmethyl)-2,3,4, 9-tetrahydro-β-carboline 5u and 0.02 mmol of 10% Pd(OH) 2 / C was dissolved in a mixed solvent of 3 milliliters of ethyl acetate and 1.2 milliliters of methanol, and the air in the reactor was replaced three times with hydrogen, and then the reaction mixture was heated at 40 o C hydrogenation reaction at normal pressure for 4 hours, and then through silica gel powder column chromatography to obtain optically active (S)-isopropyl-2,3,4,9-tetrahydro-β-carboline, the structure is shown in the figure below, and the yield 98%, 97% ee.
[0031]
[0032] HPLC [Daicel Chiralcel OD-H, n -Hexane / i -propanol = 85 / 15, 1.0 mL / min, λ = 254 nm, t (major) = 8.820 min, t (minor) = 12.766 min]. [α] D 20 = -99.0°(c = 1.03, MeOH); 1 H NMR (500 MHz, CDCl 3 ) δ 0.88 (d, J = 7.0 Hz, 3H), 1.14 (d, J = 7.0 Hz, 3H), 2.09 (br,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com