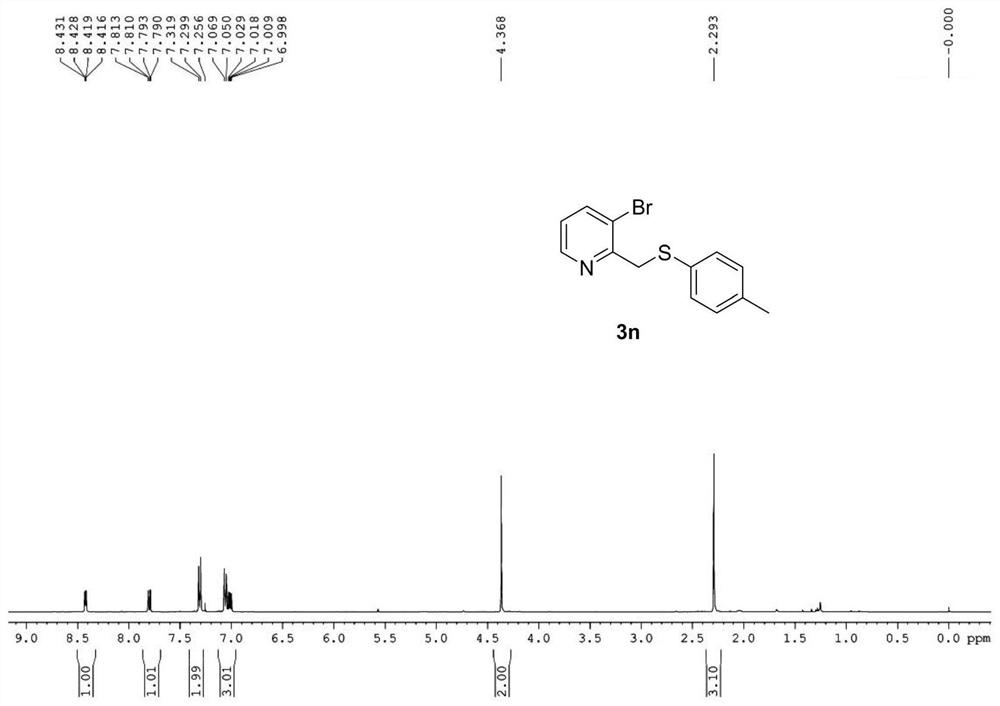
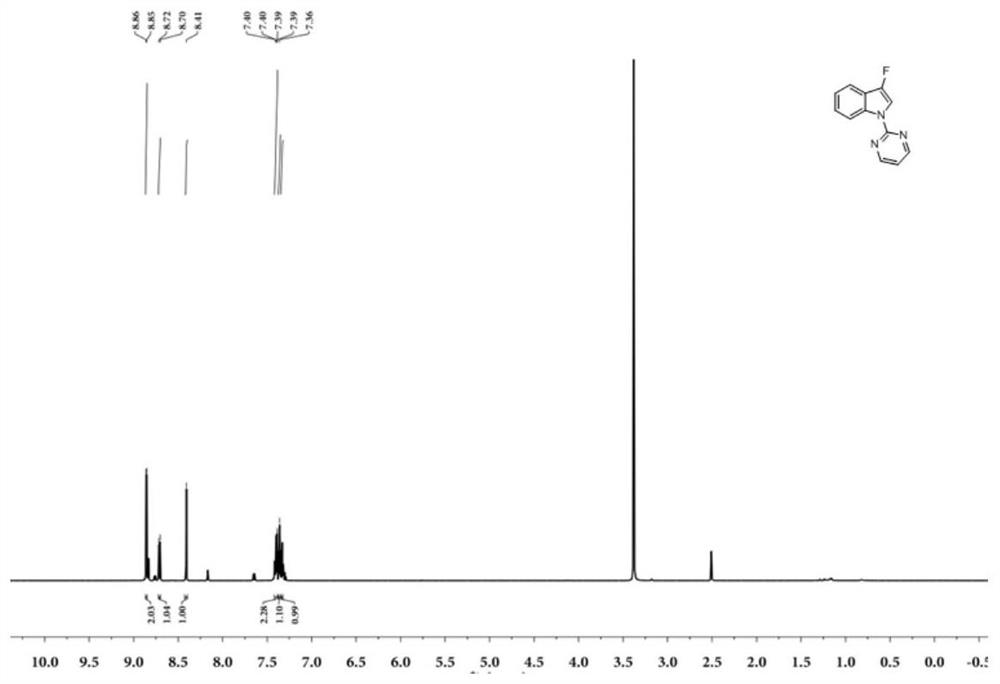
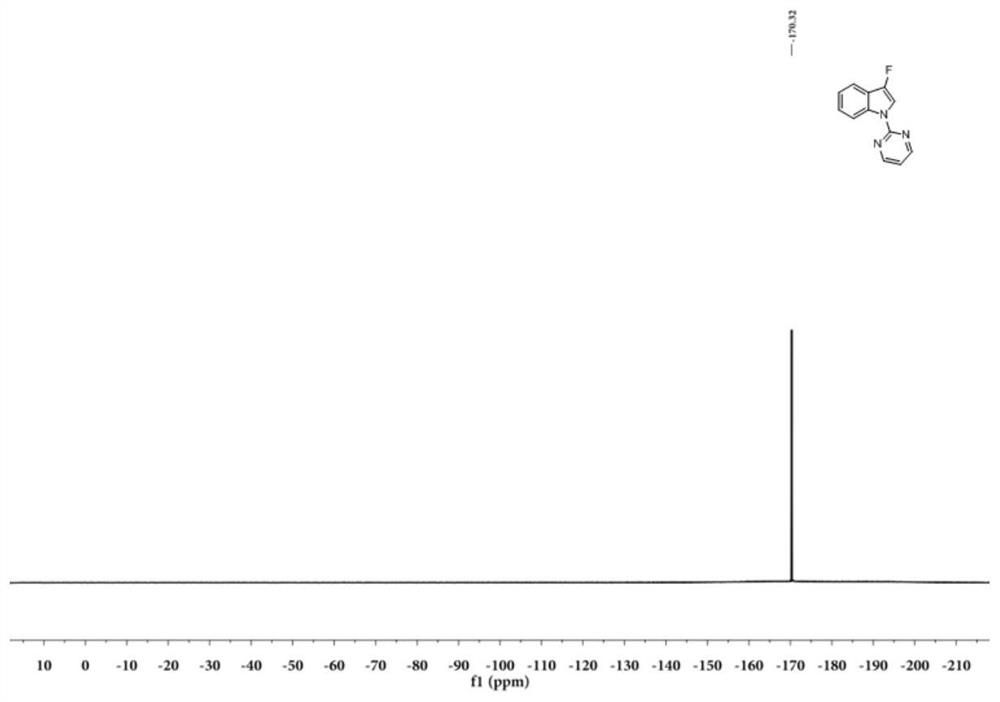
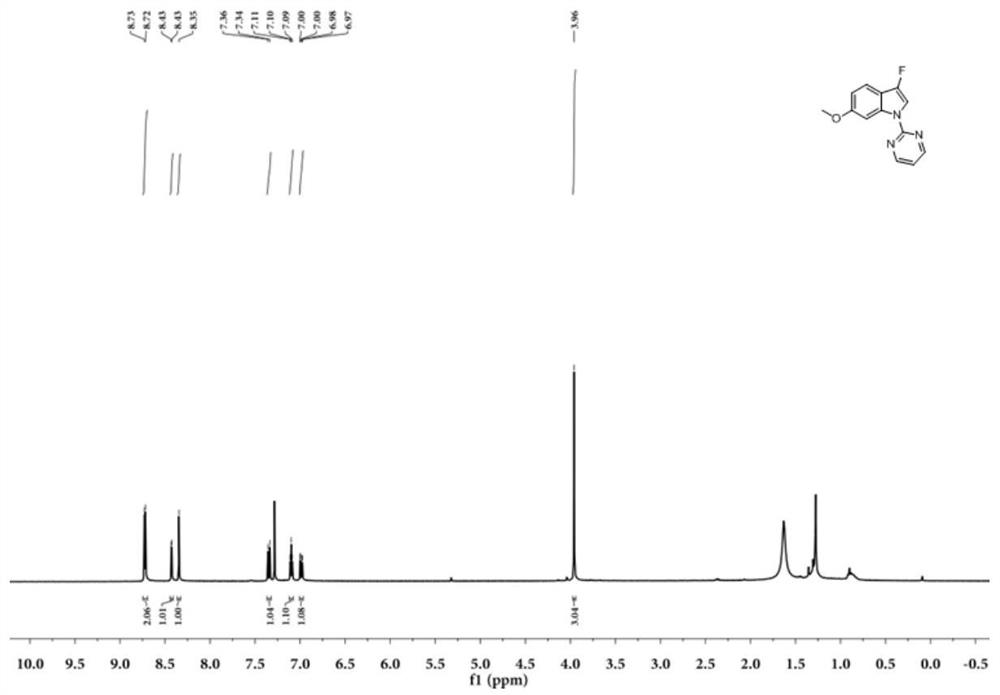
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Site selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Selectivity is the degree to which a drug acts on a given site relative to other sites. Relatively nonselective drugs affect many different tissues or organs. For example, atropine, a drug given to relax muscles in the digestive tract, may also relax muscles in the eyes and in the respiratory tract.
Live selective adaptive bandwidth
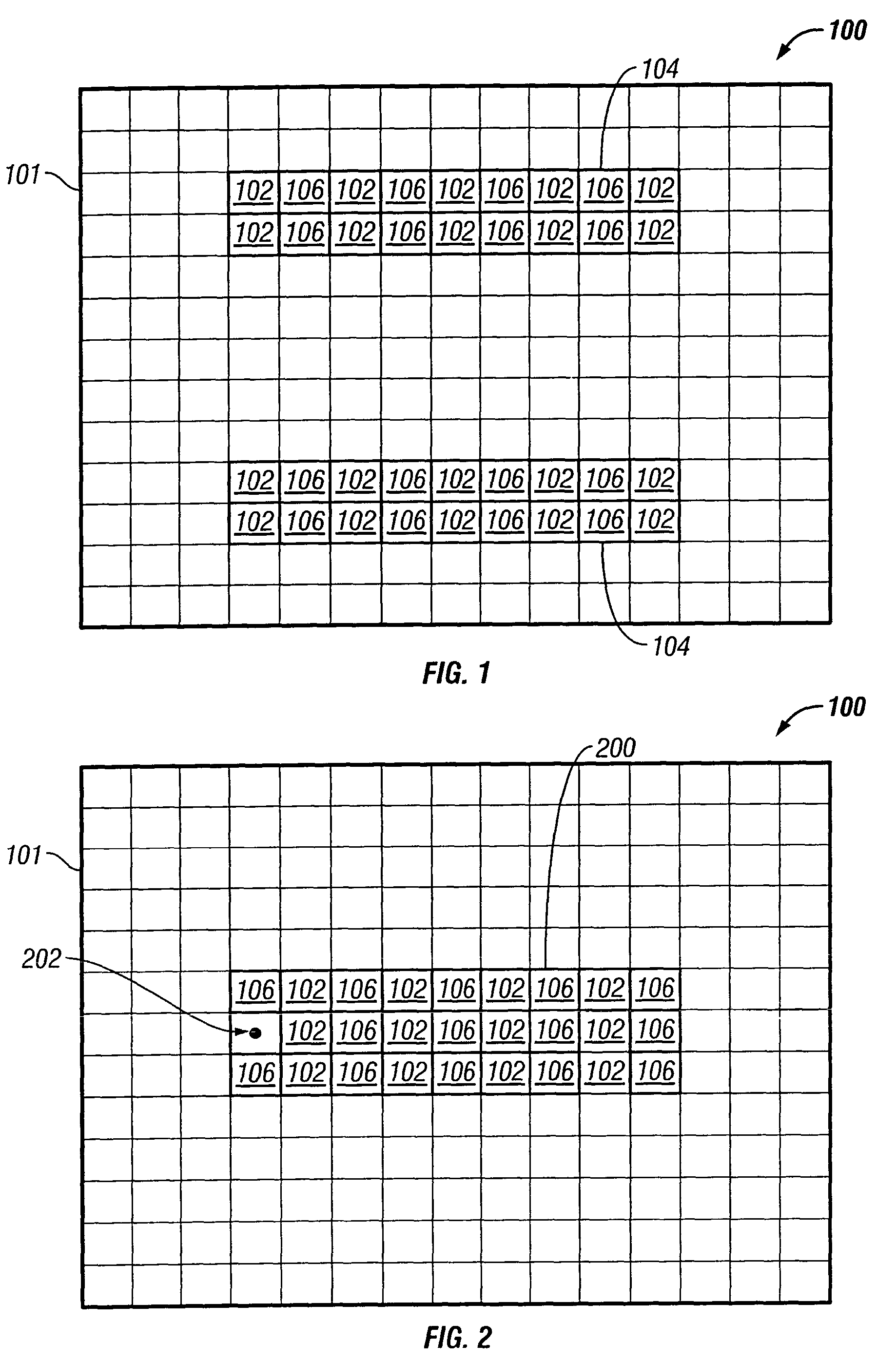
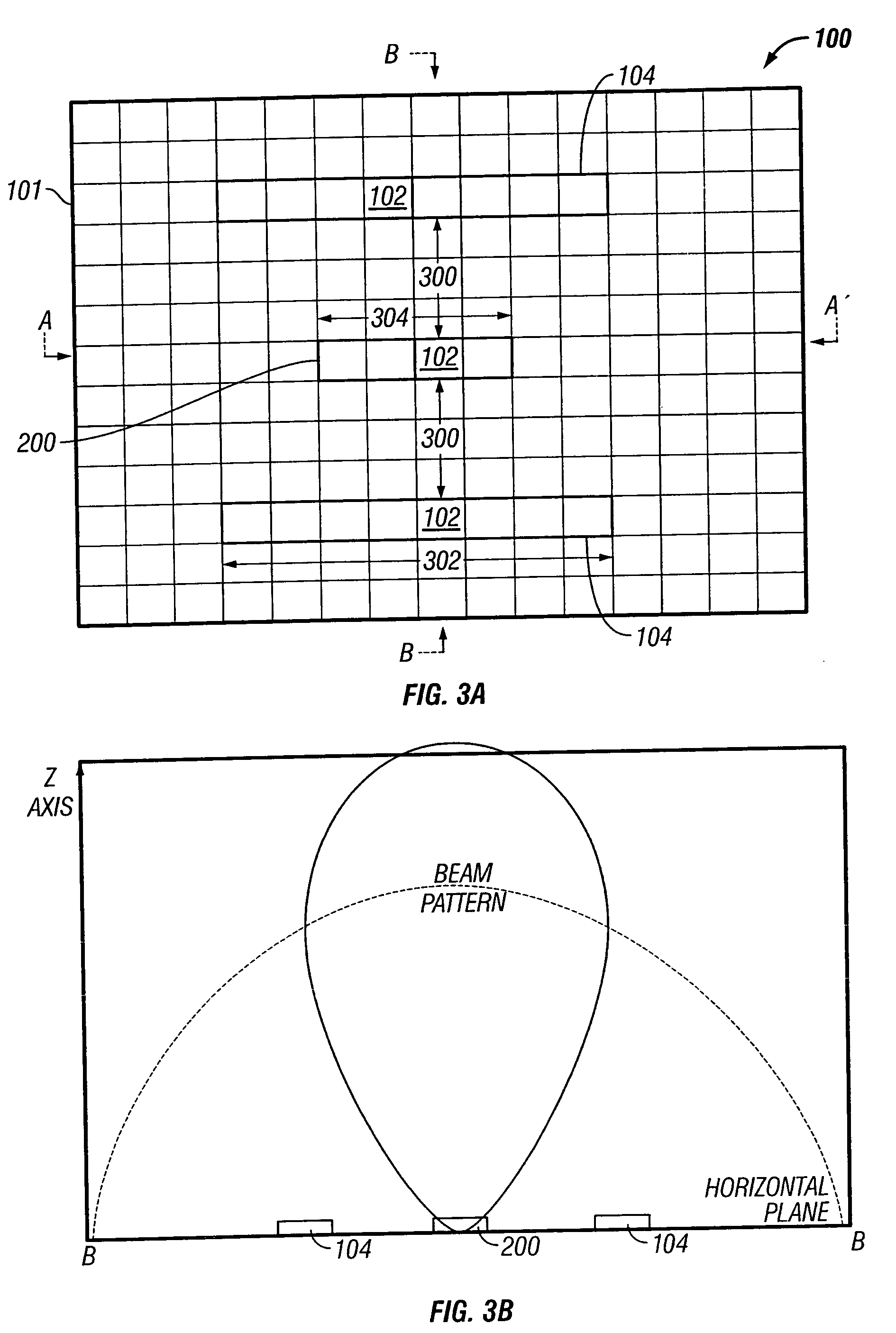
PendingUS20160150212A1High resolutionComponent with highPicture reproducers using cathode ray tubesPicture reproducers with optical-mechanical scanningImage resolutionSite selectivity
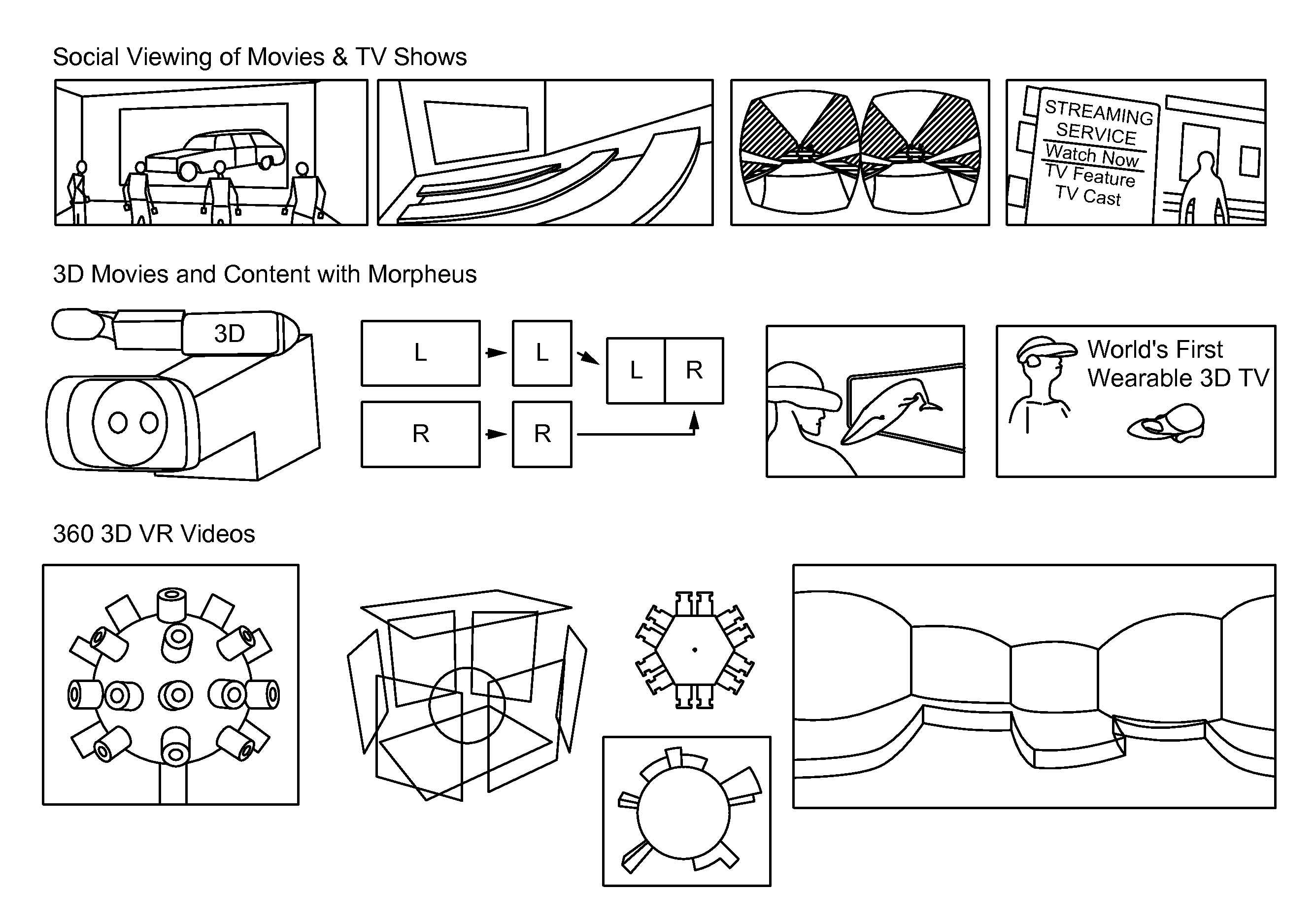
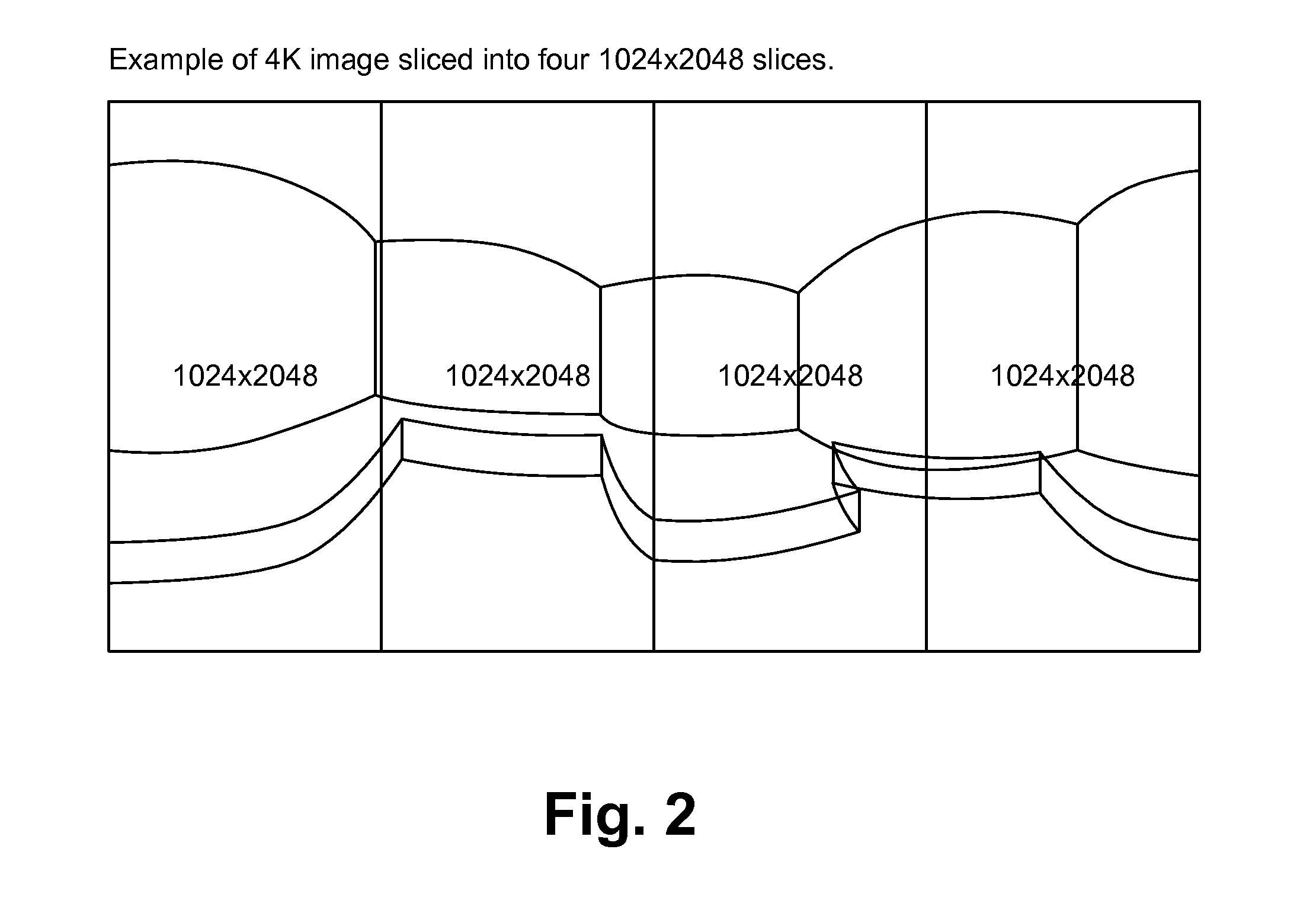
A live selective adaptive bandwidth method enables transmission of three dimensional 360 degree virtual reality content by slicing the content and utilizing different resolutions of the content, where content in the visible area of the user is a higher resolution than content in the non-visible area of the user. Additionally, network information such as available bandwidth is used in determining which resolution content to be transmitted.
Owner:SONY CORP
MEMS planar antenna array
InactiveUS20040080456A1Easy to manufactureThin profileSimultaneous aerial operationsRadiating elements structural formsSite selectivityEngineering
A MEMS planar antenna array is provided comprising a planar field of MEMSs. A lattice of parasitic elements can be formed by selectively connecting at least one MEMS in the field. An antenna active element is formed by selectively connecting MEMS in the field. Alternately, both the parasitic elements and the active elements are formed by connecting MEMS. The parasitic elements have a number, shape, length, distance from the active element, and position with respect to the active element that are formed in response to selectively connecting MEMS in the field. Further, a plurality of different parasitic element lattices can be formed in response to selectively connecting MEMS in the field. Likewise, the active element has a length, shape, and position that is formed in response to selectively connecting MEMS. Patch, monopole, and dipole antennas are among the antenna types that can be formed from the MEMS.
Owner:HANEI
Transparent pressure-sensitive adhesive product for optical use, transparent pressure-sensitive adhesive laminate for optical use and manufacturing method thereof
InactiveUS20100178496A1Good release effectSuitable for automationLamination ancillary operationsOpticsSilicone GelsSite selectivity
Owner:TAICA
Method of separating histidase enantiomer by chitosan-modified gold nanochannel film and detecting method thereof
InactiveCN103896846AEasy to separateConvenient methodOrganic chemistryRaman scatteringSelf assembleEnantiomer
The invention discloses a method of separating histidase enantiomer by a chitosan-modified gold nanochannel film. The method comprises the following steps: by taking a polycarbonate film and an aluminum oxide film as base films, adopting a chemical deposition method to prepare a gold nanochannel film; self-assembling chitosan onto the pore wall of the gold nanochannel to form a functional nanochannel film with chiral site selectivity on the surface; and separating D-histidine, L- histidine in a chiral manner by utilizing excellent separation ability of the nanochannel. During detection, silver sol is used as a surface enhanced Raman substrate to enhance SERS (surface enhanced raman scattering) effect of D-histidine, L-histidine, so that selectivity and sensitivity of the substance are improved to detect the D-histidine and the L-histidine at the same time. The invention provides a quicker and more convenient method for separating and detecting the chiral substance by constructing a coupling device of a nanochannel separating tank and an SERS detecting system, and shows unique advantages and wide application prospect of the method.
Owner:SHANGHAI NORMAL UNIVERSITY
Optical transparent pressure-sensitive adhesive body, optical transparent pressure-sensitive adhesive laminate, and method for producing the same
InactiveUS20110318577A1Superior selective peeling stabilityQuick responseLaminationLamination apparatusSilicone GelsSite selectivity
A highly pressure-sensitive adhesive optical transparent pressure-sensitive adhesive body or optical transparent pressure-sensitive adhesive laminate which stably provide peeling site selectivity between both sides of the transparent pressure-sensitive adhesive body and a release sheet in the case of pasting, whereas stably and easily express peeling site selectivity between both sides of the transparent pressure-sensitive adhesive body in the case of rework, that is, re-pasting, as well as superior adhesion stability after pasting, and a method for producing the same.An optical transparent pressure-sensitive adhesive body and the like having pressure-sensitive adhesive surface (a) and pressure-sensitive adhesive surface (b) having different pressure-sensitive adhesive property from each other, and being formed with an addition reaction type silicone gel, characterized in that pressure-sensitive adhesive performance (Ga) of pressure-sensitive adhesive surface (a) and pressure-sensitive adhesive performance (Gb) of pressure-sensitive adhesive surface (b) satisfy a relationship of Ga<Gb, and pressure-sensitive adhesive performance (Ga) and pressure-sensitive adhesive performance (Gb) have 5 to 32 in ball number and 2 to 12 in ball number difference in the tilt type ball tack test (tilt angle: 30 degrees) according to JIS Z0237; and that pressure-sensitive adhesive surface (a) and pressure-sensitive adhesive surface (b) are formed by tightly adhering an uncured raw material of said addition reaction type silicone gel to different release film (A) and release film (B) and thermal-curing them, and the release film (A) and the release film (B) have a release treatment layer comprising a fatty acid amide type additive.
Owner:TAICA
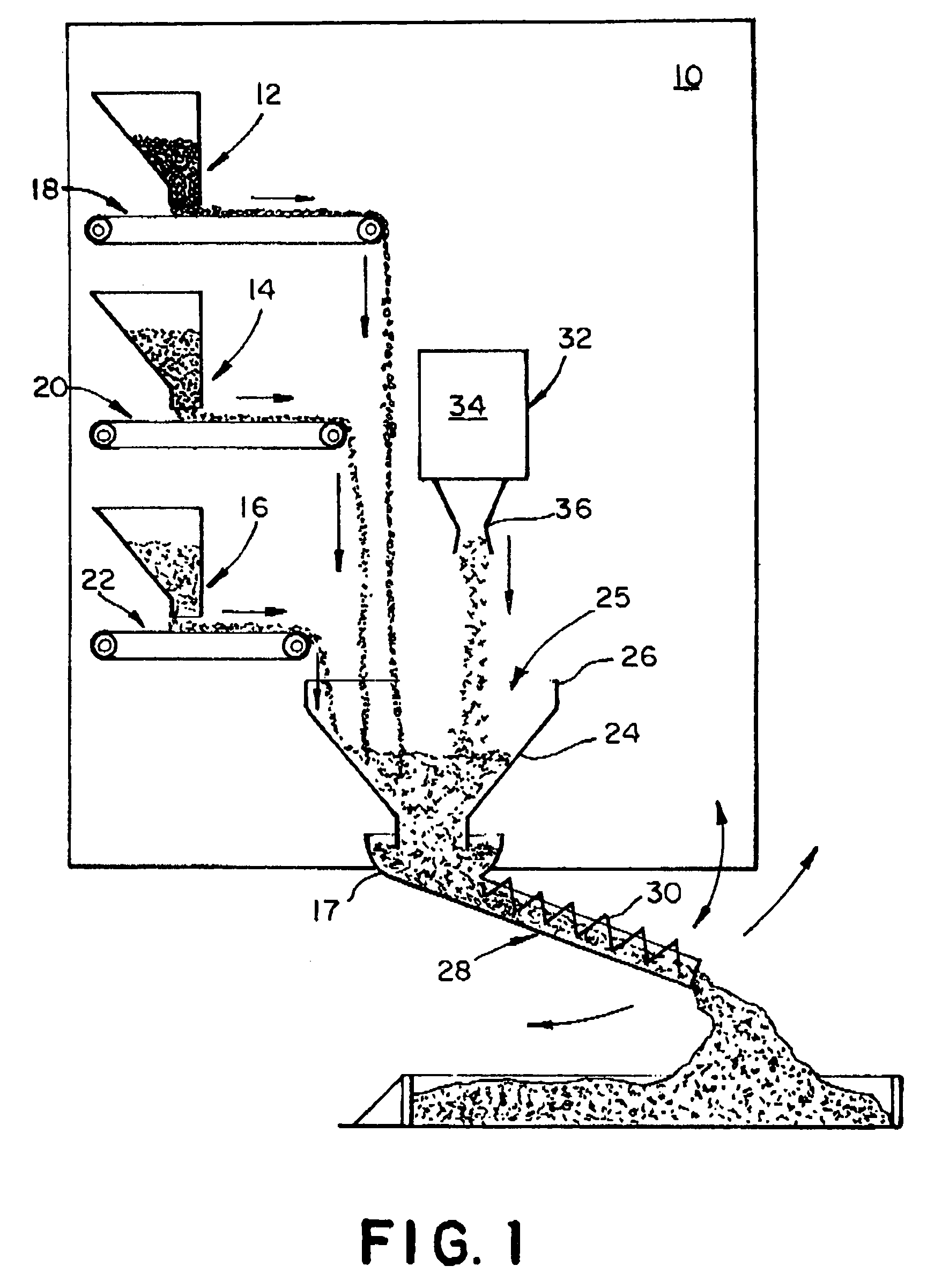
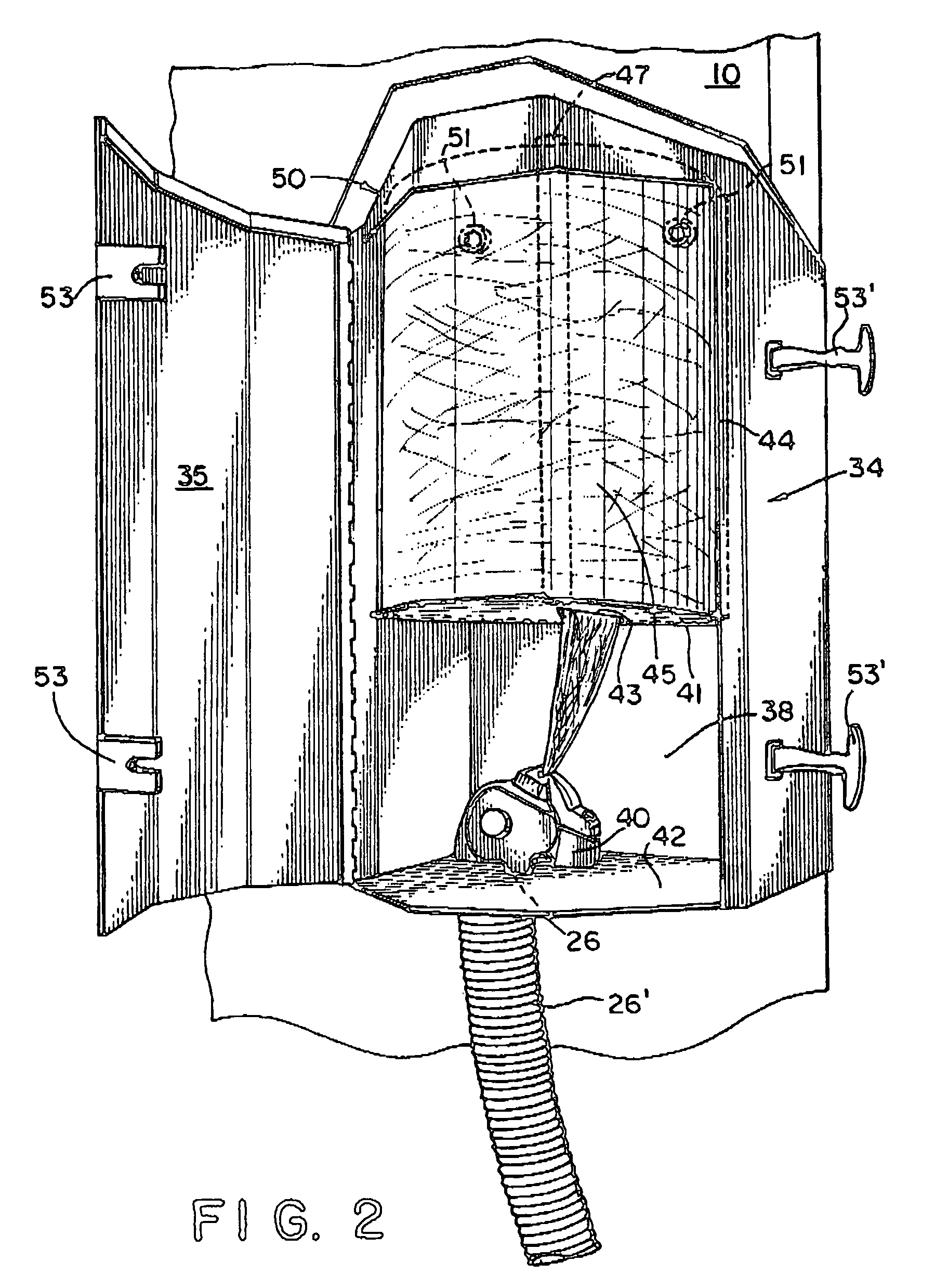
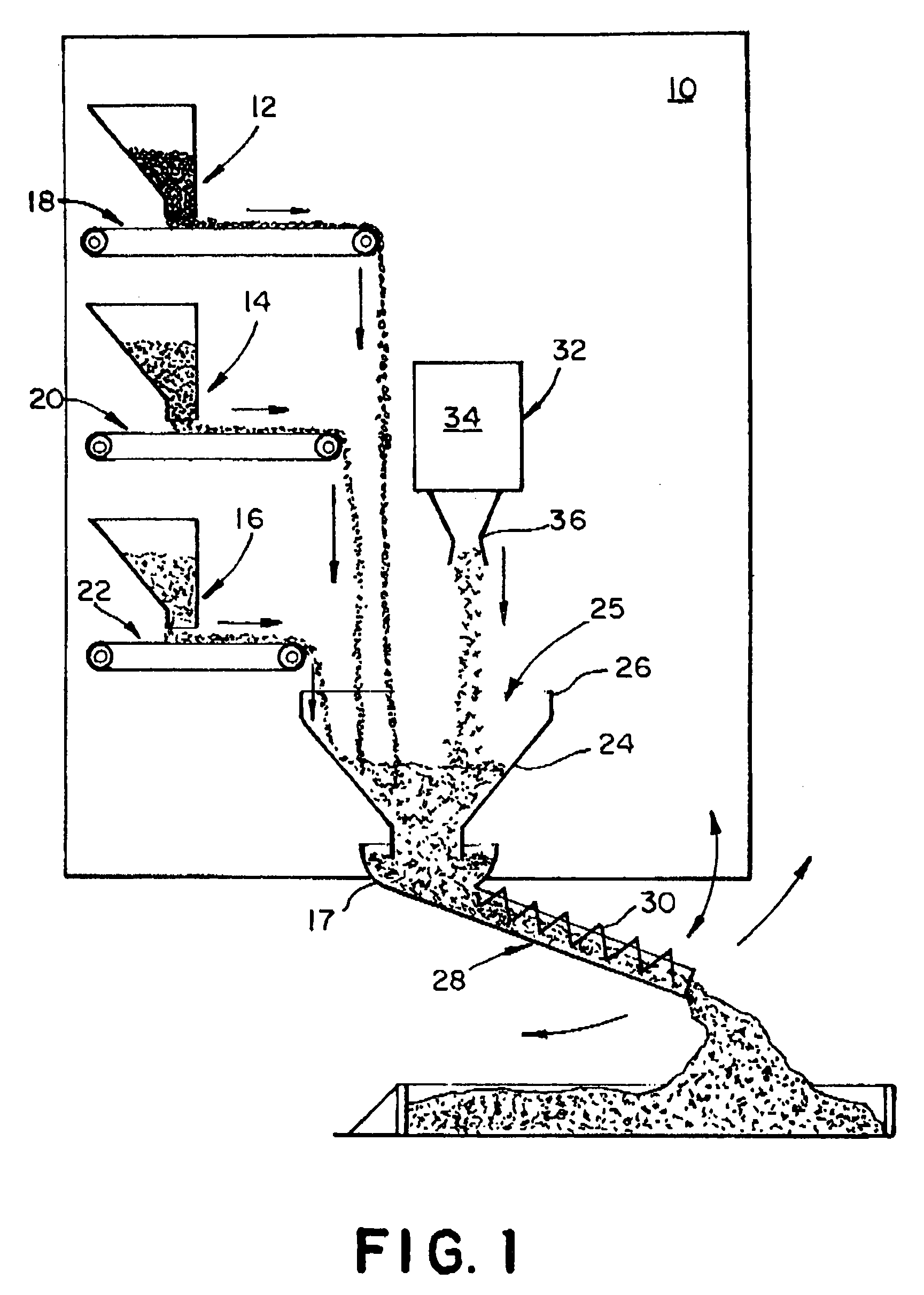
Concrete delivery truck
An apparatus and process for mixing and depositing a strand reinforced concrete mix at a job site from a concrete delivery truck including a mixer on the truck for mixing separate concrete ingredients at a job site, which truck further includes a fiber strand chopping device on the truck disposed and adapted to introduce chopped fiber strand lengths into the separate concrete ingredients and water and deposit the mix selectively at the job site without clumping and grouping of the fiber strand lengths in the mix.
Owner:FORTA LLC
Method of separating chiral drug penicillamine enantiomer based on functional gold nanochannel
Owner:SHANGHAI NORMAL UNIVERSITY
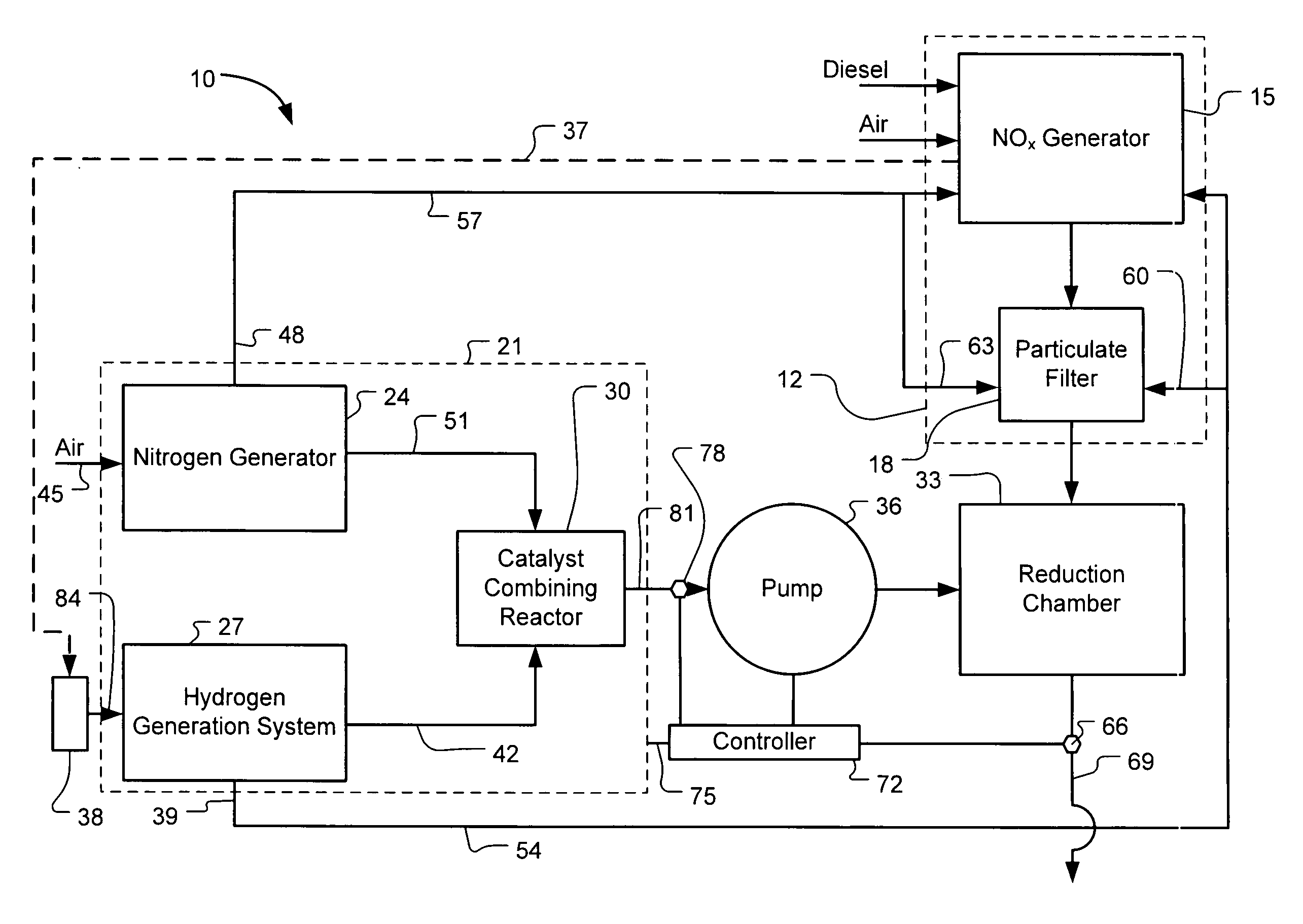
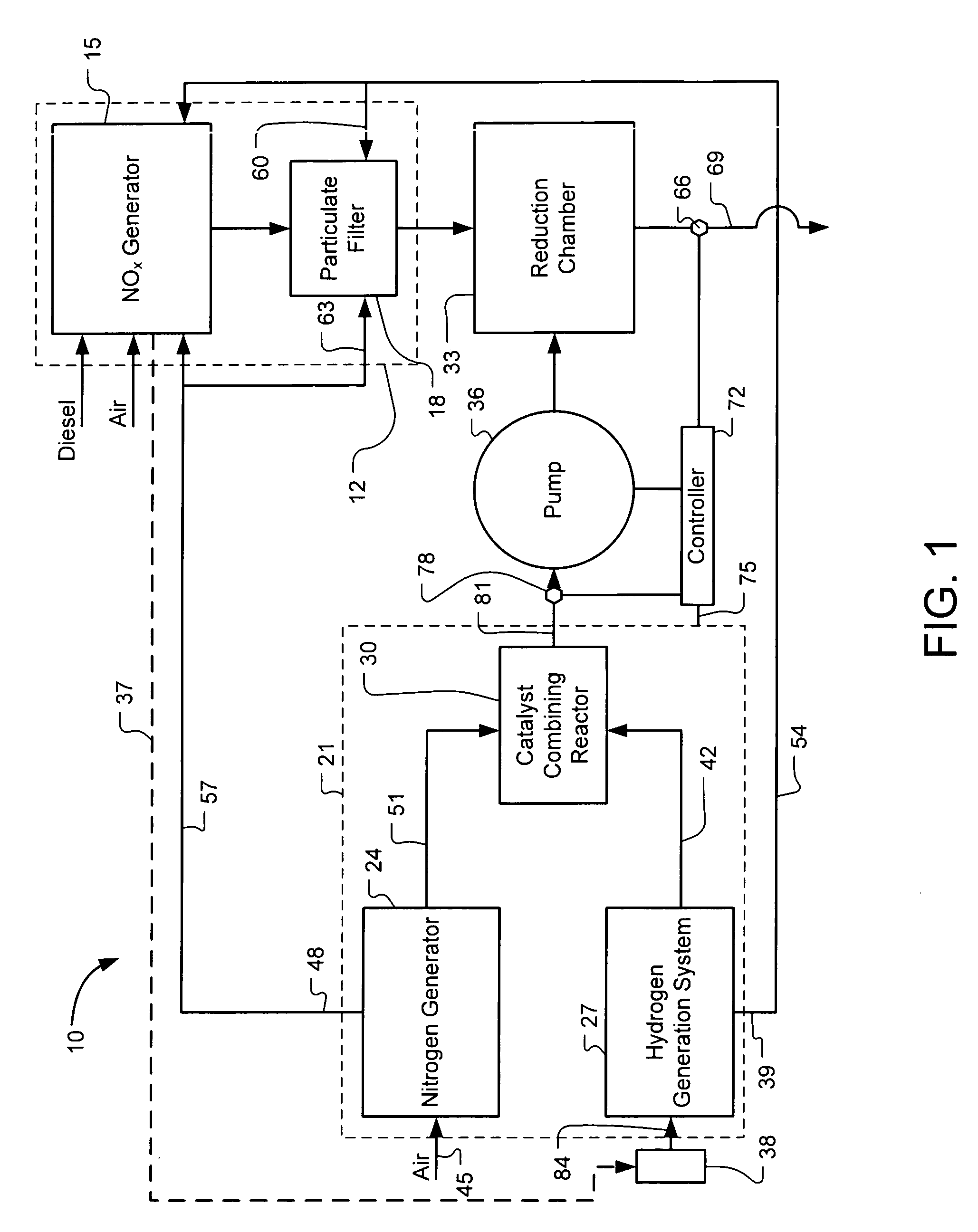
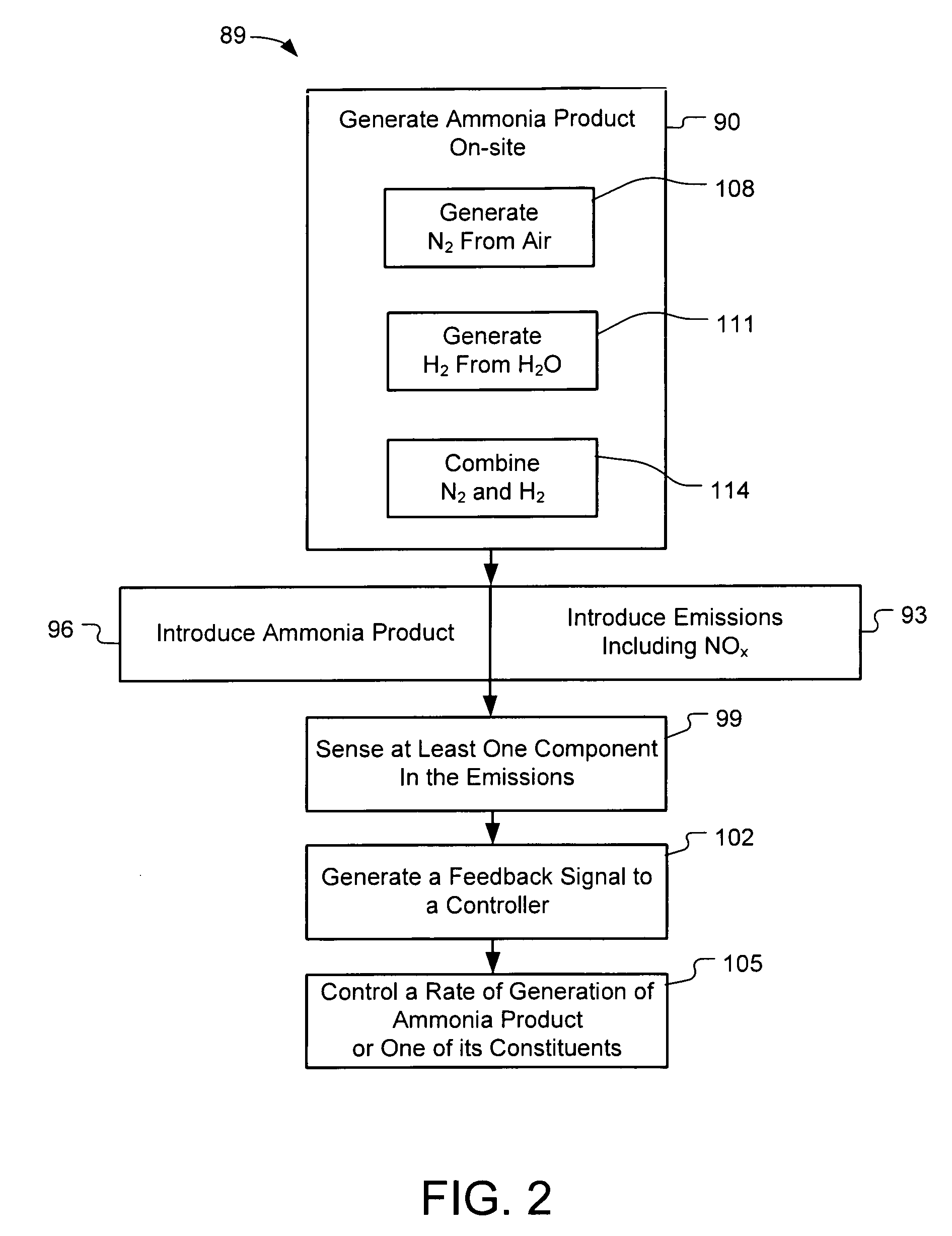
Systems and Methods for On-Site Selective Catalytic Reduction
A selective catalytic reduction (SCR) system includes an on-board ammonia generation system that produces nitrogen from air and hydrogen from a source of a hydrogen-containing compound, and generates an ammonia product from the nitrogen and hydrogen to provide the ammonia product into an exhaust from a NOx generator to reduce the NOx in the exhaust. Oxygen from one or both of the nitrogen generator and the hydrogen generation cell can be supplied to the NOx generator for cleaner combustion or to a particulate filter for cleaning the filter. H2O from the NOx generator can at least partially provide a water source for the hydrogen generation cell.
Owner:CERAMTEC
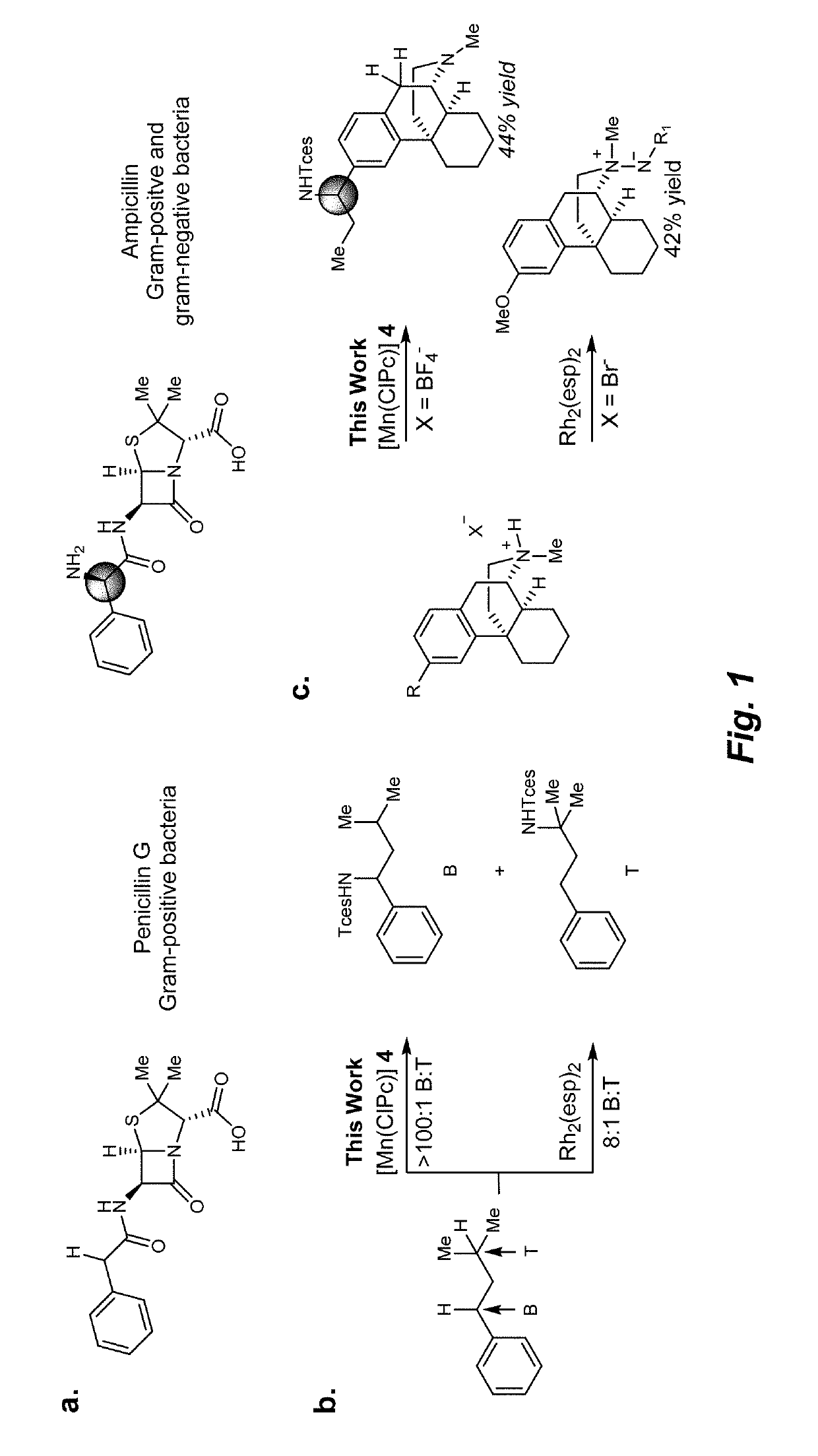
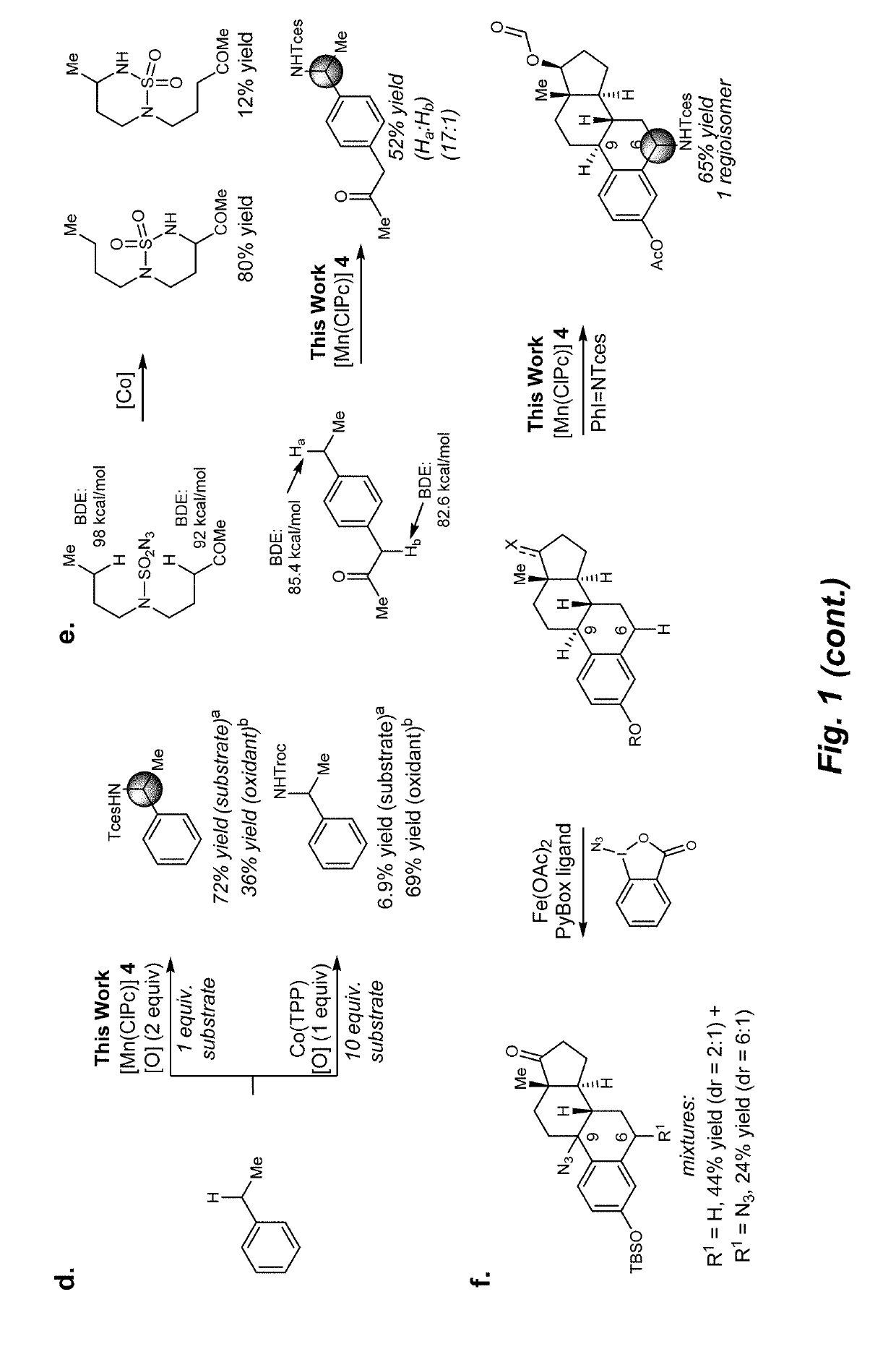
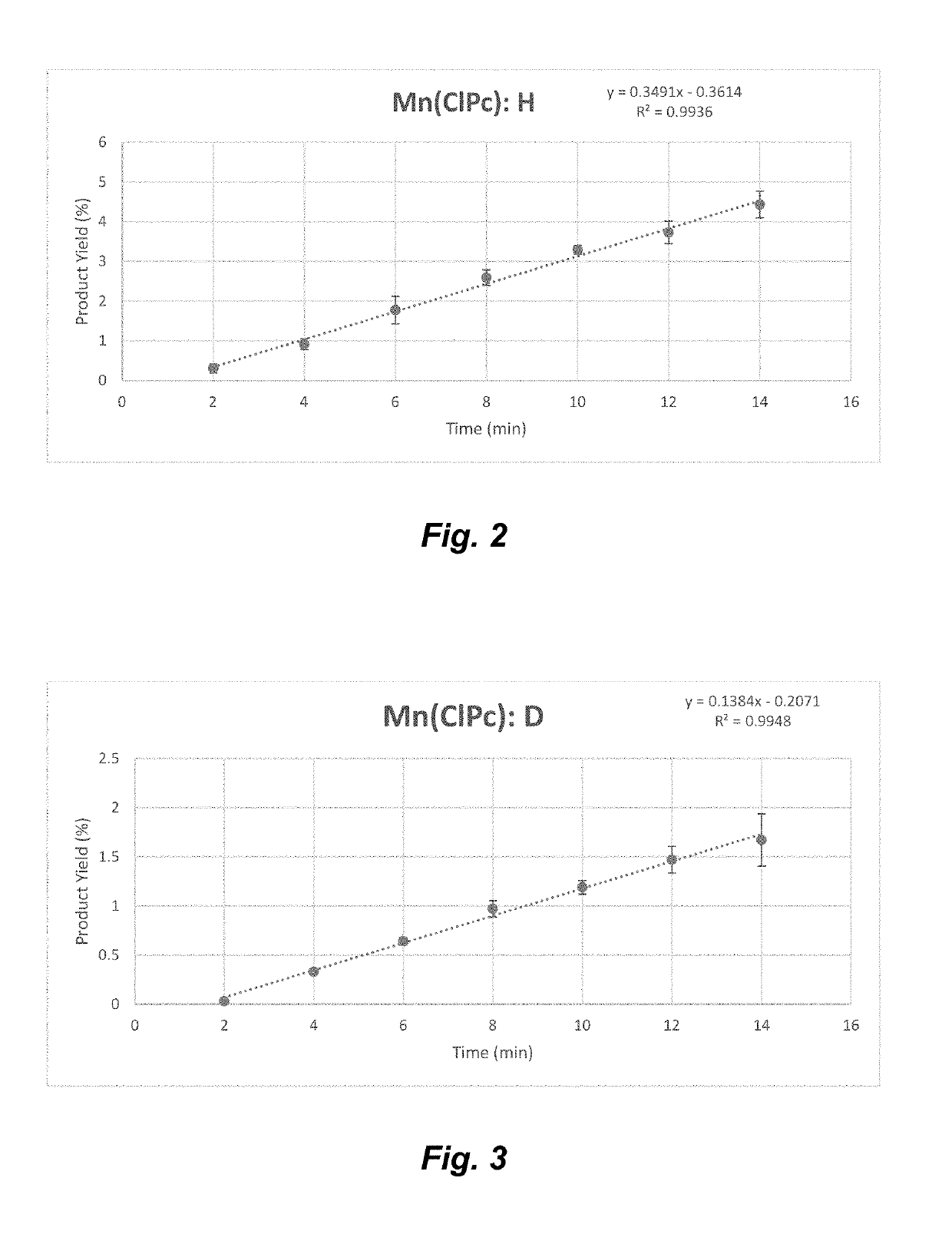
Manganese (III) catalyzed c--h aminations
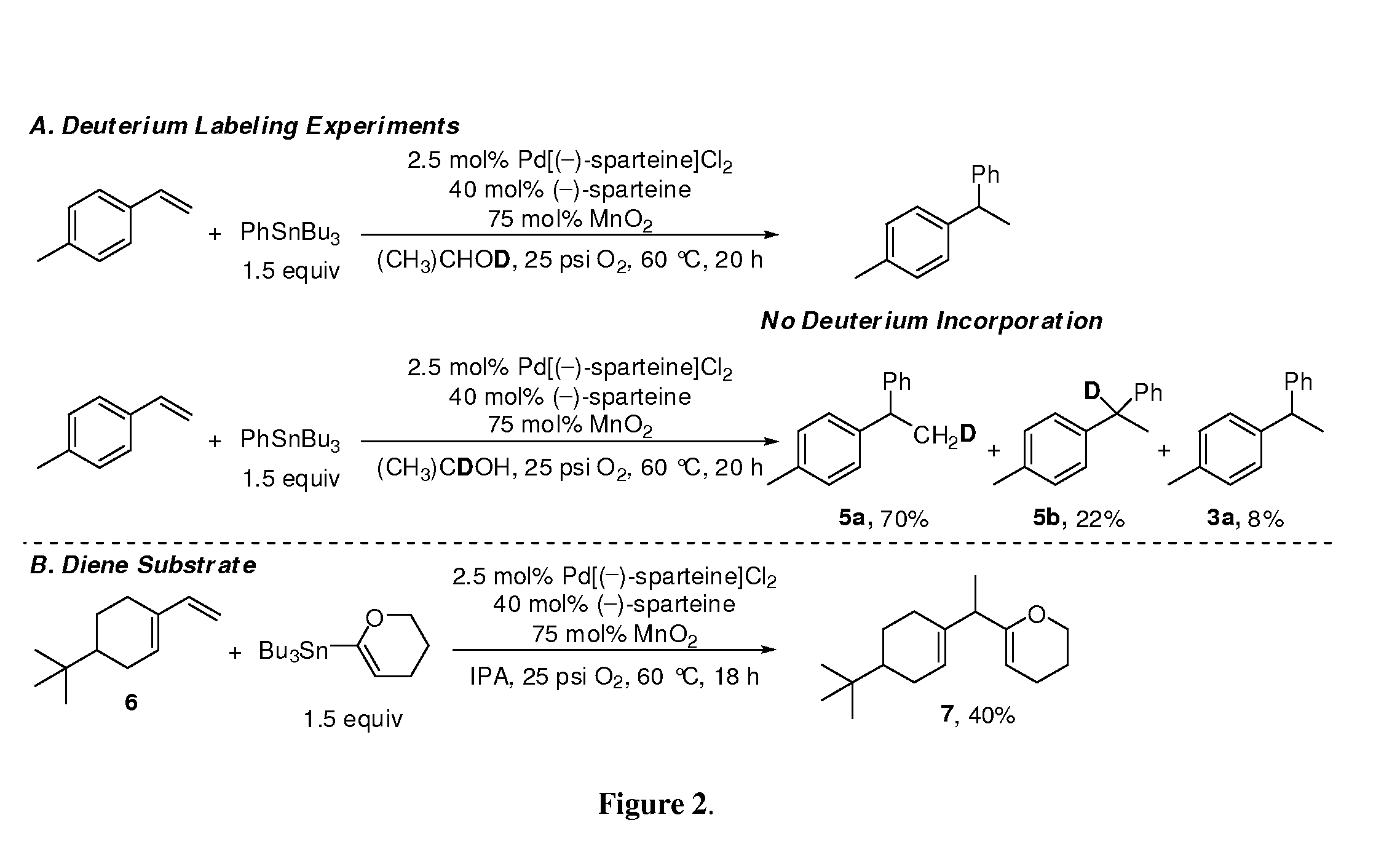
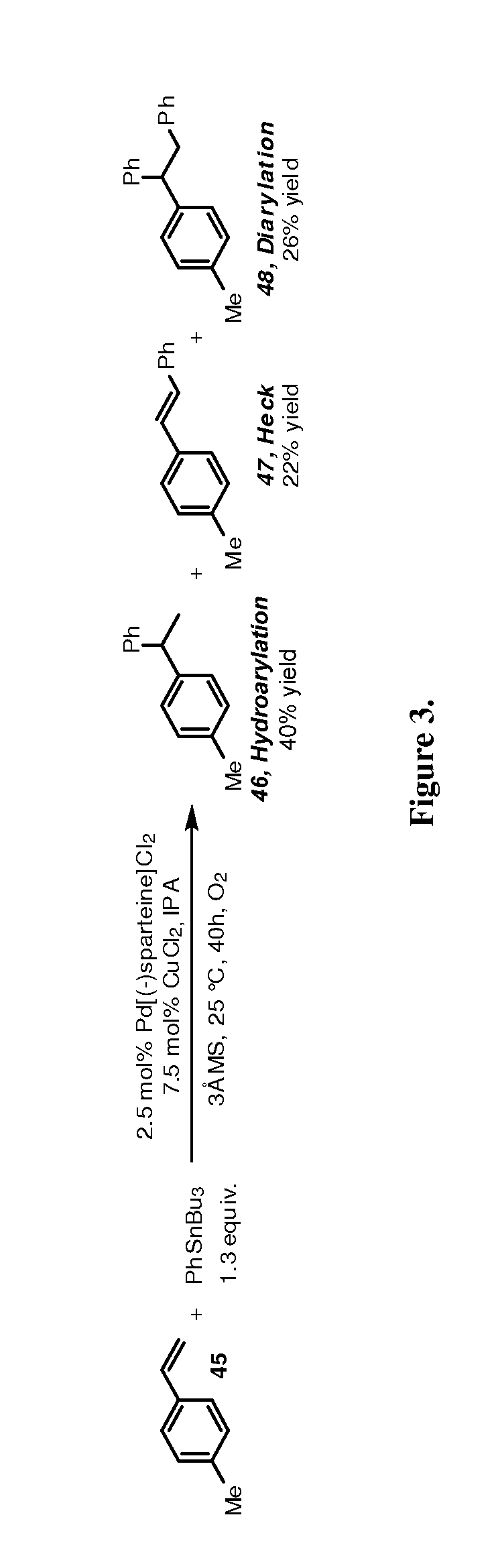
ActiveUS20190106448A1Organic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsRate-determining stepSite selectivity
Reactions that directly install nitrogen into C—H bonds of complex molecules are significant because of their potential to change the chemical and biological properties of a given compound. Selective intramolecular C—H amination reactions that achieve high levels of reactivity, while maintaining excellent site-selectivity and functional-group tolerance is a challenging problem. Herein is reported a manganese perchlorophthalocyanine catalyst [MnIII(ClPc)] for intermolecular benzylic C—H amination of bioactive molecules and natural products that proceeds with unprecedented levels of reactivity and site-selectivity. In the presence of Brønsted or Lewis acid, the [MnIII(ClPc)]-catalyzed C—H amination demonstrates unique tolerance for tertiary amine, pyridine and benzimidazole functionalities. Mechanistic studies indicate that C—H amination proceeds through an electrophilic metallonitrene intermediate via a stepwise pathway where C—H cleavage is the rate-determining step of the reaction. Collectively these mechanistic features contrast previous base-metal catalyzed C—H aminations.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Preventives/remedies for inflammatory airway diseases
InactiveUS7214672B2High site selectivityLow systemic side effectOrganic active ingredientsBiocideRespiratory tract diseaseWhole body
A preventive and / or therapeutic agent for inflammatory respiratory tract diseases containing, as an active ingredient, a steroid derivative represented by the following formula (1):(wherein R represents a hydrogen atom, a halogen atom, a hydroxy group, or —OCOR1 (wherein R1 represents a linear or branched alkyl group which may be substituted by a halogen atom or a cycloalkyl group; a cycloalkyl group; or an aryl group)).These compounds can continuously suppress respiratory tract inflammation and respiratory tract hyperreaction and show a high site selectivity and little systemic effect when administered directly to the respiratory tract. By virtue of these characteristics, these compounds are remarkably useful in clinical medicine as highly safe preventive and / or therapeutic agents for inflammatory respiratory tract diseases which can be administered for a long period of time.
Owner:NIPPON SHINYAKU CO LTD
Method for enzymatic transesterfication preparation of cocoa butter equiralent by using 33-DEG C palm oil
The invention discloses a method for enzymatic transesterfication preparation of a cocoa butter equiralent by using 33-DEG C palm oil, and belongs to the technical field of enzymatic transesterfication preparation of cocoa butter equiralent. The 33-DEG C palm oil with high food safety and wide source of raw material is used as a raw material, lipase having the advantages of good Sn-1,3 site selectivity, high catalytic efficiency, broad reaction conditions and low price is adopted for enzymatic transesterfication preparation of the cocoa butter equiralent, especially, a scientific-compounding natural antioxidant is added in an enzyme catalysis process, an acid value of the cocoa butter equiralent can be significantly reduced, and the shelf life of the product is prolonged. The physicochemical properties of the finally prepared cocoa butter equiralent component and product are extremely similar to those of natural cocoa butter, the acid value is low, the ester exchange rate is high, the acyl transfer rate is low, the shelf life is long, and the cost is low. The acid value of the prepared cocoa butter equiralent is 0.5-0.8 mg KOH / g, the ester exchange rate is 82.68-88.76%, and the acyl transfer rate is 4.41-6.61%.
Owner:BEIJING UNIV OF CHEM TECH +1
Adjustable light fixture and lighting system
ActiveUS20190049100A1Eliminating dark spotUniform light distributionLighting support devicesElongate light sourcesElectricityLight spot
An adjustable light fixture and lighting system are selectively adjustable in the field and provide even light distribution across a space. A housing is selectively adjustable to a selected housing length and is mountable to the surface. A tray mountable to the housing includes plates which are selectively adjustable relative to one another to a selected tray length. Each plate includes a plurality of electroluminescent light sources providing uniformly luminous light across the light fixture. Overlap of plates varies the tray length and blocks light from light sources on one plate by the opposite plate. Evenly distributed light is therefore provided with no bright or dark spots. A lighting system of a plurality of light fixtures electrically connected to one another includes at least one adjustable light fixture, and preferably at least one stationary light fixture of fixed length. Methods of installation are also disclosed.
Owner:FORUM LIGHTING INC
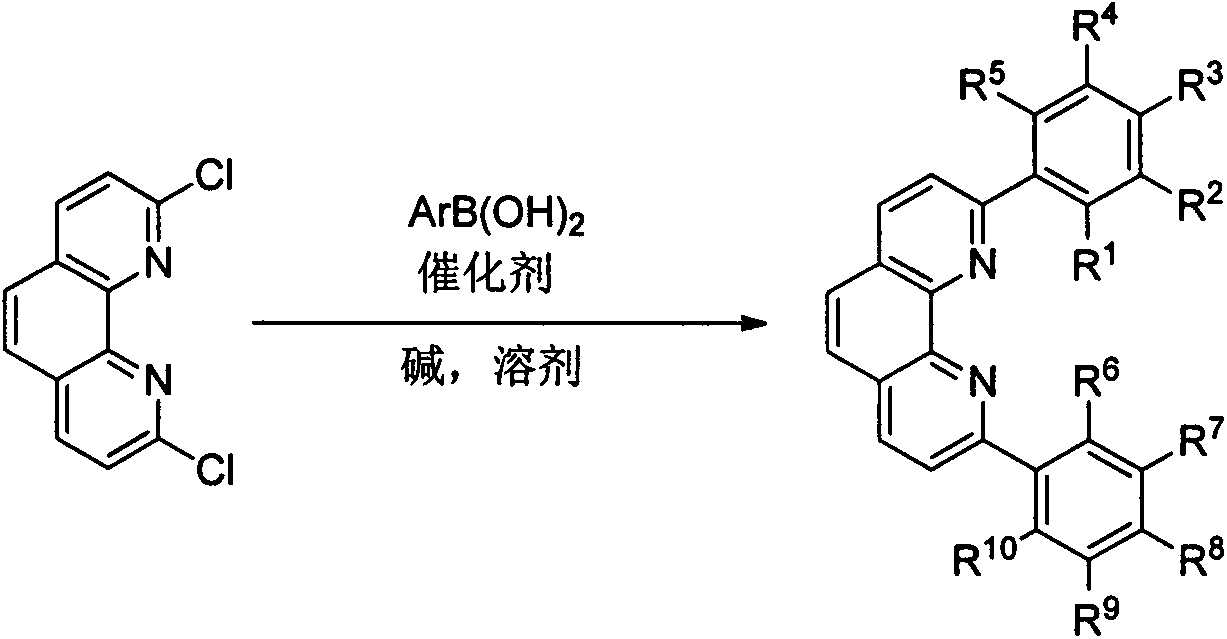
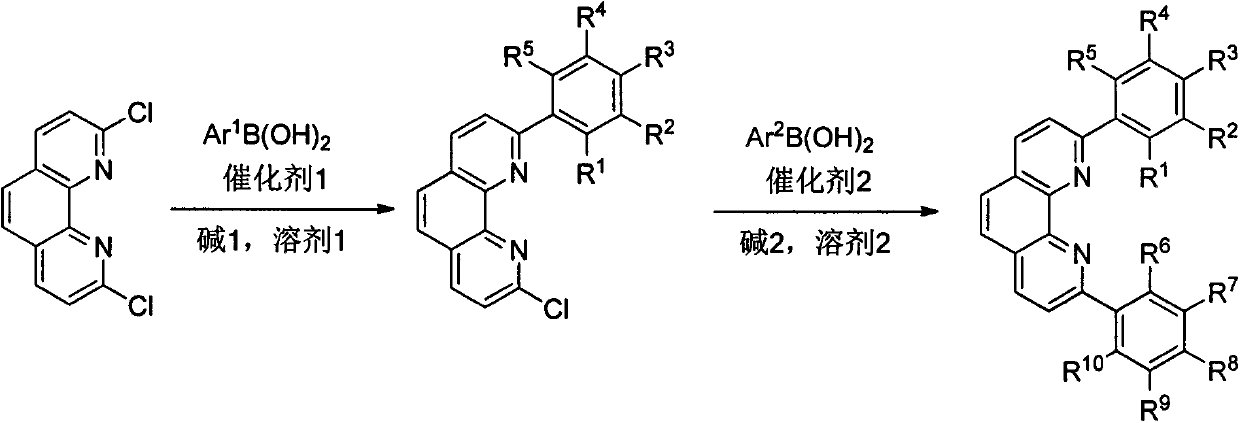
Preparation method and application of 2, 9-diaryl-substituted phenanthroline and 2, 9-diaryl-substituted phenanthroline iron complex
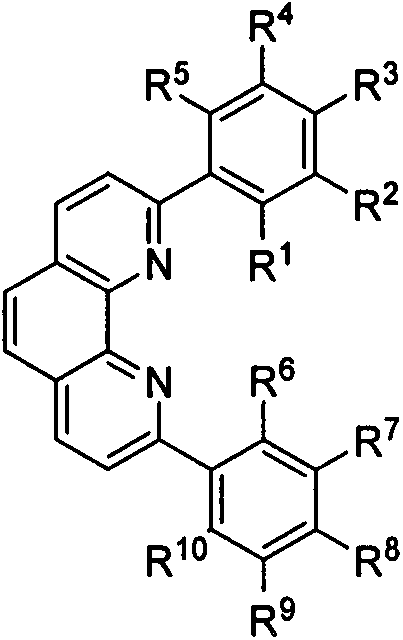
ActiveCN107586296AImprove tolerancePromote resultsSilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsIron saltsSilanes
The present invention relates to a preparation method and application of 2, 9-diaryl-substituted phenanthroline and a 2, 9-diaryl-substituted phenanthroline iron complex. Specifically, the substitutedphenanthroline is prepared by Suzuki coupling reaction of 2, 9-diaryl-dichloro-phenanthroline and arylboronic acid, and the 2, 9-diaryl-substituted phenanthroline iron complex is prepared by complexation reaction of the substituted phenanthroline and an iron salt. The phenanthroline iron complex can catalyze hydrosilation reaction of various olefins or alkynes with silane in the presence of additives, shows very high activity and selectivity especially for the hydrosilation reaction of styrene derivatives, 1-arylbutadiene and 1-alkyl-1-arylbutadiene, shows excellent 1,2-disubstituted ethylenebenzyl-site selectivity, and has a good application prospect.
Owner:NANKAI UNIV
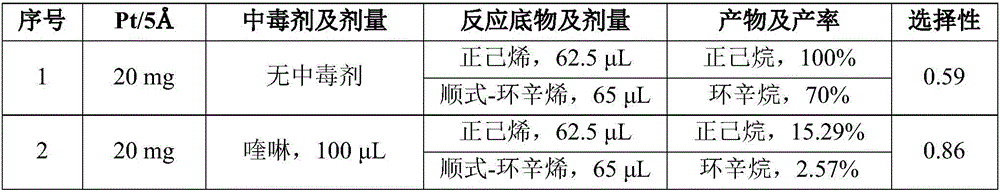
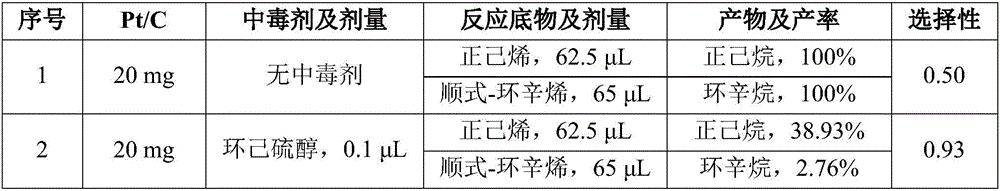
Method for improving catalytic selectivity of supported catalyst and application thereof
ActiveCN106824268AHigh selectivityMolecular sieve catalystsHydrocarbon by hydrogenationChemical reactionKinetic diameter
The invention relates to a method for improving the catalytic selectivity of a supported catalyst and application thereof. When a catalyst support is a porous one, a poisoning agent of which the kinetic diameter is greater than the dimension of a support pore and a previously prepared supported catalyst are sufficiently stirred to react; and when the catalyst support is a non-porous one, a poisoning agent of which the kinetic diameter is greater than that of a target selective reactant and the previously prepared supported catalyst are sufficiently stirred to react, thereby obtaining the poisoned supported catalyst. According to the method, the catalytic activity of internal metal nanoparticles is retained by reducing the catalytic activity of metal nanoparticles on the external surface of the support; or the effect of improving the catalytic selectivity of the supported catalyst is achieved by screening the target reactant by means of the poisoning agent. The method can effectively improve molecular dimension selectivity or chemical reaction site selectivity in catalytic hydrogenation reaction of olefin.
Owner:NANJING UNIV OF TECH
Adjustable light fixture and lighting system
ActiveUS10208933B1Equally distributedLighting support devicesElongate light sourcesElectricityLight spot
An adjustable light fixture and lighting system are selectively adjustable in the field and provide even light distribution across a space. A housing is selectively adjustable to a selected housing length and is mountable to the surface. A tray mountable to the housing includes plates which are selectively adjustable relative to one another to a selected tray length. Each plate includes a plurality of electroluminescent light sources providing uniformly luminous light across the light fixture. Overlap of plates varies the tray length and blocks light from light sources on one plate by the opposite plate. Evenly distributed light is therefore provided with no bright or dark spots. A lighting system of a plurality of light fixtures electrically connected to one another includes at least one adjustable light fixture, and preferably at least one stationary light fixture of fixed length. Methods of installation are also disclosed.
Owner:FORUM LIGHTING INC
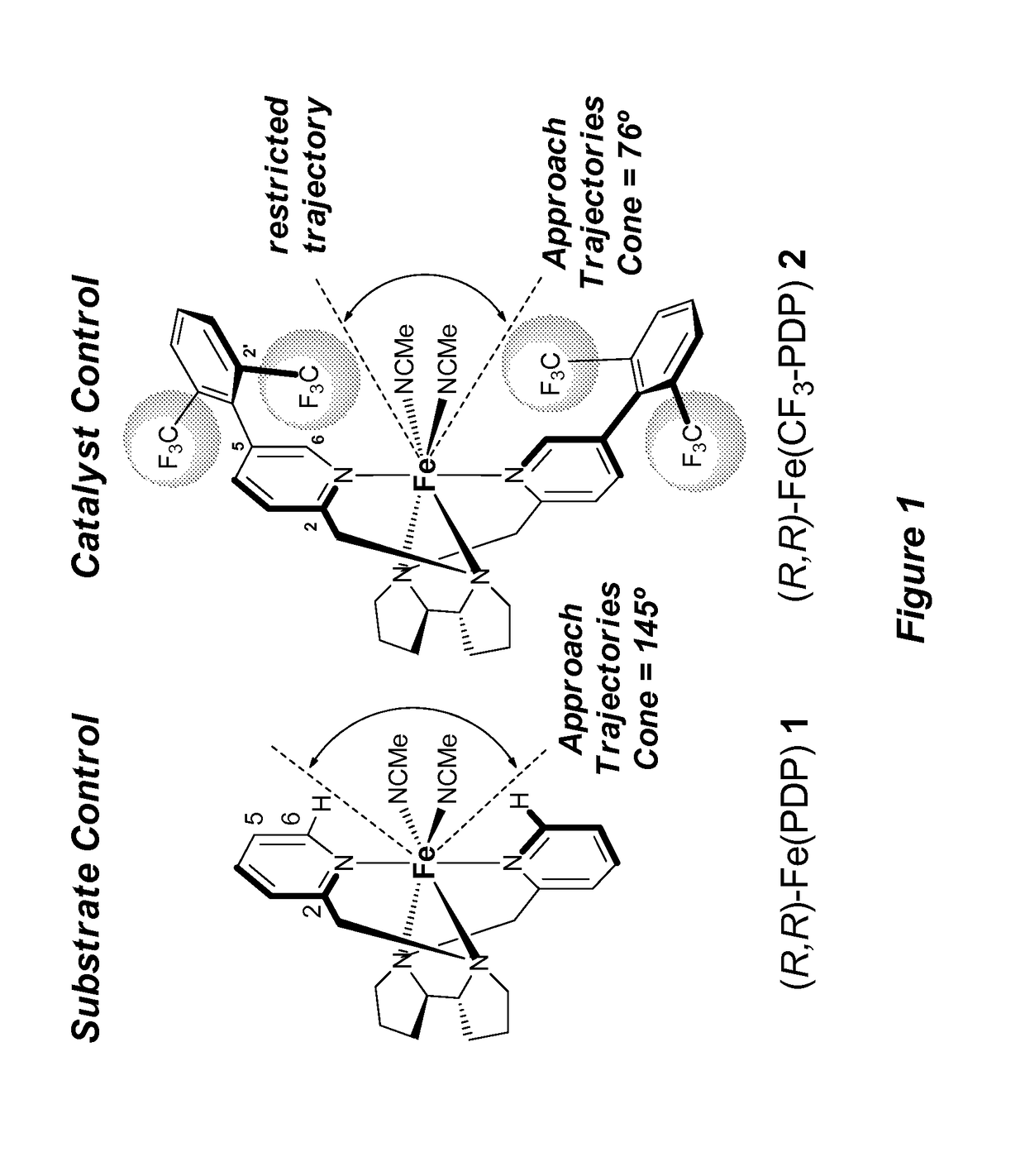
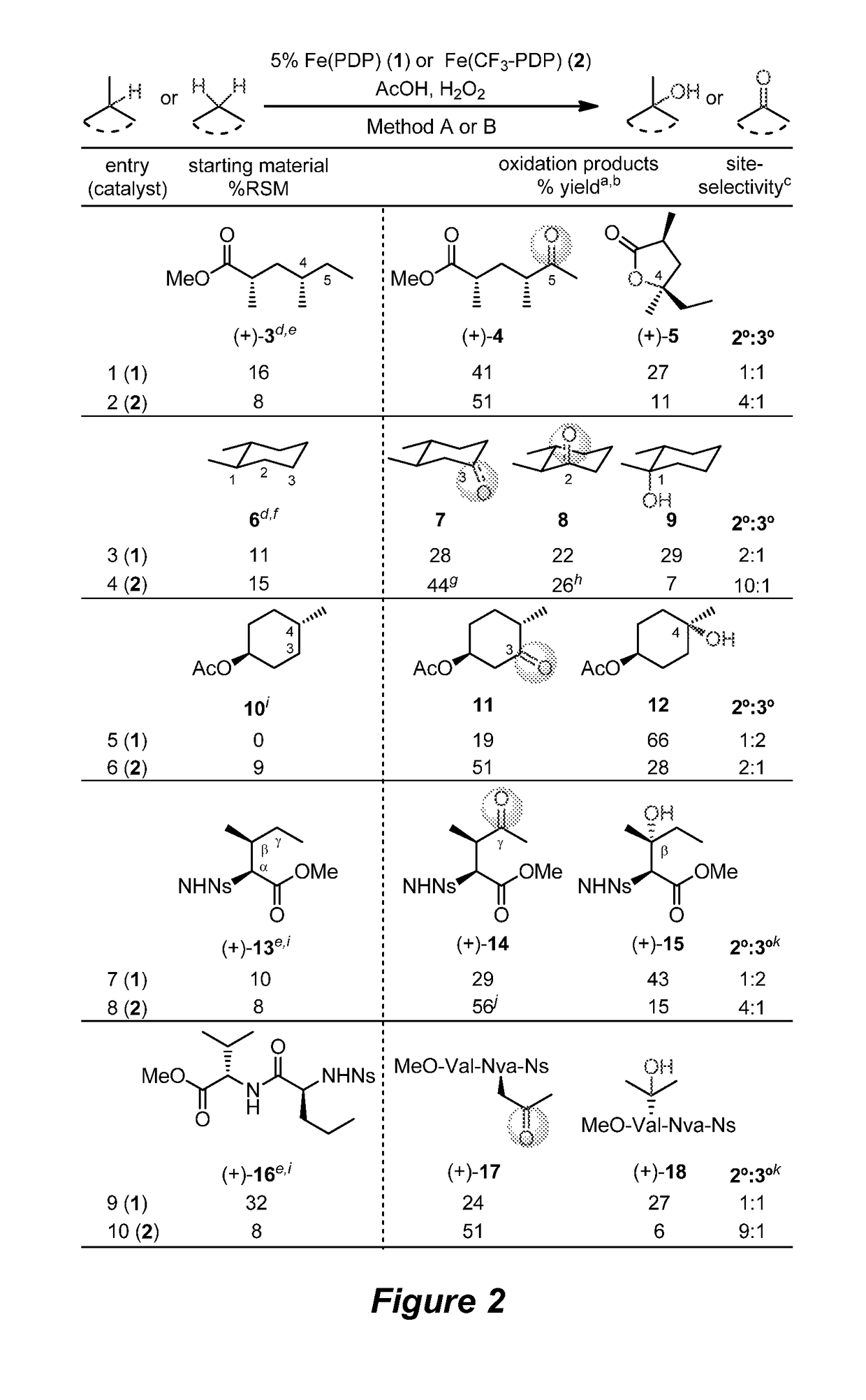
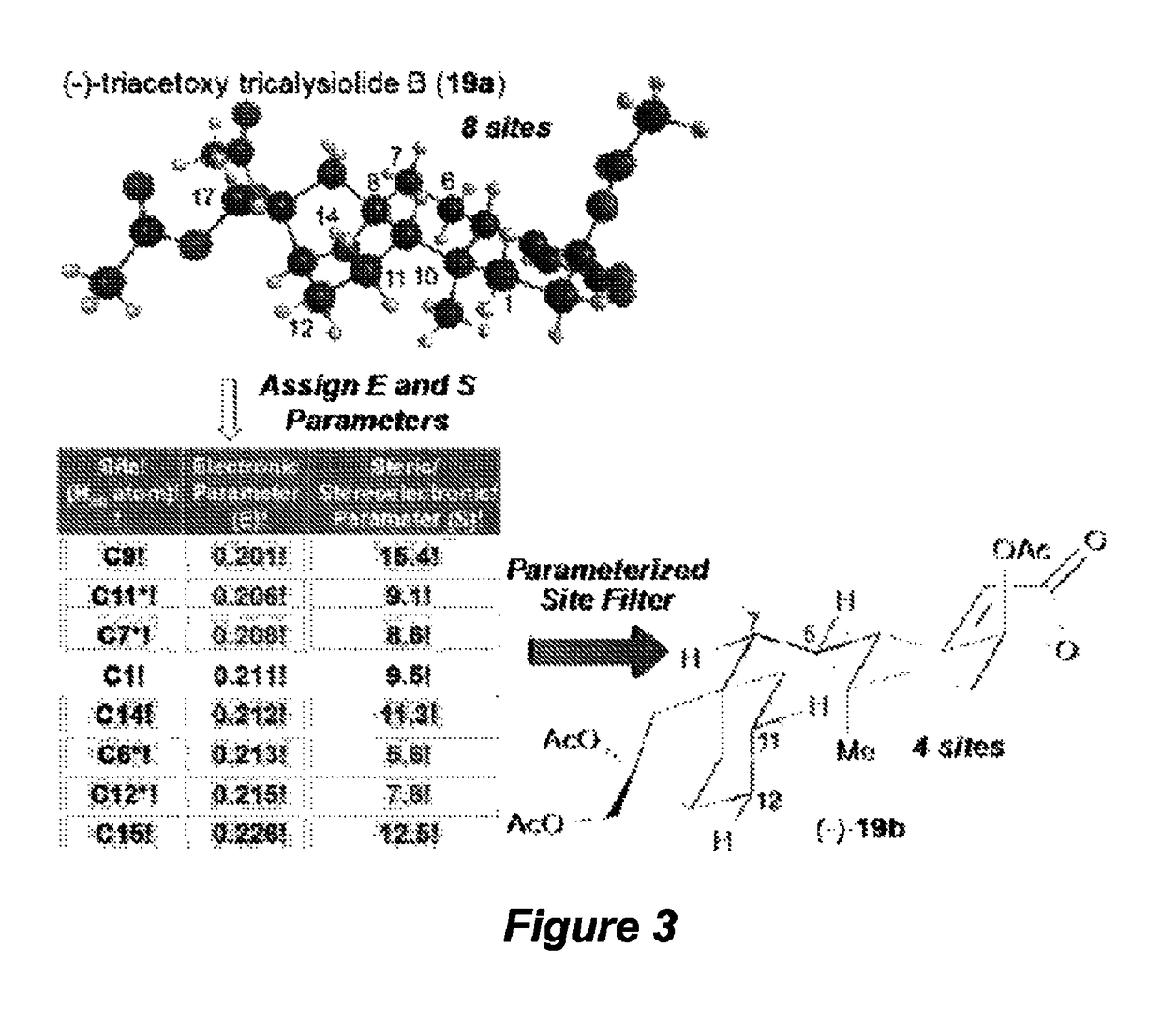
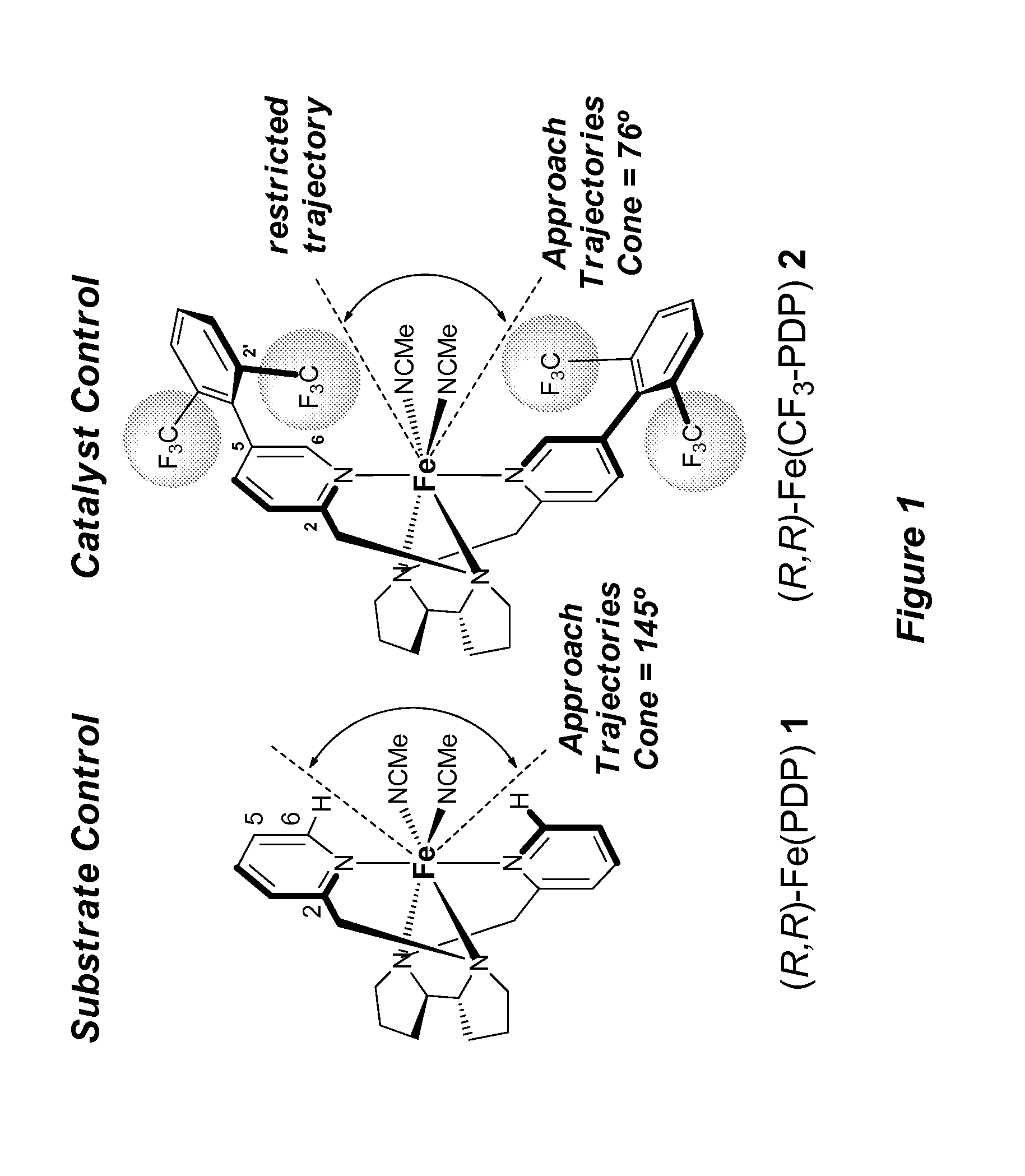
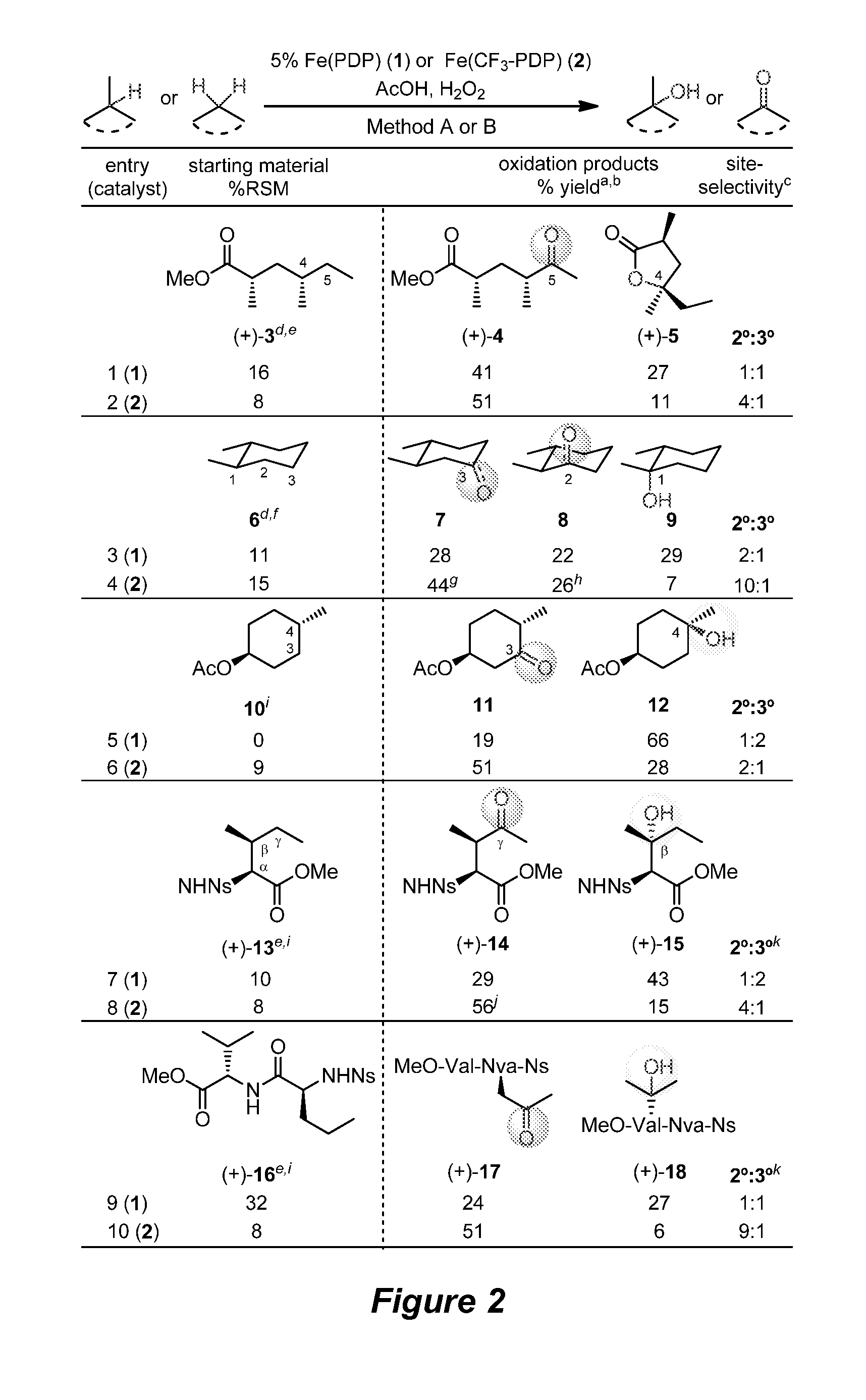
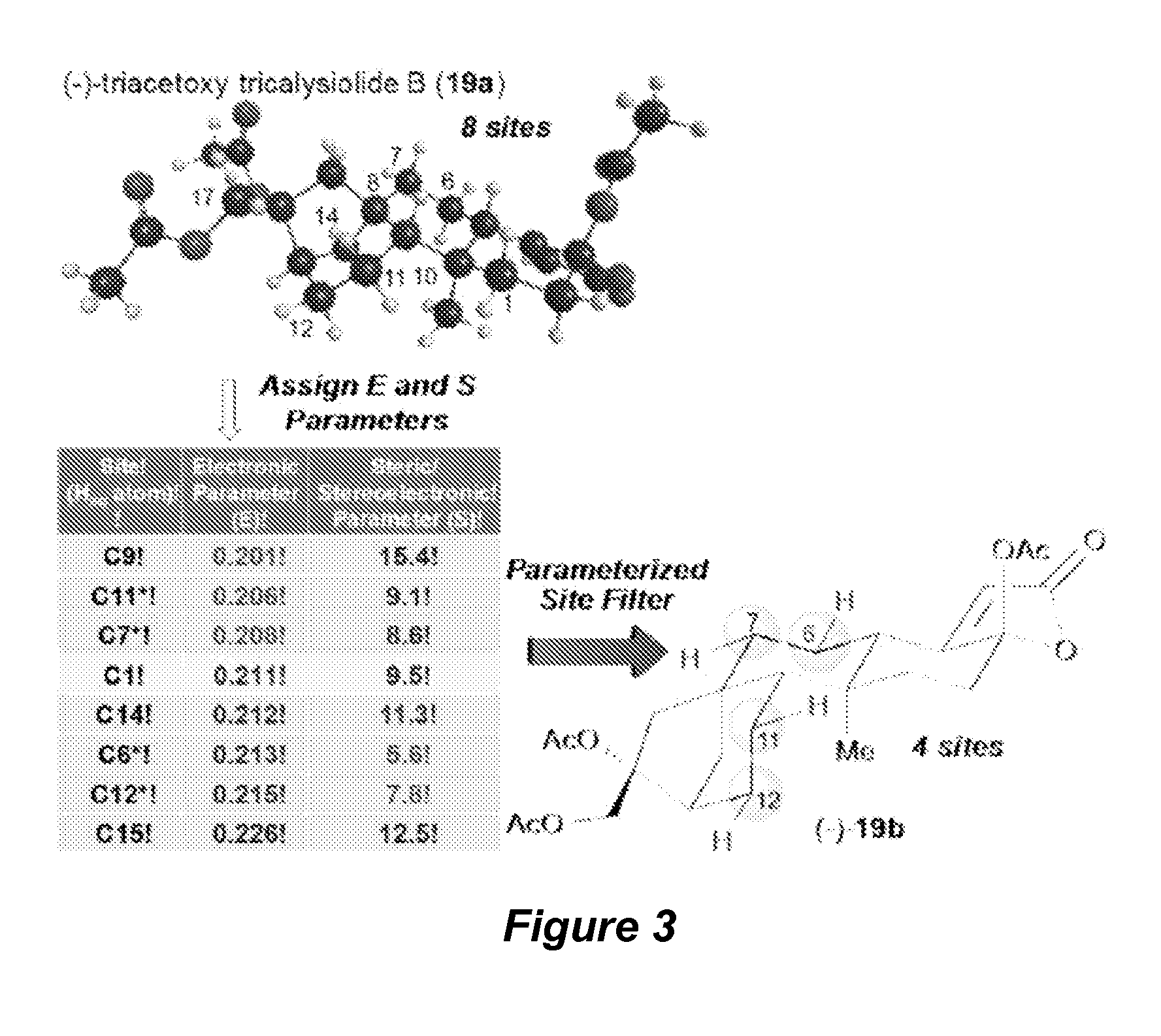
Catalyst-controlled aliphatic C—H oxidations
ActiveUS9925528B2Improve responseHigh selectivityOrganic oxidationOrganic compound preparationSite selectivityOxidizing agent
The invention provides simple small molecule, non-heme iron catalyst systems with broad substrate scope that can predictably enhance or overturn a substrate's inherent reactivity preference for sp3-hybridized C—H bond oxidation. The invention also provides methods for selective aliphatic C—H bond oxidation. Furthermore, a structure-based catalyst reactivity model is disclosed that quantitatively correlates the innate physical properties of the substrate to the site-selectivities observed as a function of the catalyst. The catalyst systems can be used in combination with oxidants such as hydrogen peroxide to effect highly selective oxidations of unactivated sp3 C—H bonds over a broad range of substrates.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
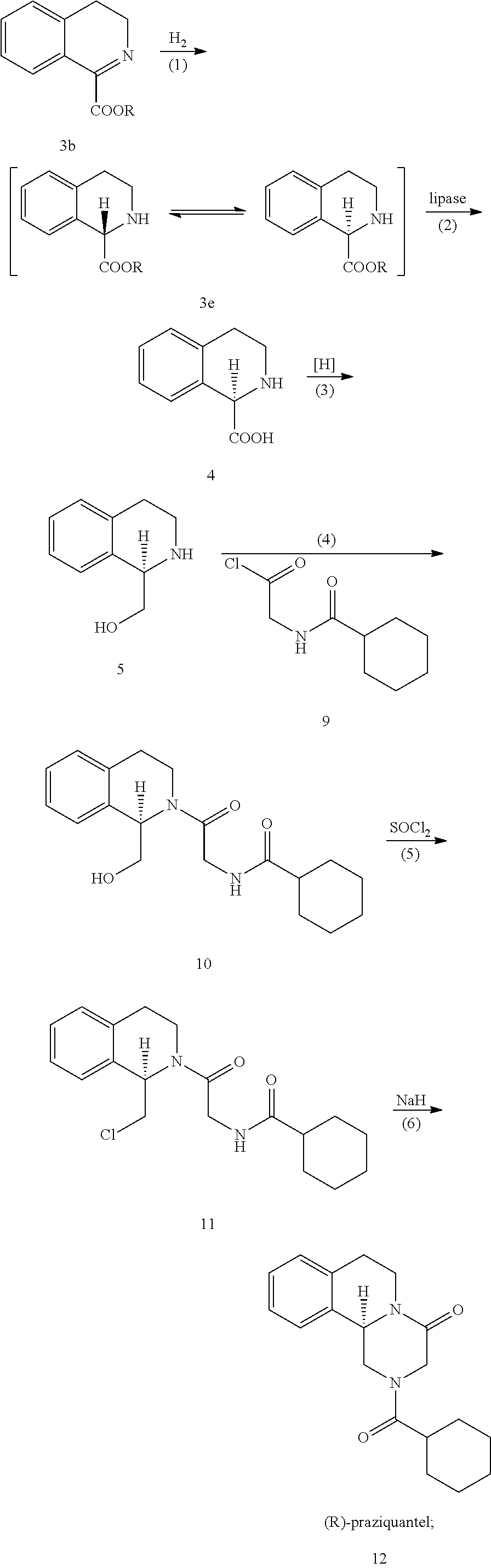
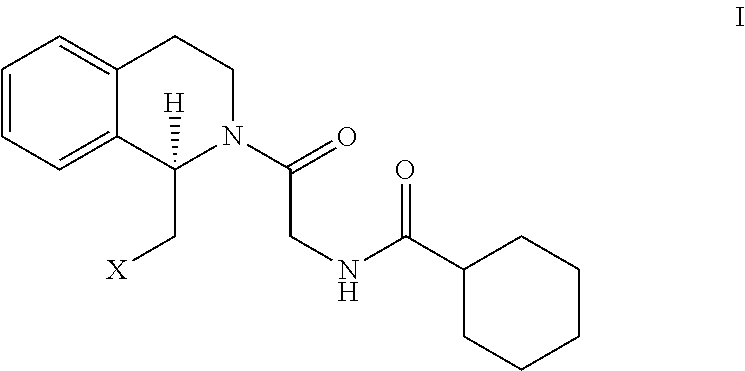
Method for preparing (r)-praziquantel
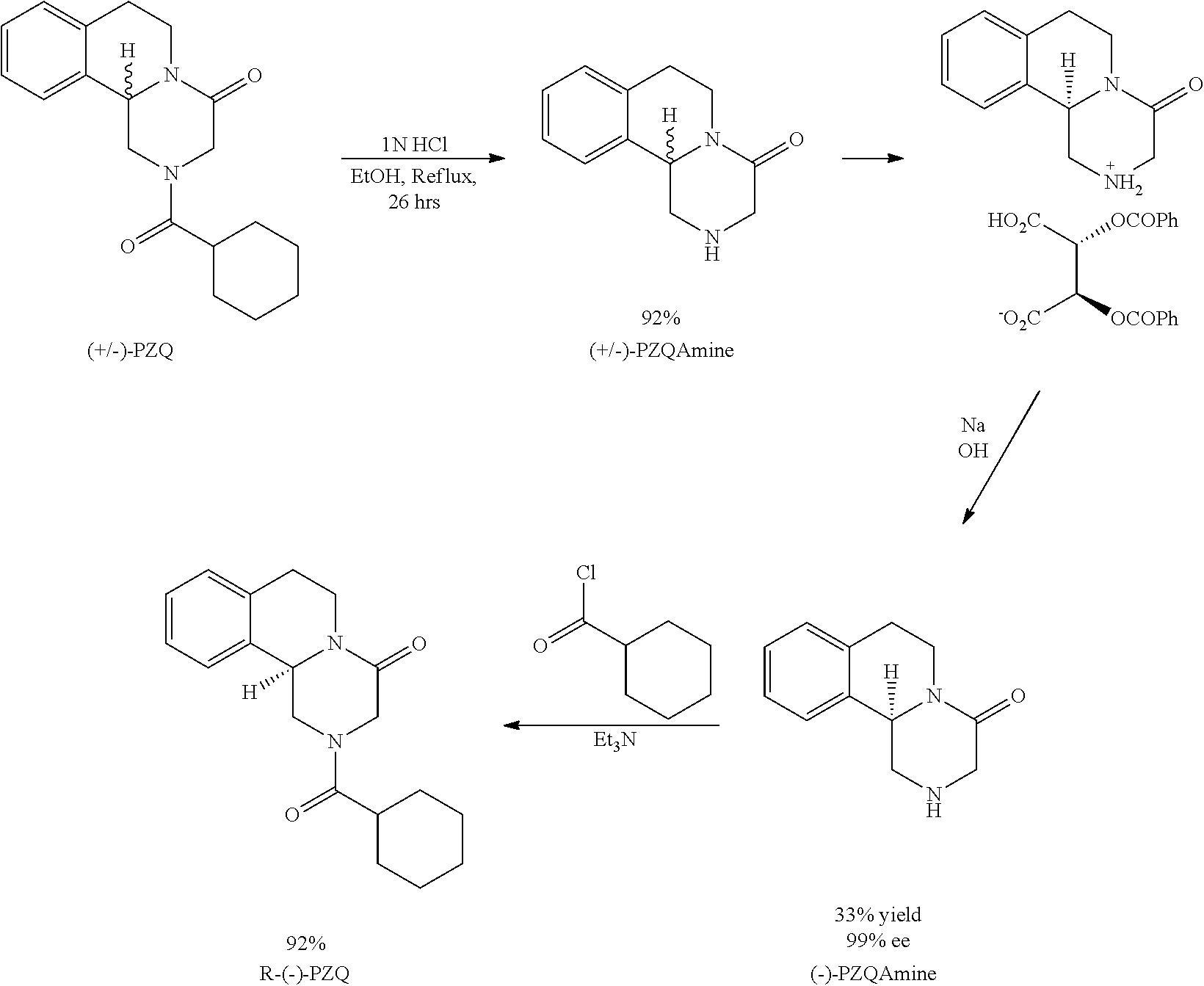
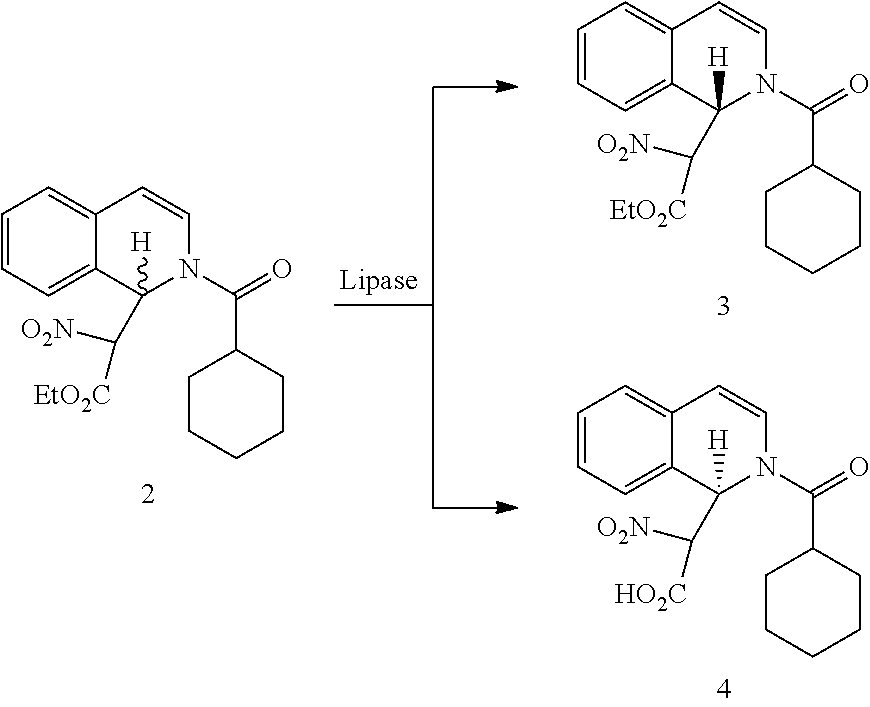
ActiveUS20140370555A1High stereoselectivityReadily availableFermentationAsymmetric synthesesChemical reactionEnantiomer
The invention relates to a new method for preparing (R)-praziquantel. In the invention, by taking advantage of the high stereo selectivity, site selectivity and region selectivity of an enzyme, an intermediate of a pure optical and chiral (R)-praziquantel are obtained by means of the dynamic kinetic resolution of an enantiomer from the synthesized racemate or derivatives thereof, and the (R)-praziquantel is obtained by using various conventional and mature organic chemical reactions with higher yield. The method of the invention has the potential advantages of easily available raw materials, low cost, environmentally safer process and convenience for large-scale production. Also, the purity of the end product can be more than 98%. By adopting the invention, the quality of the product is improved and a basis for developing high quality of active pharmaceutical ingredients and formulations is established, and thus the pending industrial problem of purifying praziquantel over 30 years becomes solvable.
Owner:TONGLI BIOMEDICAL
A method for separating histidine enantiomers with chitosan-modified gold nanochannel membrane and its detection method
InactiveCN103896846BEasy to separateShorten detection timeOrganic chemistryRaman scatteringEnantiomerSite selectivity
The invention discloses a method of separating histidase enantiomer by a chitosan-modified gold nanochannel film. The method comprises the following steps: by taking a polycarbonate film and an aluminum oxide film as base films, adopting a chemical deposition method to prepare a gold nanochannel film; self-assembling chitosan onto the pore wall of the gold nanochannel to form a functional nanochannel film with chiral site selectivity on the surface; and separating D-histidine, L- histidine in a chiral manner by utilizing excellent separation ability of the nanochannel. During detection, silver sol is used as a surface enhanced Raman substrate to enhance SERS (surface enhanced raman scattering) effect of D-histidine, L-histidine, so that selectivity and sensitivity of the substance are improved to detect the D-histidine and the L-histidine at the same time. The invention provides a quicker and more convenient method for separating and detecting the chiral substance by constructing a coupling device of a nanochannel separating tank and an SERS detecting system, and shows unique advantages and wide application prospect of the method.
Owner:SHANGHAI NORMAL UNIVERSITY
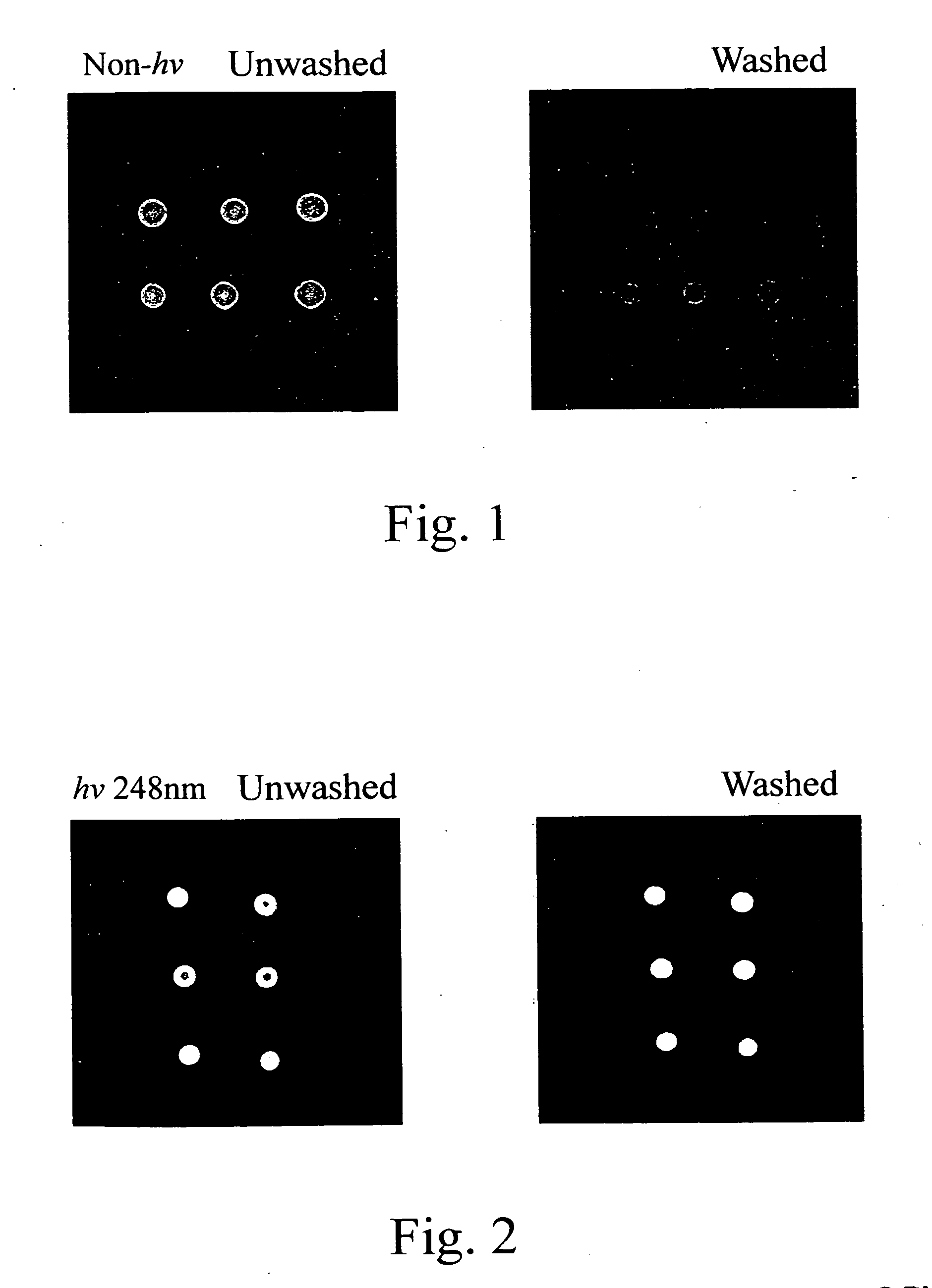
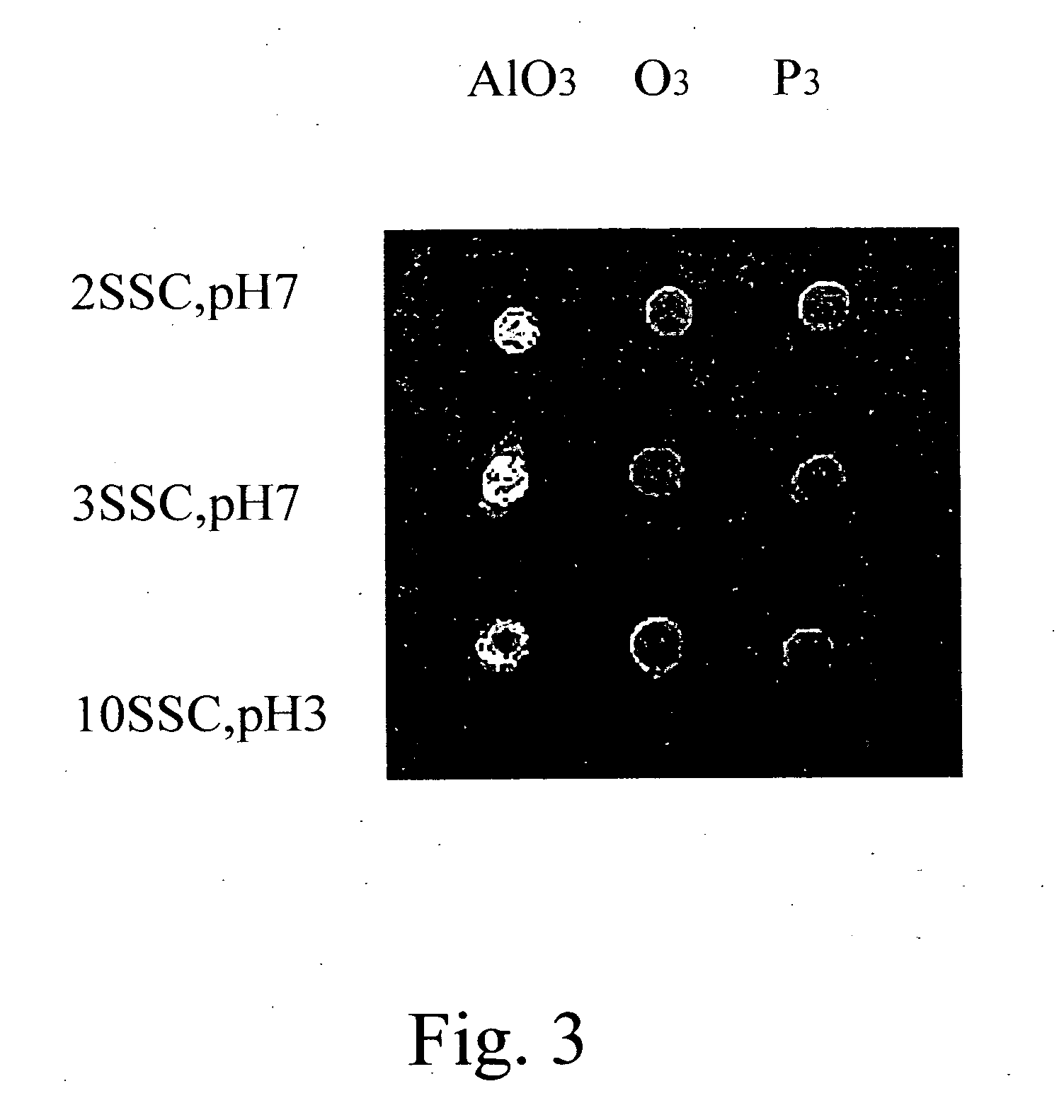
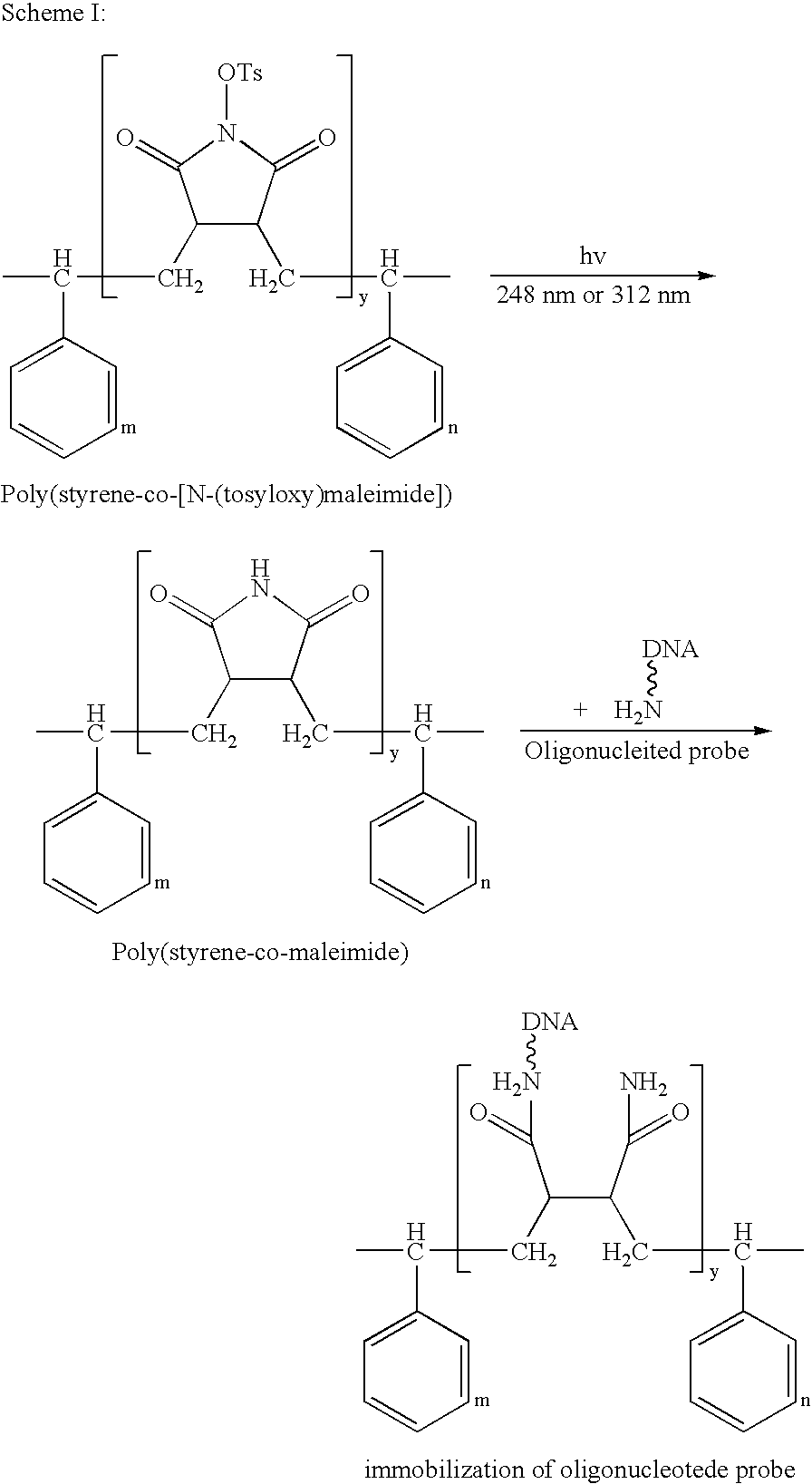
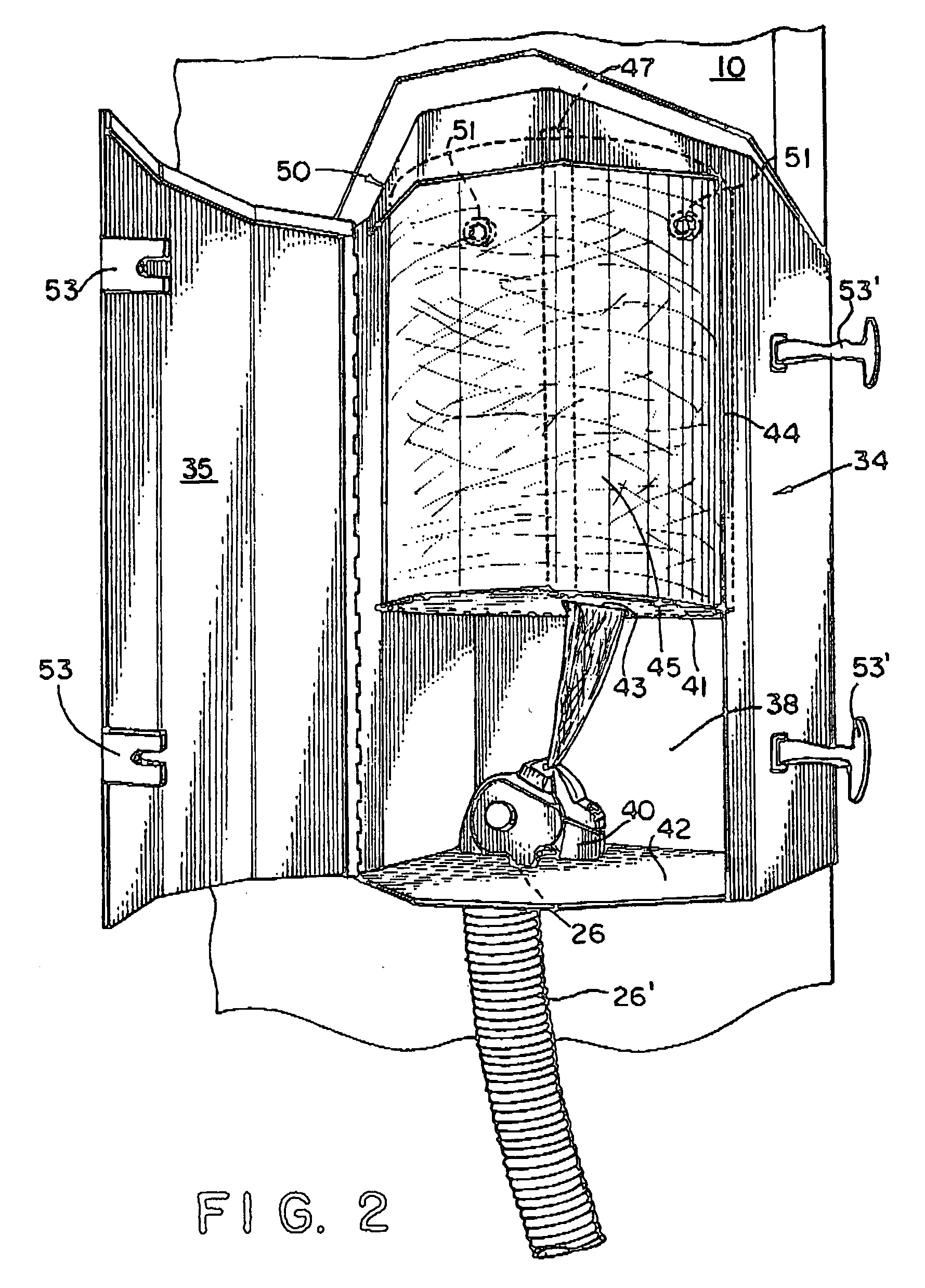
On-spot selectively activated hydrophobic slide and preparation thereof
InactiveUS7105340B2Efficient preparationMaterial nanotechnologyBioreactor/fermenter combinationsImideHigh density
Owner:IND TECH RES INST
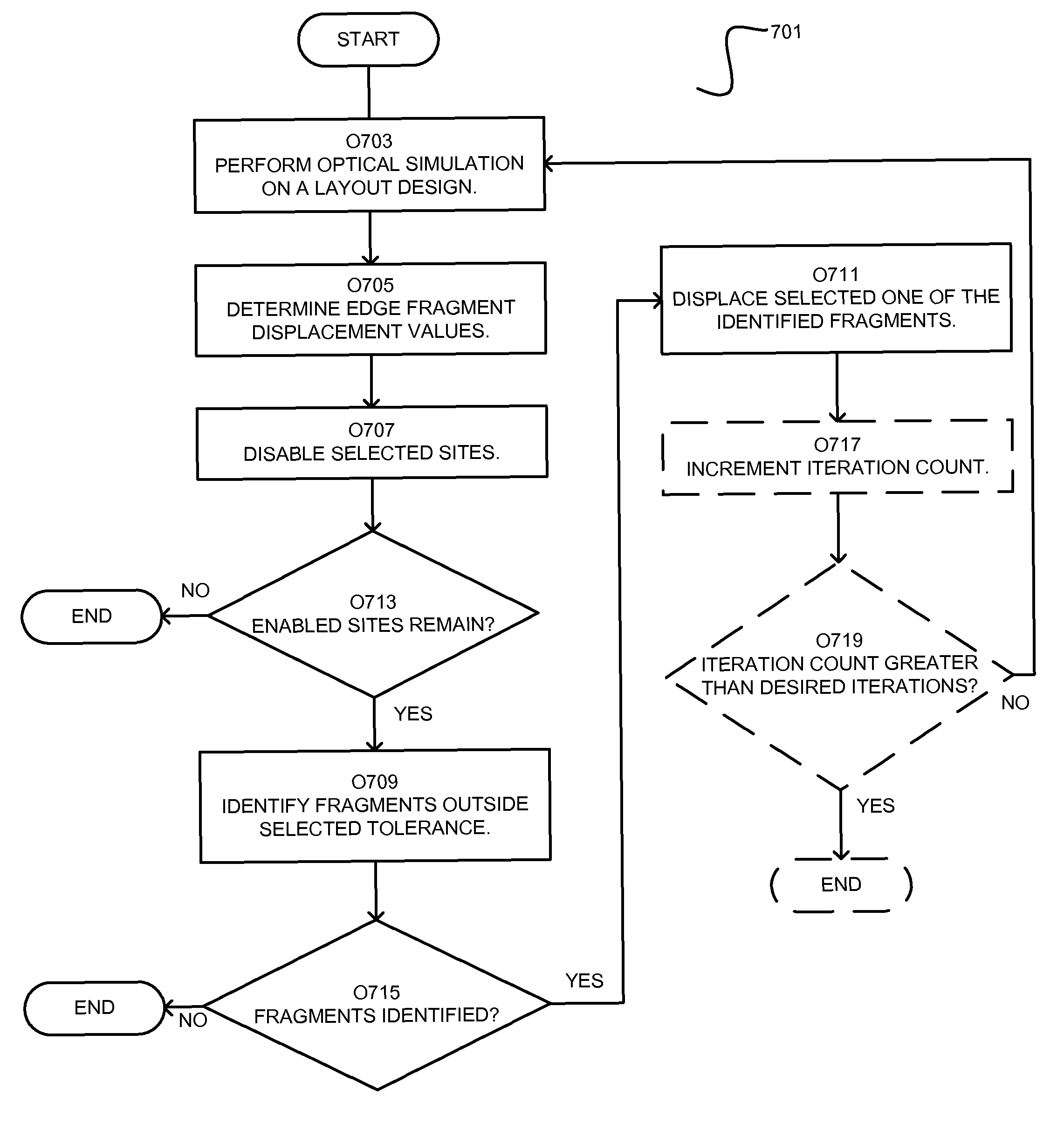
Site selective optical proximity correction
ActiveUS8191017B2Photomechanical apparatusOriginals for photomechanical treatmentEdge segmentSite selectivity
Owner:SIEMENS PROD LIFECYCLE MANAGEMENT SOFTWARE INC
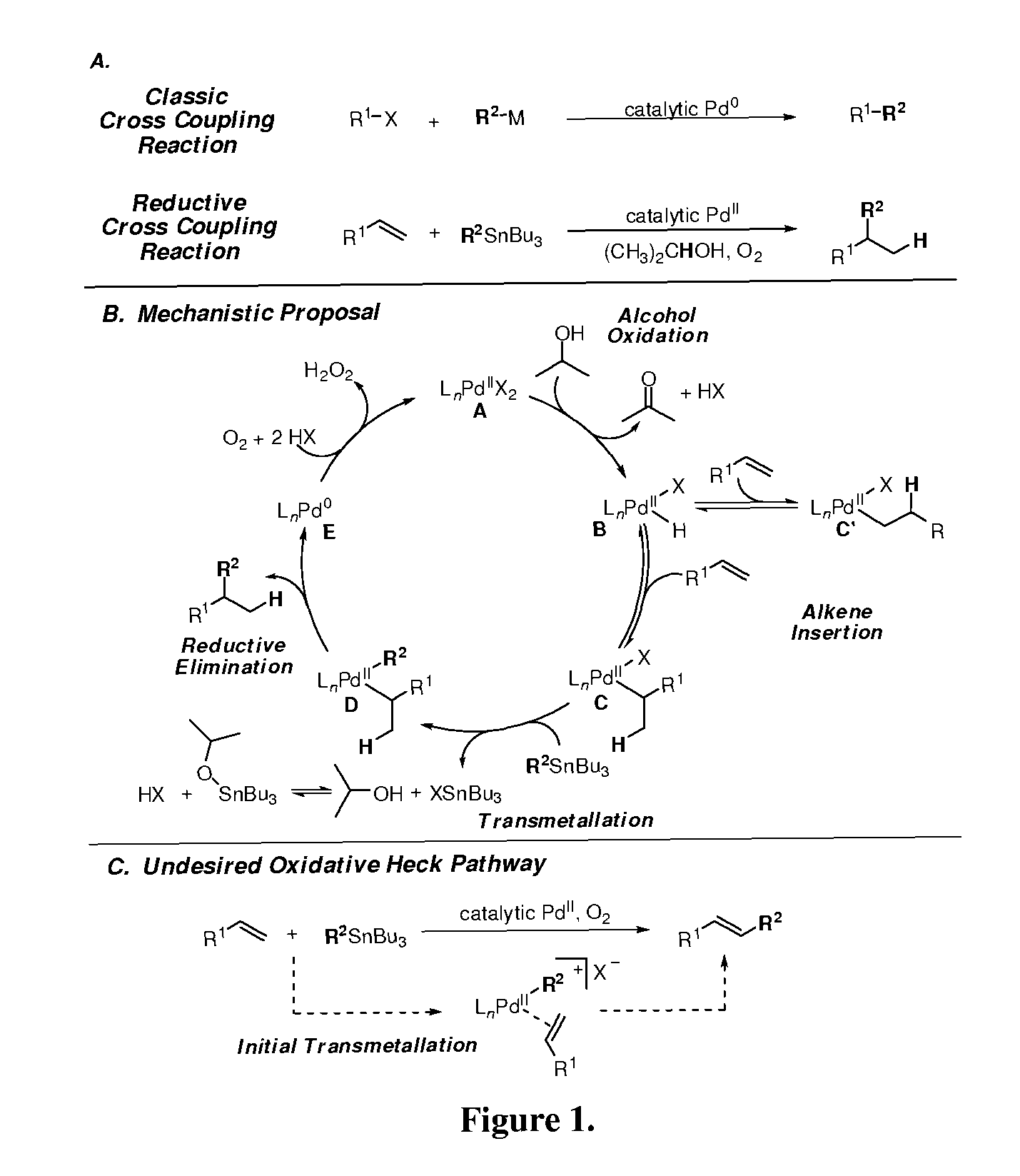
Alkene hydrofunctionalization reactions
InactiveUS8088346B2Improve versatilityAchieve functionalizationOther chemical processesRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsAlcoholRegioselectivity
Owner:UNIV OF UTAH RES FOUND
R-praziquantel preparation method
ActiveUS20140256003A1High yieldSuitable and favorable large-scale industrial productionOrganic chemistryFermentationChemical synthesisEnantiomer
Provided are R-praziquantel preparation methods, which utilize the characteristics of biological enzyme of strong stereoselectivity, site-selectivity, and regioselectivity, of high resolution efficiency, of mild reaction conditions, and of simple operations to catalyze the hydrolysis of a certain enantiomer in a chemically synthesized racemate or a derivative, thus acquiring a mixture of reacted and unreacted optical isomers. R-praziquantel prepared by the method can have a purity of 98% or more.
Owner:TONGLI BIOMEDICAL
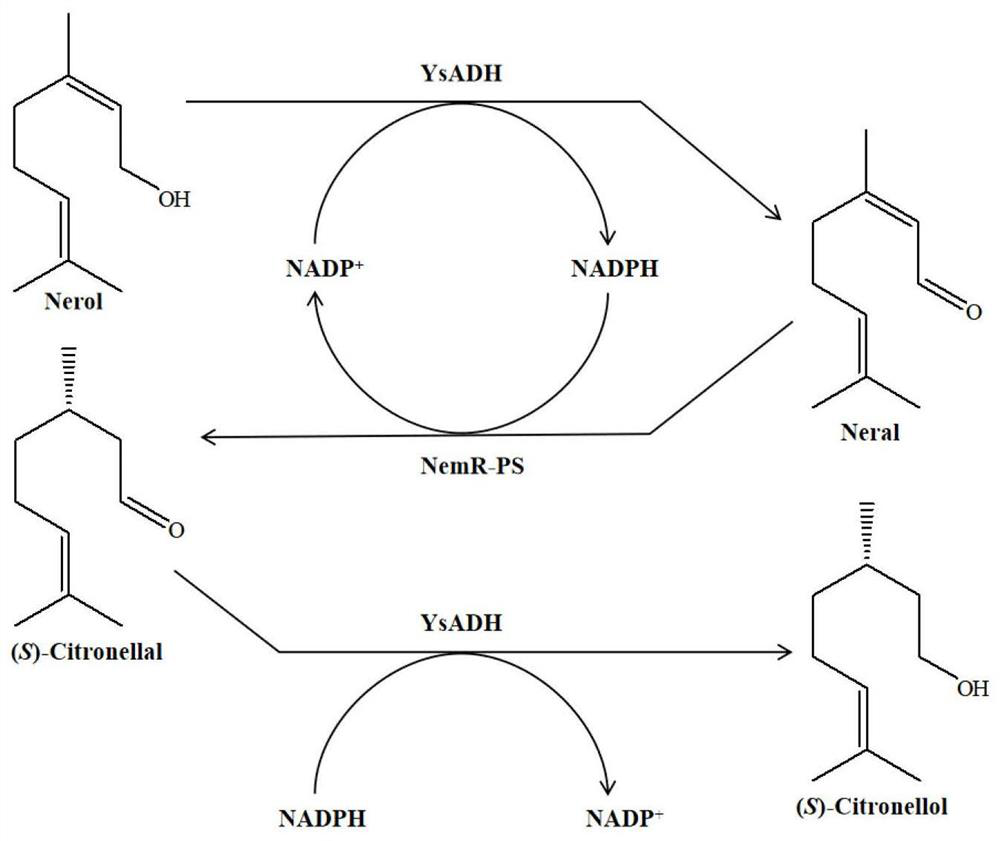
Method for synthesizing (S)-citronellol through double-enzyme coupling
PendingCN113930457AThe synthetic method is green and efficientAtom economy is highMicroorganism based processesOxidoreductasesPtru catalystSite selectivity
The invention discloses a method for synthesizing (S)-citronellol through double-enzyme coupling. The method comprises the following steps of mixing wet bacteria obtained by respectively fermenting and culturing an engineering bacterium containing an alcohol dehydrogenase YsADH gene and an engineering bacterium containing an old yellow enzyme NemR-PS gene as a catalyst, taking nerol as a substrate, taking NADP <+> as a coenzyme, and taking a buffer solution with the pH value of 6-9 as a reaction medium to form a reaction system; and after reaction is completed under the conditions of 25-55 DEG C and 0-900rpm, separating and purifying reaction liquid to finally obtain the (S)-citronellol. When 100 mM nerol is used as the substrate, the conversion rate of the product (S)-citronellol after 12 hours of reaction is as high as 99.74%, and the e.e. value of the product is greater than 99%. Compared with the prior art, the established method for synthesizing the (S)-citronellol is green and efficient, is high in atom economy, and has excellent site selectivity, chemical selectivity and enantiomer selectivity.
Owner:ZHEJIANG UNIV OF TECH
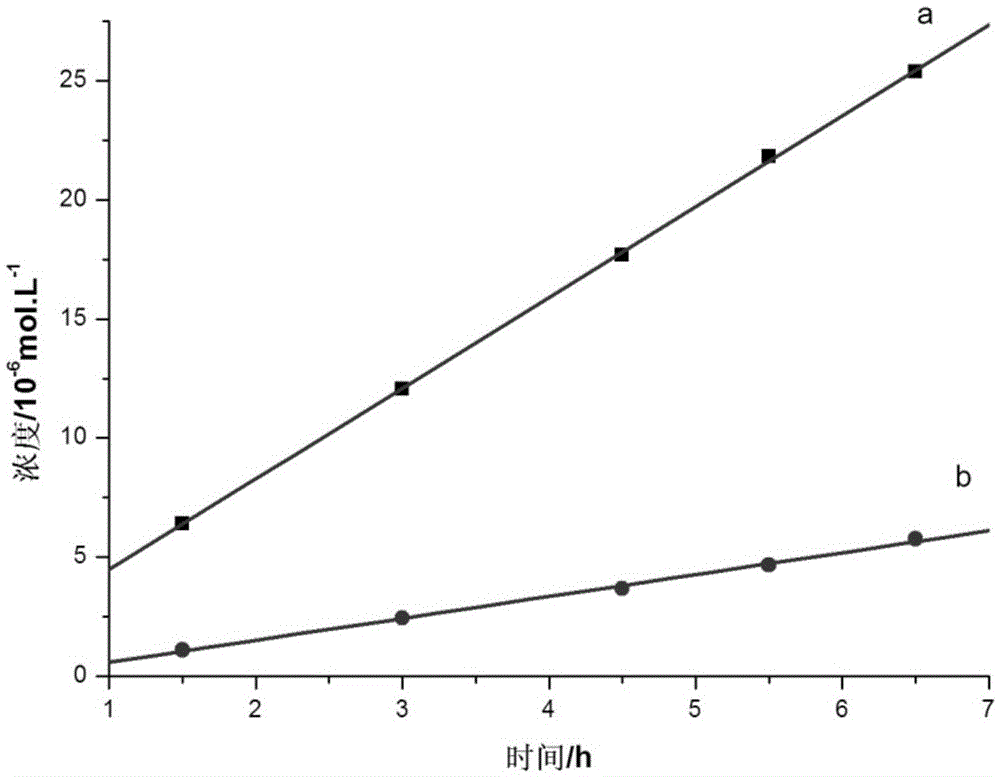
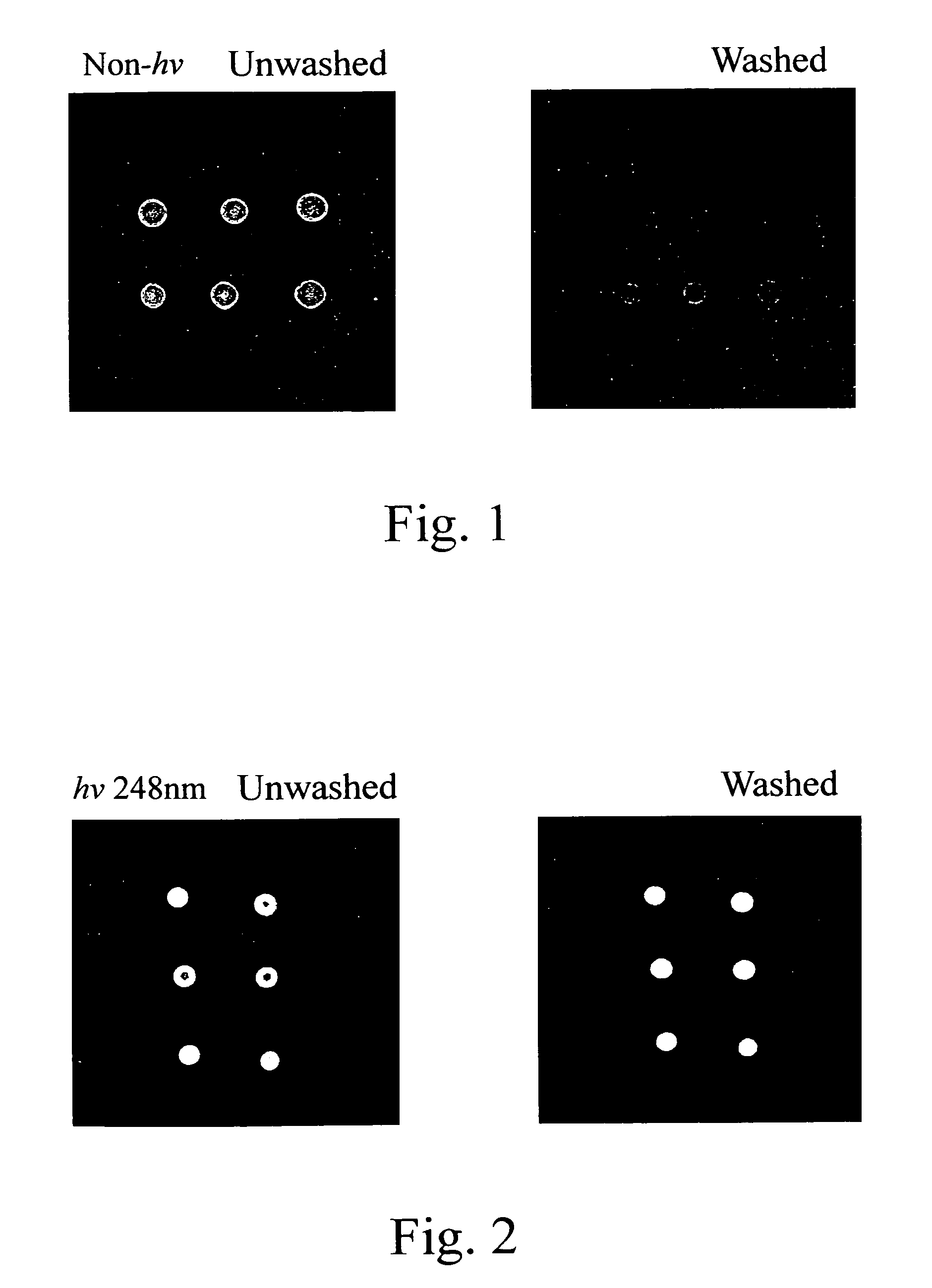
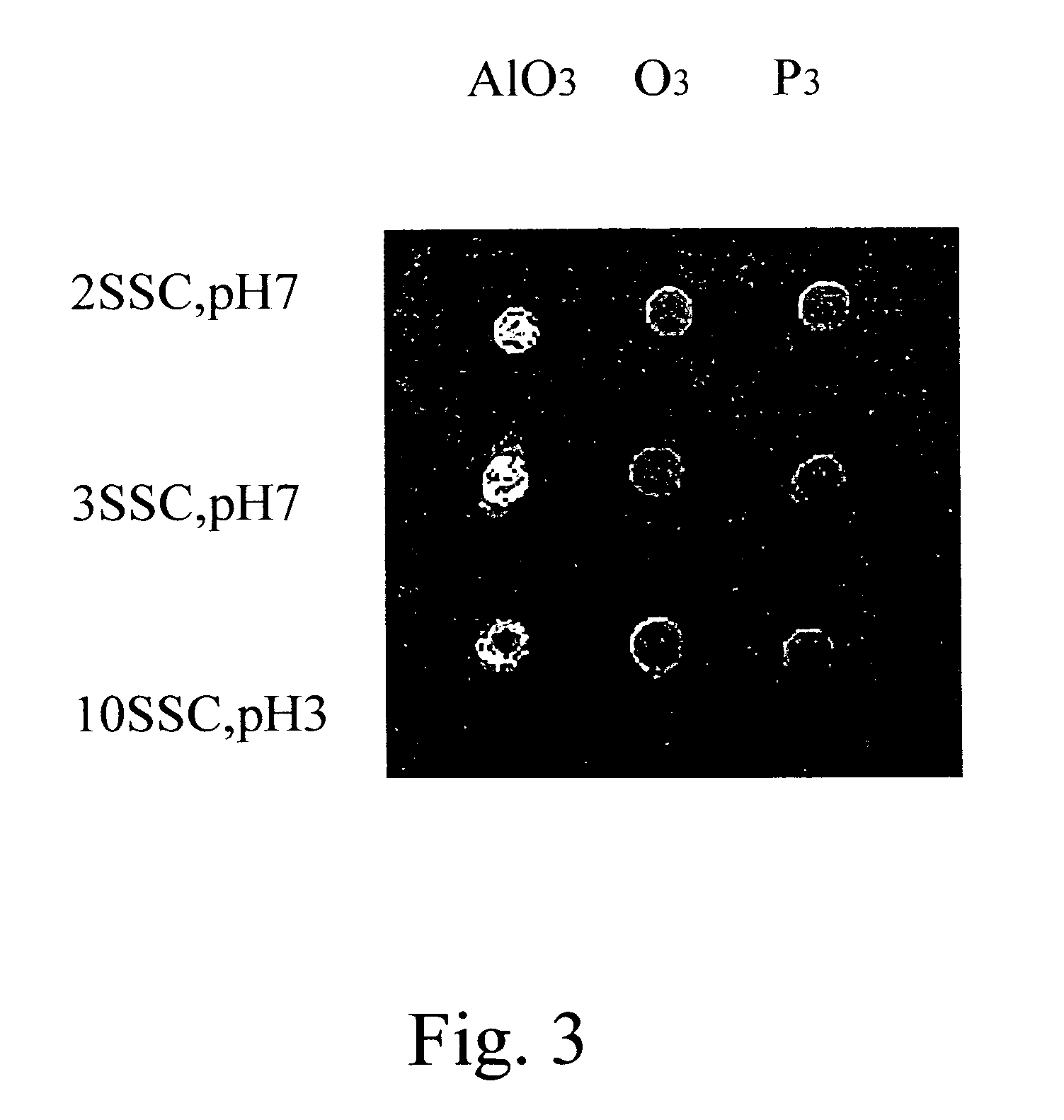
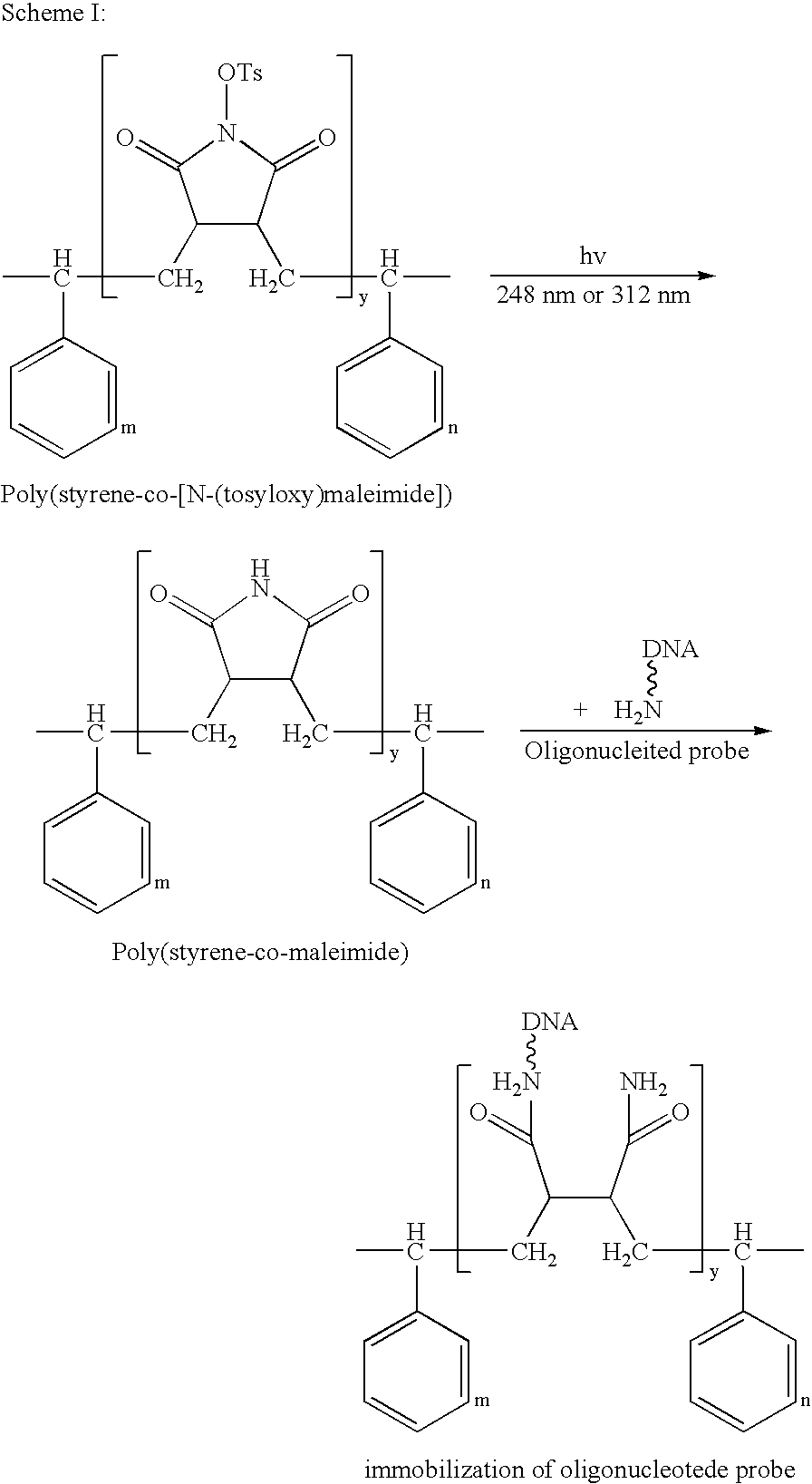
On-spot selectively activated hydrophobic slide and preparation thereof
InactiveUS20060029974A1Efficient preparationMaterial nanotechnologyBioreactor/fermenter combinationsImideHigh density
An on-spot selectively activated hydrophobic slide / microarray. The preparation method relates to a hydrophobic copolymer prepared by blending, grafting or co-polymerization of a hydrophobic material and a compound bearing a functional group protected by a protecting group, wherein the functional group is imide or cyclic amide, and the protecting group is a photo acid group such as a tosyloxy group. The hydrophobic copolymer coated on a substrate is then subjected to selective photolithographical activation so that the slide will have functional active copolymer spots separated by inactive copolymers. The resulting slide is suitable for the preparation of high-density and high-efficiency bio-chip / microarray.
Owner:IND TECH RES INST
Concrete ingredient delivery truck having a mixing means and a fiber strand chopping device for mixing concrete ingredients and short fiber strand lenghts at a job site and depositing the mixture
An apparatus and process for mixing and depositing a strand reinforced concrete mix at a job site from a concrete delivery truck including a mixing means on the truck for mixing separate concrete ingredients at a job site, which truck further includes a fiber strand chopping device on the truck disposed and adapted to introduce chopped fiber strand lengths into the separate concrete ingredients and water and deposit the mix selectively at the job site without clumping and grouping of the fiber strand lengths in the mix.
Owner:FORTA LLC
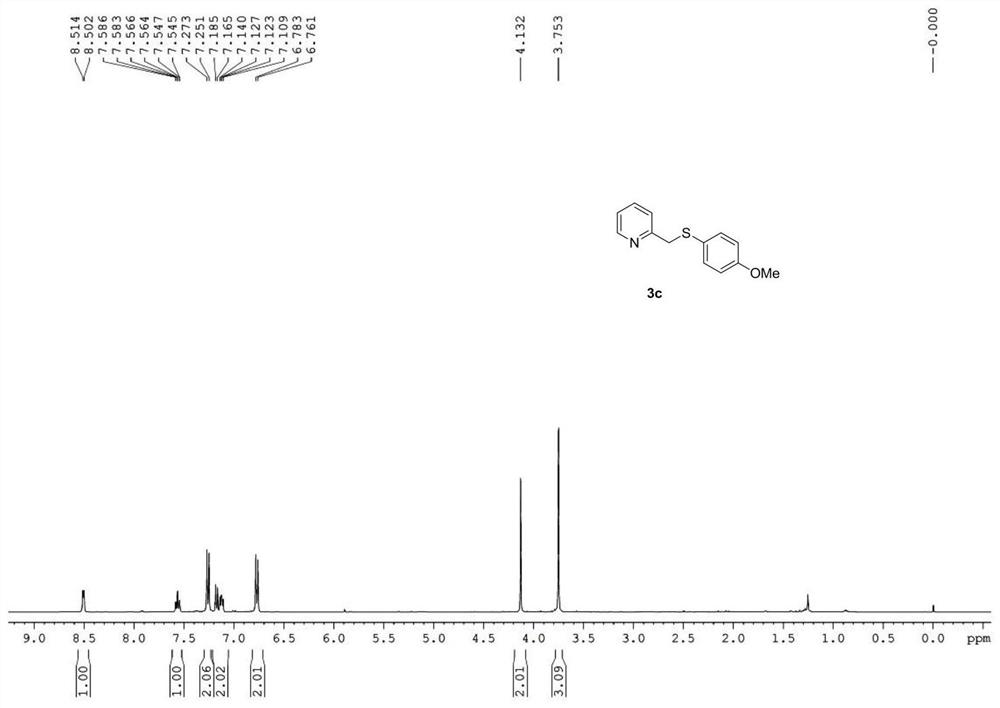
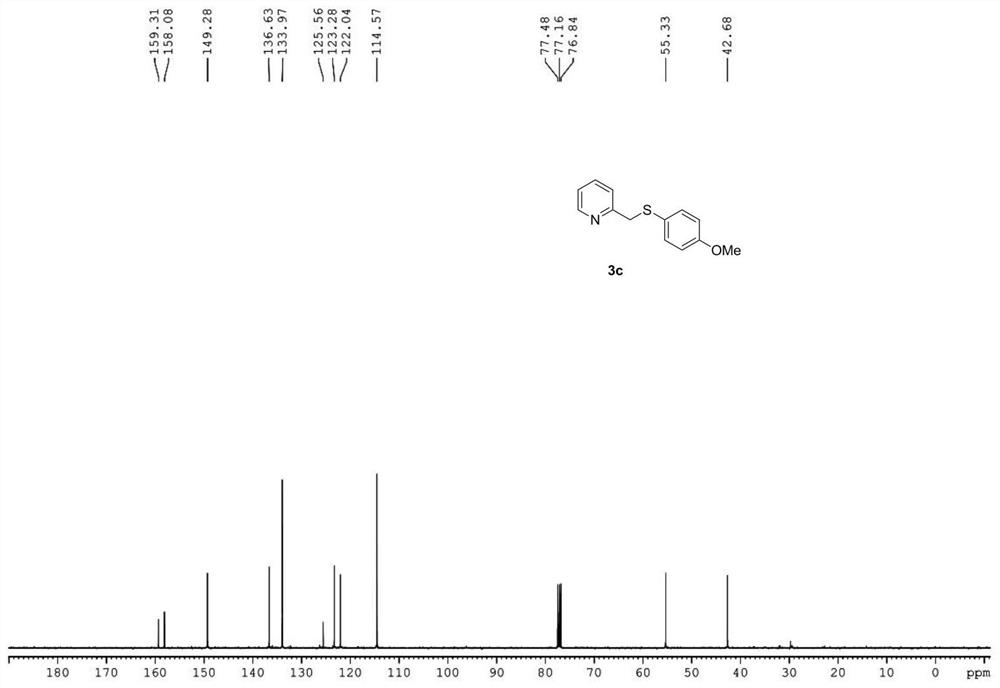
Synthetic method of 2-pyridylmethyl sulfide and synthetic process of related drugs
ActiveCN109134354BStrong position selectivityHigh yieldOrganic chemistryLithium bromideSite selectivity
The invention relates to a concise synthesis method of 2-picoline methyl sulfide and related medicines, specifically using 2-picoline nitrogen oxide as a raw material, ethyl acetate or dichloromethane as a solvent, and reacting with trifluoroacetic anhydride The trifluoroacetate intermediate is obtained without purification, under the catalysis of lithium bromide or tetrabutylammonium bromide, using toluene or ethyl acetate as a solvent and then reacting with thiophenol to generate 2-picolyl sulfide. The method has the obvious advantages of simple operation, cheap and easy-to-obtain reagents, mild reaction conditions, wide substrate applicability, good site selectivity and high yield. In addition, the method has been successfully applied to the synthesis of omeprazole sulfide and rabeprazole sulfide, and the synthesis process does not require any catalyst.
Owner:TIANJIN UNIV OF SCI & TECH
N-2-pyrimidinyl-3-fluoroindole compound and its preparation method and application
A preparation method of N-2-pyrimidinyl-3-fluoroindole compound shown in formula (I), described method comprises the following steps: take acetonitrile solvent as reaction medium, add N shown in formula (III) ‑2‑pyrimidinyl indole compound, selectfluor, CuI and photocatalyst, react at room temperature under blue light irradiation for 2 to 5 hours, after the reaction is complete, the resulting reaction mixture is post-processed to obtain N‑2‑pyrimidinyl represented by formula (I) ‑3‑fluoroindoles. This type of compound is a kind of inhibitor compound that can significantly inhibit the activity of monoamine oxidase, especially I-2 and I-3 have high selectivity to MAO-A, which provides a research basis for the screening of antidepressant and anti-Parkinson drugs . The invention uses a photocatalyst to react under blue light irradiation and room temperature, has mild reaction conditions, high site selectivity, high reaction efficiency, greenness and environmental protection, and the reaction yield can reach 85%.
Owner:ZHEJIANG UNIV OF TECH
Optical transparent pressure-sensitive adhesive material, optical transparent pressure-sensitive adhesive laminate, and process for producing same
InactiveCN102421864BReduce pollutionTransparency and easy controlLaminationLamination apparatusSilicone GelsSite selectivity
A highly pressure-sensitive adhesive optical transparent pressure-sensitive adhesive body or optical transparent pressure-sensitive adhesive laminate which stably provide peeling site selectivity between both sides of the transparent pressure-sensitive adhesive body and a release sheet in the case of pasting, whereas stably and easily express peeling site selectivity between both sides of the transparent pressure-sensitive adhesive body in the case of rework, that is, re-pasting, as well as superior adhesion stability after pasting, and a method for producing the same. An optical transparent pressure-sensitive adhesive body and the like having pressure-sensitive adhesive surface (a) and pressure-sensitive adhesive surface (b) having different pressure-sensitive adhesive property from each other, and being formed with an addition reaction type silicone gel, characterized in that pressure-sensitive adhesive performance (Ga) of pressure-sensitive adhesive surface (a) and pressure-sensitive adhesive performance (Gb) of pressure-sensitive adhesive surface (b) satisfy a relationship of Ga < Gb, and pressure-sensitive adhesive performance (Ga) and pressure-sensitive adhesive performance (Gb) have 5 to 32 in ball number and 2 to 12 in ball number difference in the tilt type ball tack test (tilt angle: 30 degrees) according to JIS Z0237; and that pressure-sensitive adhesive surface (a) and pressure-sensitive adhesive surface (b) are formed by tightly adhering an uncured raw material of said addition reaction type silicone gel to different release film (A) and release film (B) and thermal-curing them, and the release film (A) and the release film (B) have a release treatment layer comprising a fatty acid amide type additive.
Owner:TAICA
Catalyst-controlled aliphatic c-h oxidations
ActiveUS20160214097A1Improve responseHigh yieldOrganic oxidationOrganic compound preparationSite selectivityOxidizing agent
The invention provides simple small molecule, non-heme iron catalyst systems with broad substrate scope that can predictably enhance or overturn a Substrate Control Catalyst Control substrate's inherent reactivity preference for sp3-hybridized C—H bond oxidation. The invention also provides methods for selective aliphatic C—H bond oxidation. Furthermore, a structure-based catalyst reactivity model is disclosed that quantitatively correlates the innate physical properties of the substrate to the site-selectivities observed as a function of the catalyst. The catalyst systems can be used in combination with oxidants such as hydrogen peroxide to effect highly selective oxidations of unactivated sp3 C—H bonds over a broad range of substrates.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Preparation method of trifluoromethylated polypeptide compounds
The invention discloses a method for preparing a trifluoromethylated polypeptide compound represented by formula (II), the method comprising the following steps: using a mixed solvent of DMF and water with a volume ratio of 1:1-4 As the reaction medium, the polypeptide compound as shown in formula (I) and 1-(trifluoromethyl)-1,2-phenyliodyl-3(1H)-ketone react 3-3 at room temperature under blue light in photocatalyst After 8 hours, after the reaction is complete, the resulting reaction mixture is post-treated to obtain trifluoromethylated polypeptide compounds represented by formula (II). The present invention drives the reaction under visible light irradiation, and the reaction solvent is a mixed solvent of DMF and water, which is more in line with the concept of green chemistry, green, and environmentally friendly. The product can be prepared in one step, the operation process is simple, and the source of raw materials has been commercialized and easily obtained. Using photocatalyst, it has good catalytic performance, mild reaction conditions and high site selectivity.
Owner:杭州肽佳生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com