Preparation method of trifluoromethylated polypeptide compounds
A technology of trifluoromethylation and peptide compounds, applied in the fields of peptides and organic chemistry, which can solve the problems of reducing functional group compatibility and atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
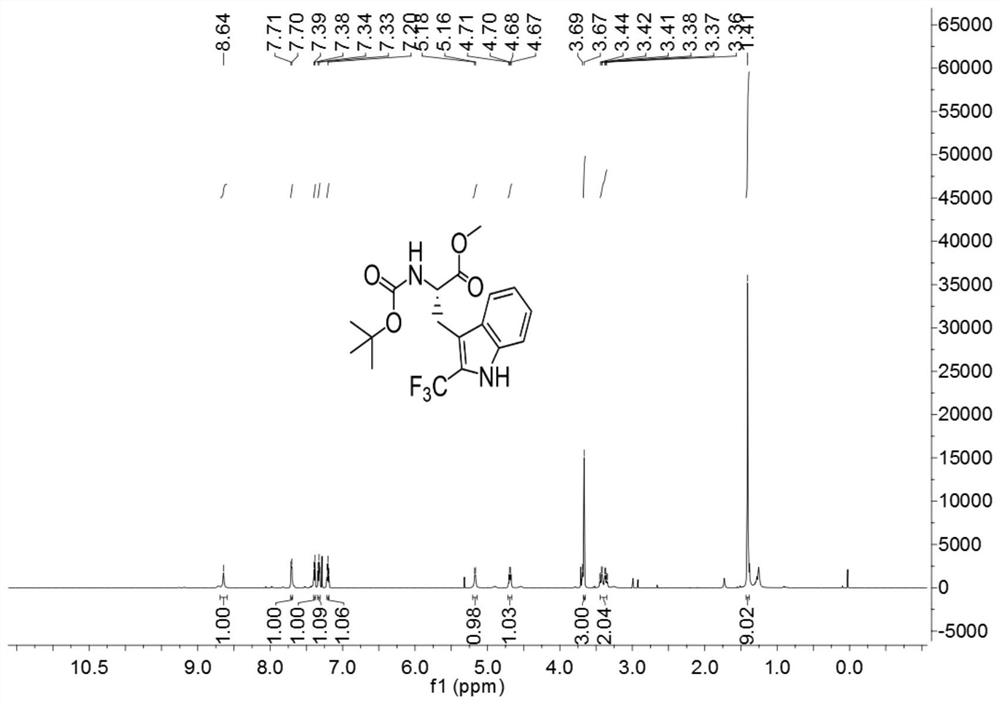
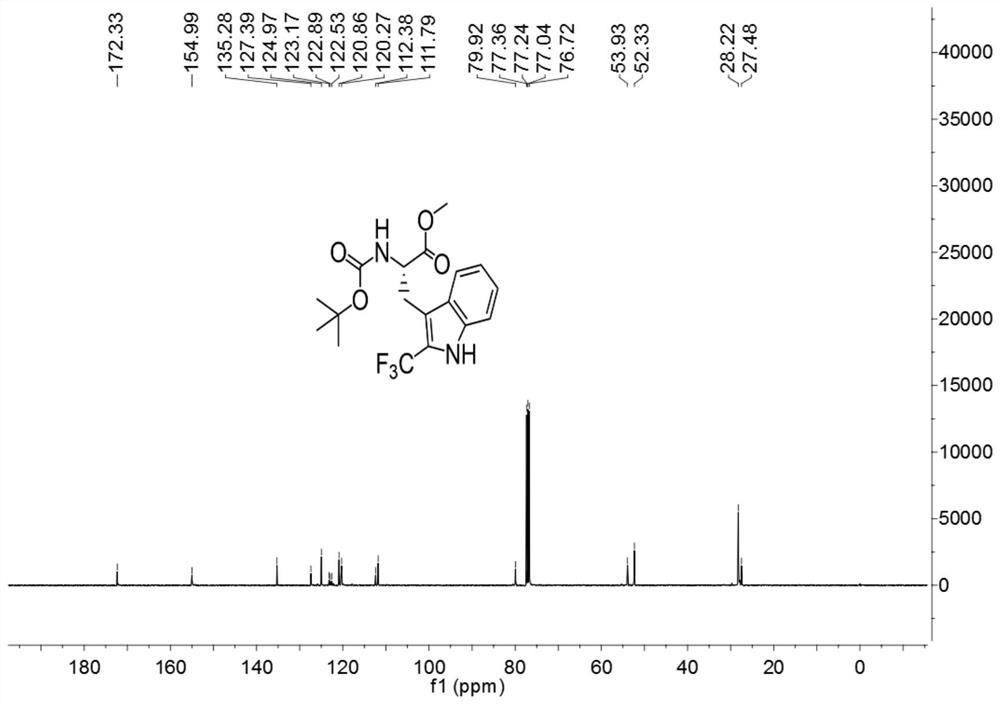
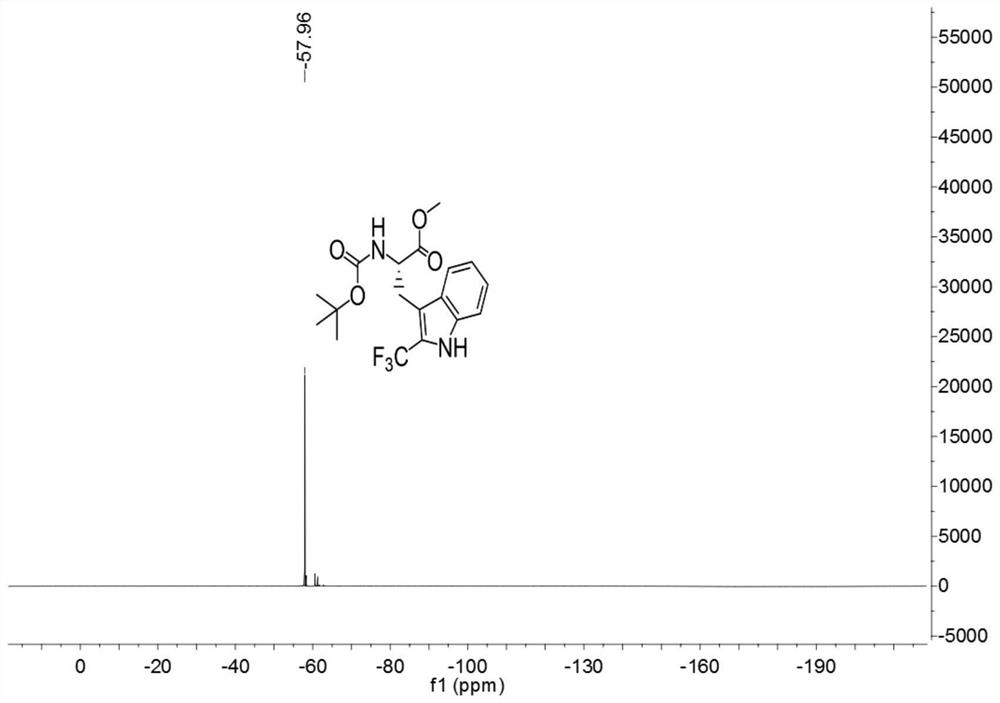
[0033]
[0034] Add 3ml of a mixed solvent of DMF and water to 127.2mg (0.4mmol) of Boc-Trp-OMe (purchased from Shanghai Beide Pharmaceutical Technology Co., Ltd., article number: BD118484-10g, batch: ALZ812, bottle number: N16-418) (The volume ratio of DMF to water is 1:4), add 252mg (0.8mmol) 1-(trifluoromethyl)-1,2-phenyliodide-3(1H)-one, 5.3mg (0.016 mmol) fluorescein, react for 3 hours under the irradiation of 9w blue LED light at room temperature, after the reaction, carry out the extraction operation: 1. Add saturated NaCl aqueous solution and ethyl acetate to the reaction solution for extraction twice, 2. Add for the first time Saturated NaCl aqueous solution 40mL, ethyl acetate 30mL, conduct one extraction, 3. After the first extraction is completed, collect the organic layer, take the water phase layer and add 20ml ethyl acetate for the second extraction, after the extraction is completed, take the organic layer and pass through anhydrous sulfuric acid Sodium-drie...
Embodiment 2
[0036] Except that the mass and moles of 1-(trifluoromethyl)-1,2-phenyliodyl-3(1H)-one were changed to 126.4 mg (0.4 mmol), and the mass and moles of fluorescein were changed to 1.328 mg (0.004mmol), other operation is constant, and operating procedure is with embodiment 1, obtains compound pure product 46.32mg shown in formula 2a, and reaction yield is 30%.
Embodiment 3
[0038] In addition to changing the mass and moles of 1-(trifluoromethyl)-1,2-phenyliodyl-3(1H)-one to 379.2 mg (1.2 mmol), the mass and moles of fluorescein to 66.4 mg (0.2mmol), other operation is constant, and operating procedure is with embodiment 1, obtains the compound pure product 58.67mg shown in formula 2a, and reaction yield is 38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com