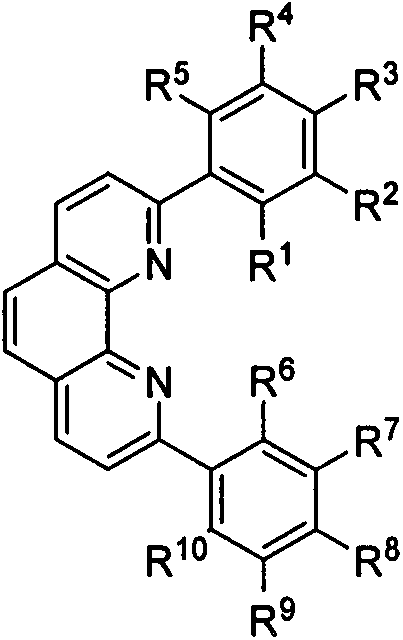
Preparation method and application of 2, 9-diaryl-substituted phenanthroline and 2, 9-diaryl-substituted phenanthroline iron complex
A technology of o-phenanthroline and phenanthroline, applied in the field of o-phenanthroline iron complexes, can solve the problems of large substrate limitations, less iron catalysts, and low catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
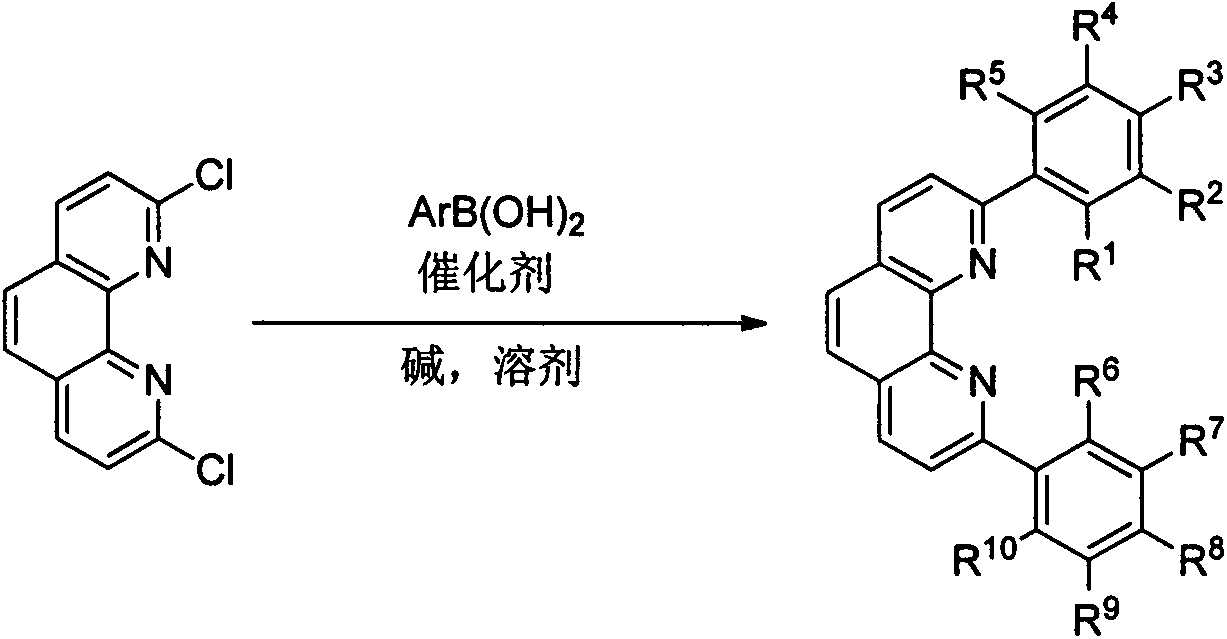
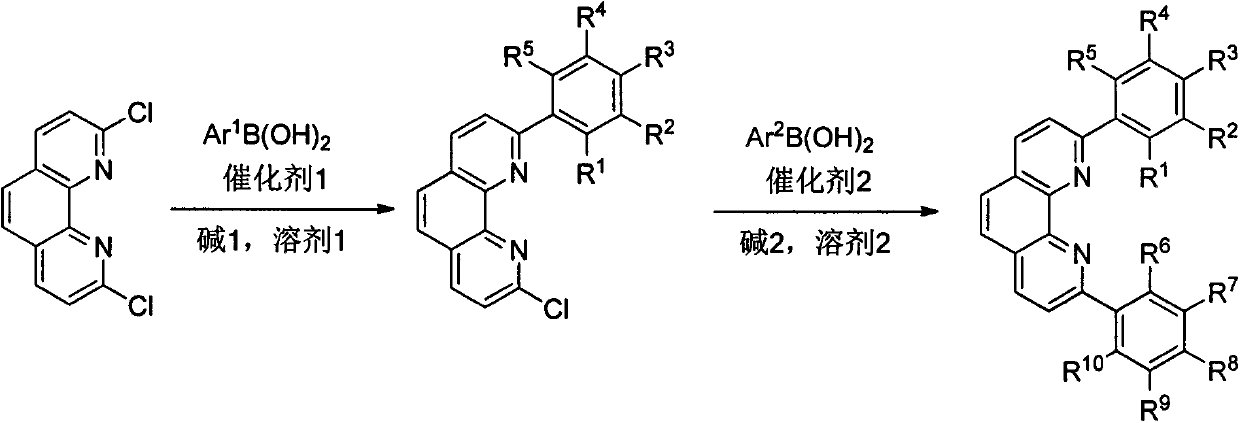
[0070] Example 1: Preparation of 2,9-diaryl substituted o-phenanthroline 2a-2c
[0071]
[0072]Reactant 1 (747 mg, 3 mmol), 2,4,6-triethylphenylboronic acid (1.86 g, 9 mmol), Ba( Oh) 2 ·8H 2 O (4.74g, 15mmol), PdCl 2 (dPpf) (329mg, 0.45mmol), replace the system with nitrogen atmosphere on the vacuum line, add degassed toluene (100mL) and water (5mL) under nitrogen flow, start stirring, and heat the oil bath to 110°C. After heating and stirring for 15 h, TLC confirmed that the reactant was completely consumed, and the heating was stopped and cooled to room temperature. Remove the insoluble matter by filtration, wash the residue with 40mL DCM, and remove the filtrate under vacuum, dissolve the residual brown-black solid with 100mL DCM, transfer to a separatory funnel, wash with saturated saline, dry over anhydrous sodium sulfate, and remove the organic phase under vacuum Post-dry loading column chromatography (PE / EA=5:1 as eluent) to obtain the target product 2,9-bis-2,4...
Embodiment 2
[0080] Example 2: Preparation of 2,9-diaryl substituted o-phenanthroline 2d, 2e
[0081]
[0082] Into a 250mL two-necked bottle equipped with a reflux condenser, an air extraction head, and a rubber stopper, the reactant 1 (498mg, 2mmol), 2,4,6-triisopropylphenylboronic acid (596mg, 2.4mmol), K 3 PO 4 ·3H 2 O (2.66g, 10mmol), Pd (PPh 3 ) 4 (346 mg, 0.3 mmol), the system was replaced by nitrogen atmosphere on the vacuum line, degassed DME (70 mL) and water (2.5 mL) were added under nitrogen flow, stirring was started, and the temperature of the oil bath was raised to 95 °C. After heating and stirring for 15 h, TLC confirmed that the reactant was completely consumed, and the heating was stopped and cooled to room temperature. Remove the insoluble matter by filtration, wash the residue with 20mL DCM, vacuum precipitation, dissolve the residue with 50mL DCM and transfer to a separatory funnel, wash with saturated saline, dry over anhydrous sodium sulfate, dry the organic p...
Embodiment 3
[0087] Example 3: Preparation of 2,9-diaryl substituted o-phenanthroline iron complexes
[0088]
[0089] In a glove box, weigh 2,9-bis-2,4,6-triisopropylphenyl-1,10-phenanthroline (2a) (1.001 g, 2 mmol) and FeCl into a 25 mL reaction vial 2 (253.5mg, 2mmol), add 20mL tetrahydrofuran, react at room temperature for 24 hours, vacuum pump to remove part of tetrahydrofuran (about 5mL remaining in the system), add 15mL n-hexane, orange-red solid precipitates, filter, and use n-hexane (3× 5mL) to wash the filter cake, the resulting solids were collected, and the high vacuum pump was drained to obtain the target product 2,9-bis-2,6-diethylphenyl-1,10-phenanthroline ferrous chloride (3a), Orange powder, yield 83%, decomposition temperature 282-286 ℃. 1 HNMR (400MHz, CDCl 3 )δ53.85(s, 2H), 27.88(s, 2H), 3.76(s, 2H), 1.87(s, 2H), 1.10(s, 4H), 0.27(s, 6H), -3.58(s, 12H), -10.77(s, 4H), -12.86(s, 4H), -16.74(s, 2H).IR(neat): 3555m, 3481s, 3416s, 3237w, 2967w, 2933, 2033w, 1639m, 161...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com