N-2-pyrimidinyl-3-fluoroindole compound and its preparation method and application
A technology for pyrimidinyl indole and compounds, which is applied in the field of N-2-pyrimidinyl-3-fluoroindole compounds, and can solve the problems of harsh conditions, low selectivity of non-corresponding forms, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
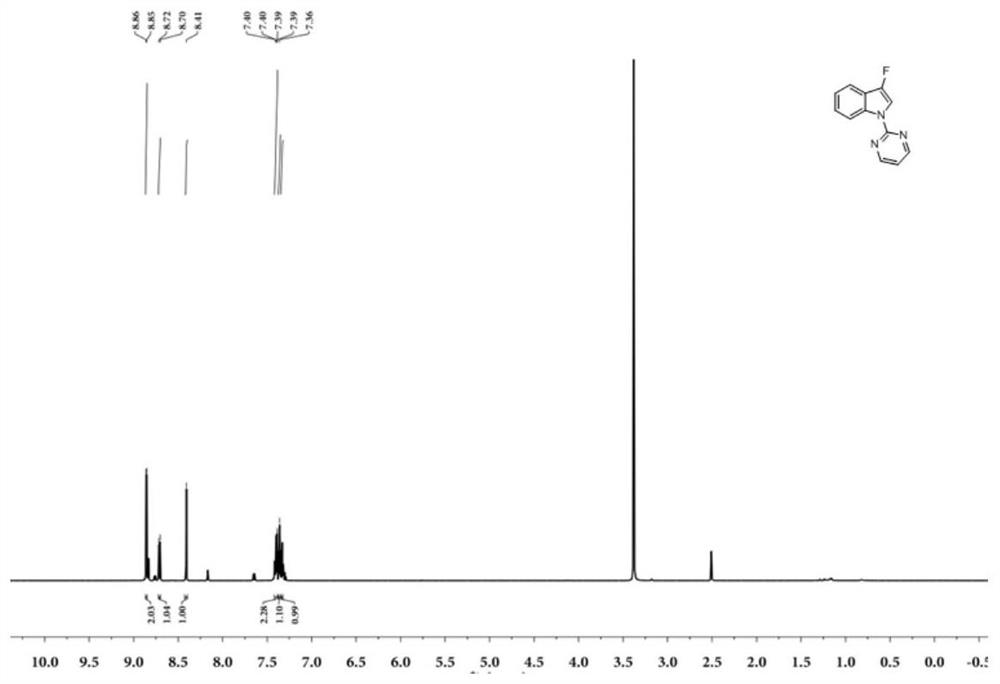
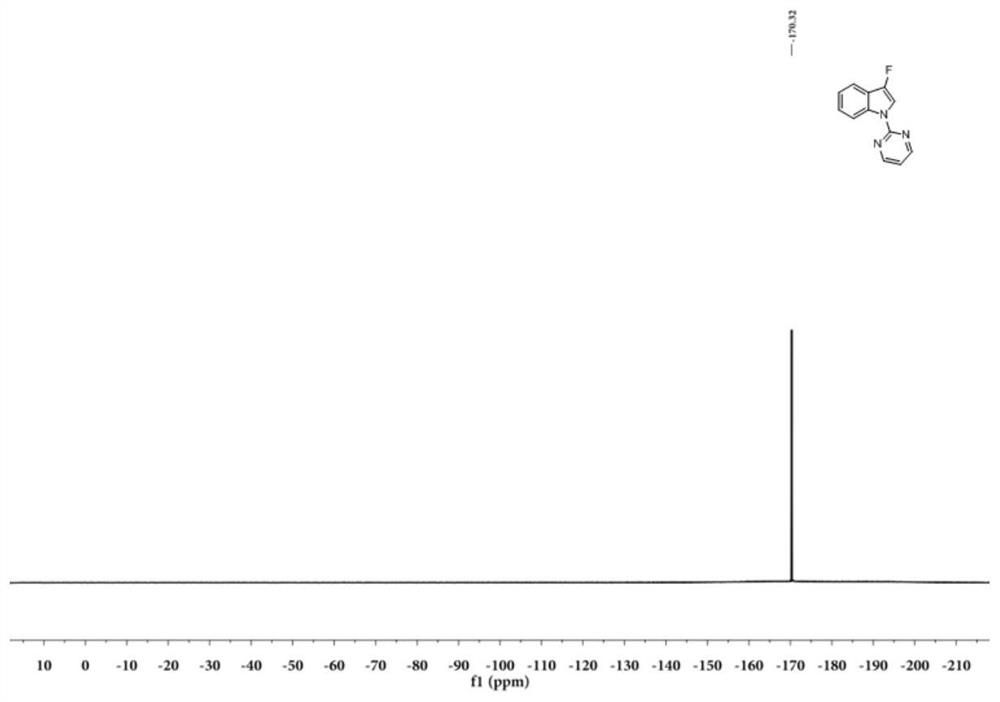
[0048] Example 1: Preparation of N-2-pyrimidinyl-3-fluoroindole (I-1)
[0049] The reaction formula is as follows:
[0050]
[0051] Dissolve 0.058g (0.3mmol) of N-2-pyrimidinylindole (Ⅲ-1) in 1mL of acetonitrile, add 0.126g (0.36mmol) of Selectfluor (F source, purchased from Beijing Bailingwei Technology Co., Ltd.), and then add 0.010g (0.015mmol) Eosin Y (photocatalyst, purchased from Beijing Bailingwei Technology Co., Ltd.), finally add 0.017g (0.09mmol) CuI (purchased from Beijing Bailingwei Technology Co., Ltd.), under the irradiation of 9w blue LED light, 25 °C React for 2.0 hours, follow the reaction by TLC, the N-2-pyrimidinyl indole (Ⅲ-1) point disappears, the reaction is complete, dilute with 10mL ethyl acetate, wash 3 times with 10ml saturated aqueous sodium chloride solution, collect the organic phase, and use Dry over anhydrous sodium sulfate and concentrate to obtain the crude product containing (I-1), which is separated by thin-layer chromatography (with a m...
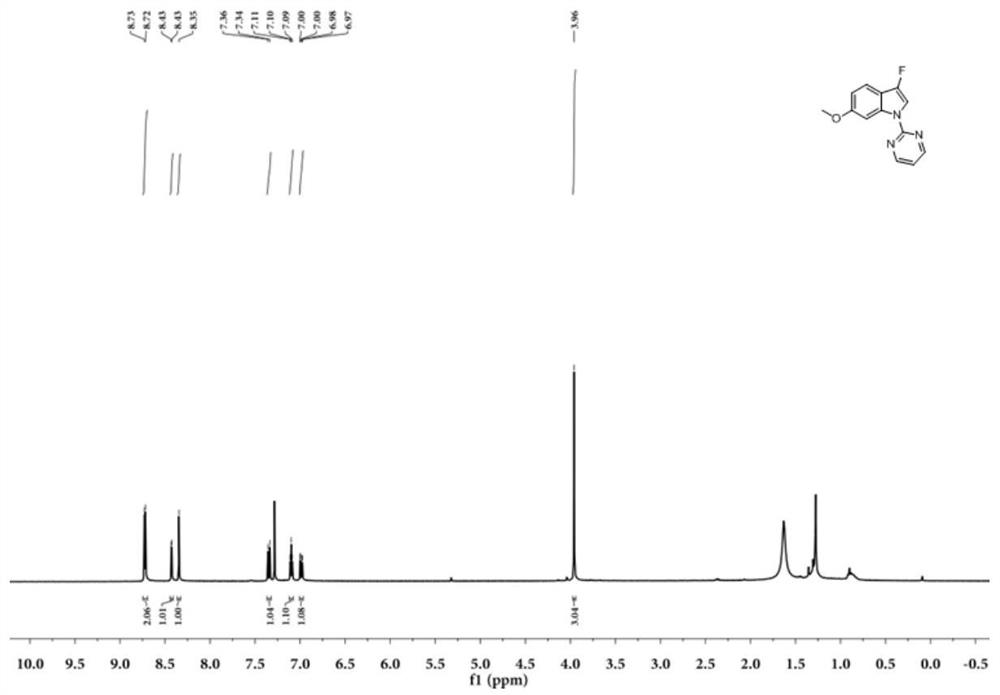
Embodiment 2~9
[0053] Embodiment 2~9 substituent screening:
[0054] R in formula (V), (VI) 3 For tert-butoxycarbonyl, pivaloyl, benzyl, acetyl, benzoyl, H, methyl.
Embodiment 2
[0056] Dissolve 0.065g (0.3mmol) N-tert-butoxycarbonylindole (VI-1, purchased from Beijing Bailingwei Technology Co., Ltd.) in 1mL acetonitrile, add 0.126g (0.36mmol) Selectfluor (F source), and then add 0.010g (0.015mmol) eosin Y (photocatalyst), finally add 0.017g (0.09mmol) CuI, under the irradiation of 9w blue LED lamp, react at 25°C for 4.0 hours, follow the reaction by TLC, N-tert-butoxycarbonylindole ( VI-1) point disappears, producing a lot of by-products. The reaction solution was diluted with 10 mL of ethyl acetate, washed 3 times with 10 mL of saturated aqueous sodium chloride solution, the organic phase was collected, dried over anhydrous sodium sulfate, and concentrated to obtain the crude product (V-1), which was used on a large plate of thin layer chromatography (with The mixture of petroleum ether and ethyl acetate with a volume ratio of 25:1 is used as a developing solvent) to separate, collect the silica gel powder containing the target compound, and use a mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com