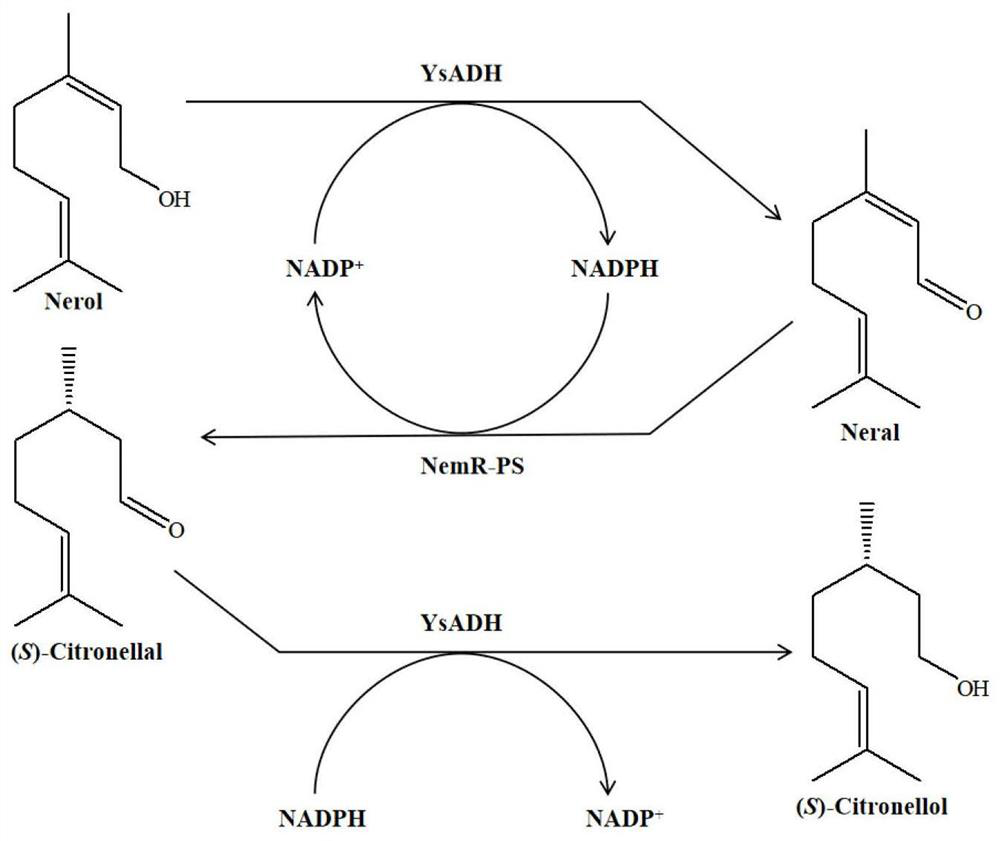
Method for synthesizing (S)-citronellol through double-enzyme coupling
A technology of citronellol and old yellow enzyme, which is applied in the field of biocatalysis, can solve the problems of reduced atom economy, lack of high activity, and can not meet the requirements of practical application, and achieves superior site selectivity and high atom economy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
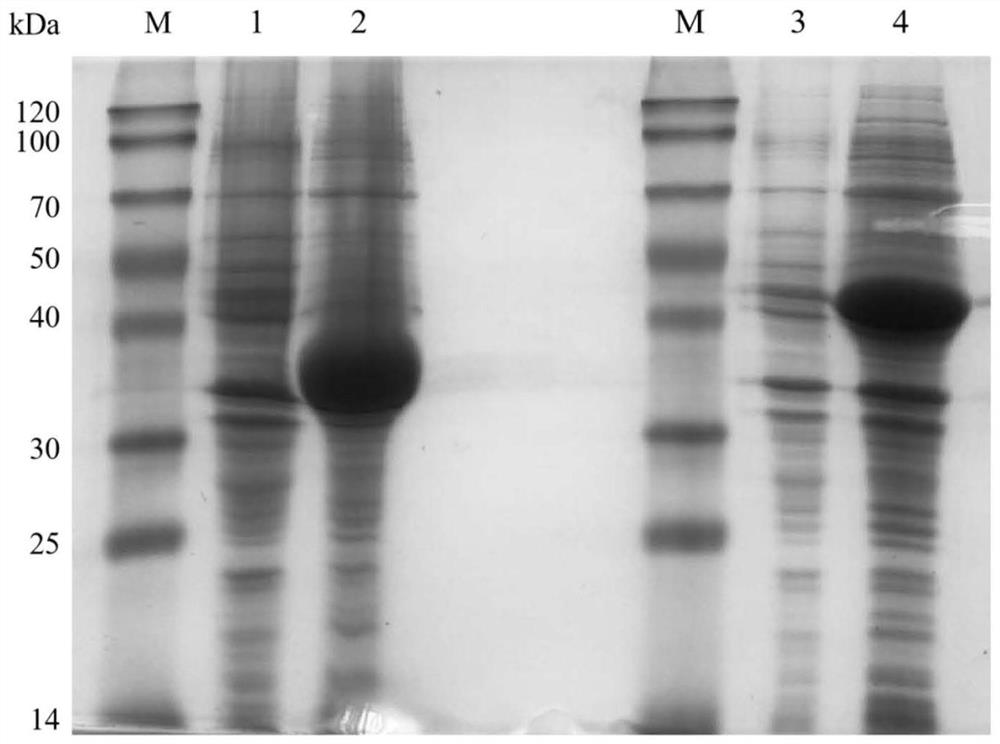
[0034] Example 1: Preparation of wet bacteria and crude enzyme solution expressing alcohol dehydrogenase YsADH and old yellow enzyme NemR-PS
[0035] 1. Construction of Alcohol Dehydrogenase YsADH Genetic Engineering Bacteria
[0036] The artificially synthesized disclosed source is from Yorkella sp.WZY002; preserved in the China Type Culture Collection Center, address: Wuhan, China, Wuhan University, postcode: 430072, preservation number: CCTCC No: M2013099, preservation date: 2013 On March 22, the gene encoding alcohol dehydrogenase YsADH was disclosed in the patent application CN201310188883.9), and its amino acid sequence and nucleotide sequence are respectively shown in SEQ ID NO.1 and SEQ ID NO.2.
[0037] The construction of genetically engineered bacteria E.coli BL21(DE3) / pET-28b-YsADH: Insert the gene encoding alcohol dehydrogenase YsADH shown in SEQ ID NO.2 into the Nco I and Hind III restriction enzyme sites of the pET28b vector, The recombinant vector pET28b-YsADH...
Embodiment 2
[0059] Example 2: Construction of initial reaction system for synthesizing (S)-citronellol based on coenzyme self-cycle with nerol as substrate
[0060] The wet thallus of alcohol dehydrogenase YsADH prepared in step 3 of Example 1 and the wet thallus of old yellow enzyme NemR-PS are mixed in a buffer (50mM Tris-HCl) in a certain proportion as a catalyst, and nerolidol is used as a substrate. Add coenzyme NADP + , constituting a total reaction system of 10 mL. The substrate nerolidol is preformed into a 1M solution with ethanol as the solvent, and then added appropriately according to the final concentration of the substrate in the reaction system.
[0061] The initial reaction system without condition optimization is as follows: the final concentration of substrate nerol is 60mM, the coenzyme NADP + The final concentration is 0.4mM, the final concentration of wet cells is 120g / L (the wet cells of alcohol dehydrogenase YsADH and the wet cells of old yellow enzyme NemR-PS are...
Embodiment 3
[0066] Embodiment 3: Taking nerol as the substrate based on the optimum temperature of coenzyme self-circulation synthesis (S)-citronellol
[0067] Select 25°C, 30°C, 35°C, 40°C, 45°C, 50°C and 55°C, and other operations and reaction conditions are the same as in Example 2. Three sets of parallel experiments were performed each time, and the average value and standard error were calculated. The results were as follows: Figure 5 shown. At 25°C, the conversion rate of (S)-citronellol was 43.75%; when the catalytic temperature was 40°C, the conversion rate had reached 72.39%. When the temperature increased to 45 and 50 °C, the conversion decreased to 65.78% and 55.27%. In summary, the optimum reaction temperature is 40°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com