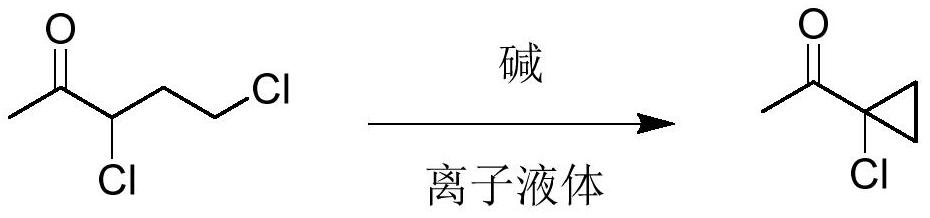
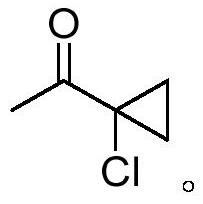
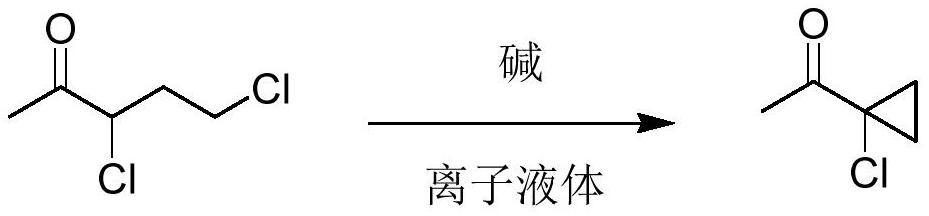
Novel synthesis method of 1-acetyl-1-chlorocyclopropane
A synthesis method and chlorocyclopropane technology are applied in the preparation of carbon-based compounds, chemical instruments and methods, organic cyclization, etc., and can solve problems such as high cost and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]Add raw material 3,5-dichloro-2-pentanone (100.0g, 0.63mol) and imidazole ionic liquid 1-butyl-3-methylimidazolium bromide (1.0g) to the reaction flask, add dropwise 30% Sodium hydroxide aqueous solution (92.0g, 0.69mol), control the temperature at 30-40°C, add to complete the reaction for 1h, filter the reaction solution to remove salt, stand to separate layers, and distill the organic phase under reduced pressure to obtain the product 1-acetyl-1 - 68.5 g of chlorocyclopropane, with a purity of 96.5%, a yield of 88.5%, and the aqueous phase containing the ionic liquid can be applied mechanically.
Embodiment 2
[0048] Add raw material 3,5-dichloro-2-pentanone (100.0g, 0.63mol) and the above-mentioned aqueous phase containing ionic liquid to the reaction flask, add sodium hydroxide solid (27.8g, 0.69mol) in batches, and control the temperature At 30-40°C, the reaction was completed for 1h, the reaction solution was filtered to remove salt, and the layers were separated. The organic phase was distilled under reduced pressure to obtain 69.2g of the product 1-acetyl-1-chlorocyclopropane, with a purity of 97.1%, and a yield of 90. %, the aqueous phase containing the ionic liquid can be applied.
Embodiment 3
[0050] Add solvent dichloromethane (100g) in reaction flask, raw material 3,5-dichloro-2-pentanone (100.0g, 0.63mol) and imidazoles ionic liquid 1-butyl-3-methylimidazolium bromide ( 1.0g), add 30% sodium hydroxide aqueous solution (92.0g, 0.69mol) dropwise, control the temperature at 30-40°C, and react for 1h after addition, filter the reaction solution to remove salt, let it stand for stratification, and recover the organic phase by precipitation first The organic solvent was then distilled under reduced pressure to obtain 69.8 g of 1-acetyl-1-chlorocyclopropane with a purity of 97.3% and a yield of 90.9%. The aqueous phase containing the ionic liquid can be used mechanically.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com