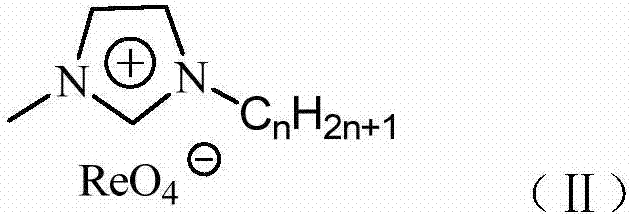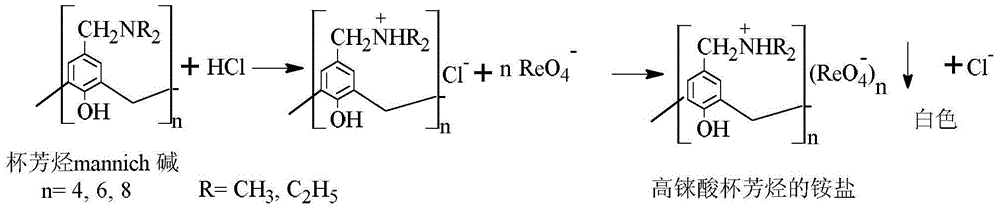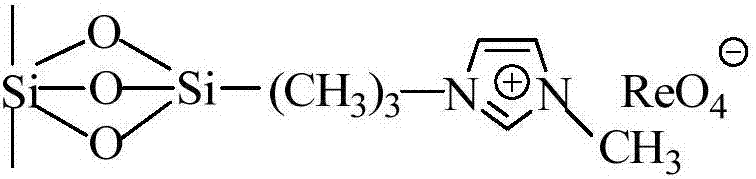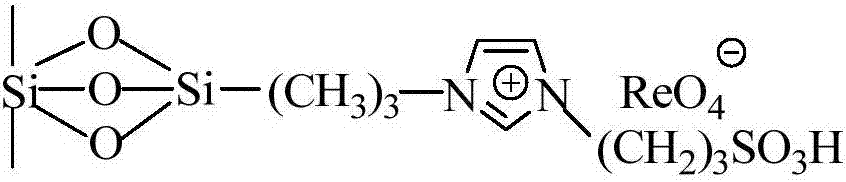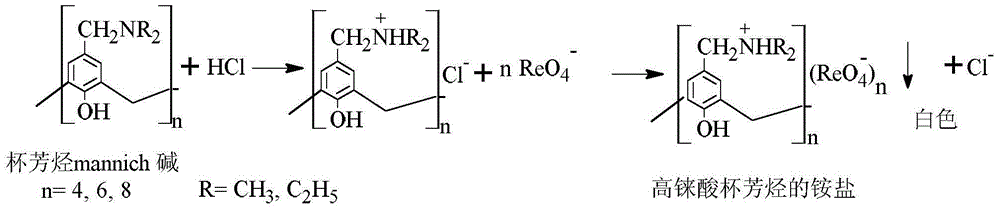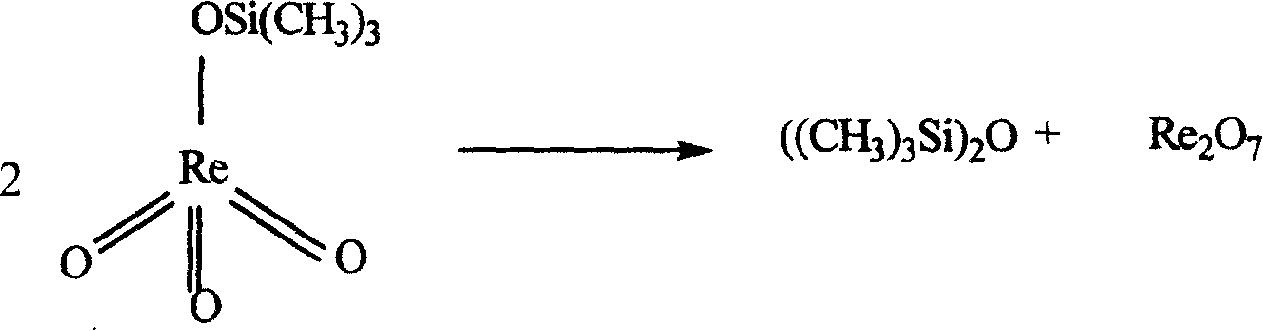Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Perrhenic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Perrhenic acid is the chemical compound with the formula Re₂O₇(OH₂)₂. It is obtained by evaporating aqueous solutions of Re₂O₇. Conventionally, perrhenic acid is considered to have the formula HReO₄, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of Re₂O₇ is kept for a period of months, it breaks down and crystals of HReO₄·H₂O are formed, which contain tetrahedral ReO⁻₄ For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.
Preparation method of high purity ammonium rhenate
ActiveCN108408785ALow Tl contentMeet the requirements of low Tl contentRhenium compoundsRheniumPerrhenic acid
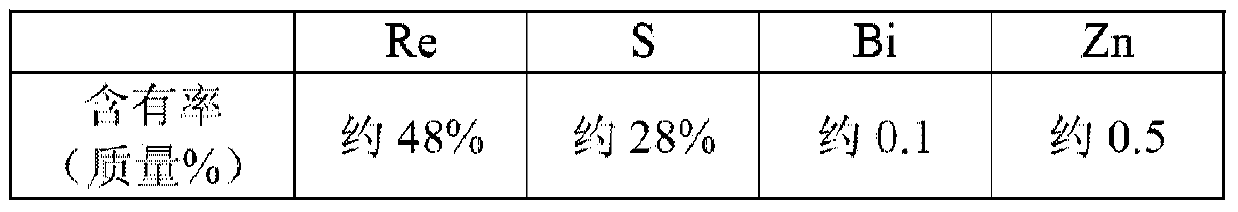
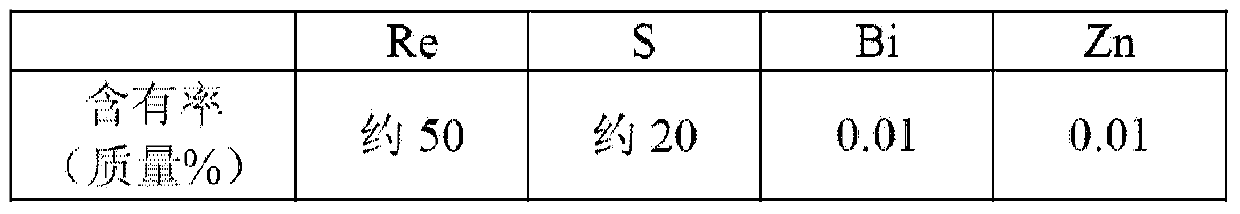
The invention discloses a preparation method of high purity ammonium rhenate, the method comprises the following steps of S1, dissolving ammonium rhenate in pure water to obtain an ammonium rhenate solution and adjusting pH value to 7 to 10, then performing oxidation and precipitation in sequence, and centrifuging to obtain an ammonium rhenate solution without containing TI; S2, after performing concentration and crystallization on the ammonium rhenate solution without containing TI, performing oxidation volatilization to obtain a perrhenic acid solution; S3, exchanging the perrhenic acid solution by adopting a cation exchange resin for removing impurities; S4, adding ammonia water into the perrhenic acid solution subjected to adsorption and impurities removal and performing concentrationand crystallization to obtain the high purity ammonium rhenate. According to the preparation method of the high purity ammonium rhenate disclosed by the invention, TI<+> in the ammonium rhenate solution is oxidized into TI<3+>, a harmful impurity TI is removed through a synergistic effect of hydrolytic precipitation of TI<3+> and adsorption packaging of a flocculating agent, then trace TI and other impurity elements are removed through oxidation volatilization, ion exchange and concentration crystallization to obtain the high purity ammonium rhenate with low TI content, the high purity ammonium rhenate meets the requirements of rhenium with low TI content in aeronautical material.
Owner:NORTHWEST INSTITUTE FOR NON-FERROUS METAL RESEARCH
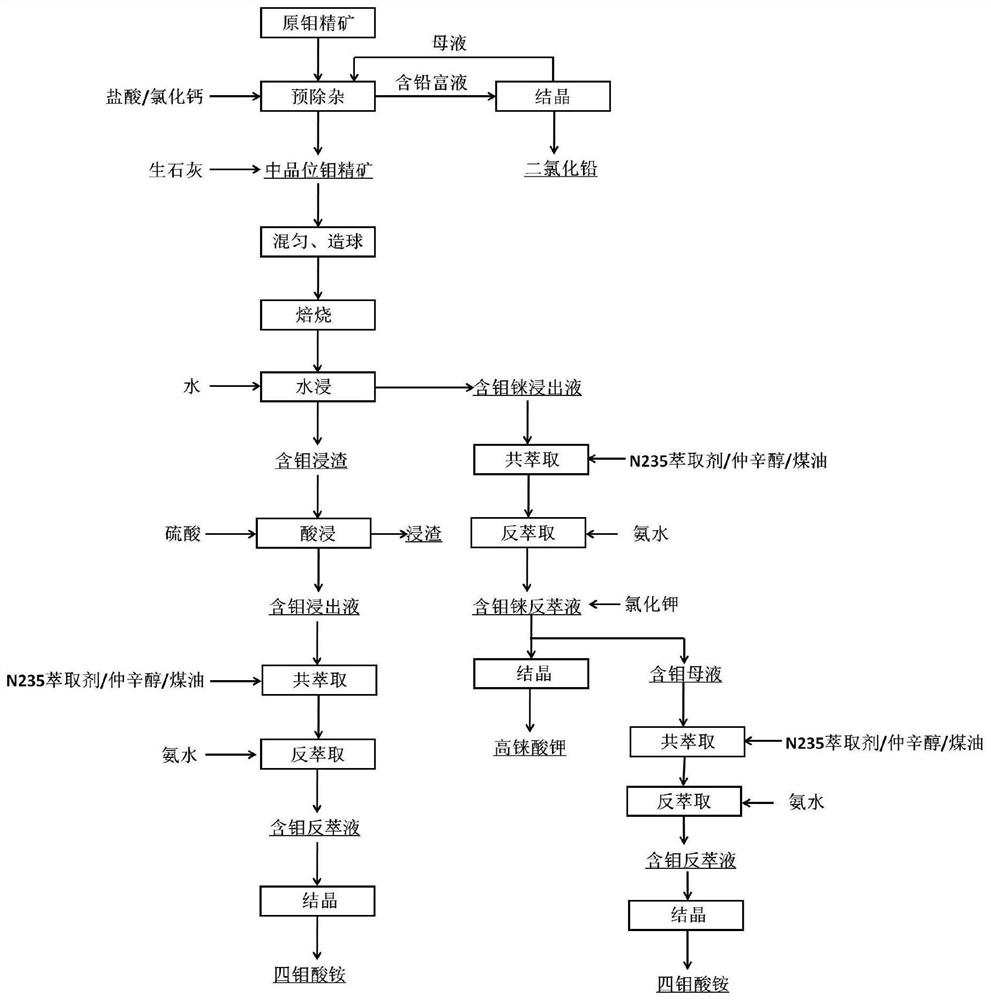
Method for comprehensive recovery of molybdenum and rhenium from high molybdenum-rhenium concentrate
ActiveCN104232941AImprove leaching rateSimple processProcess efficiency improvementRheniumPerrhenic acid
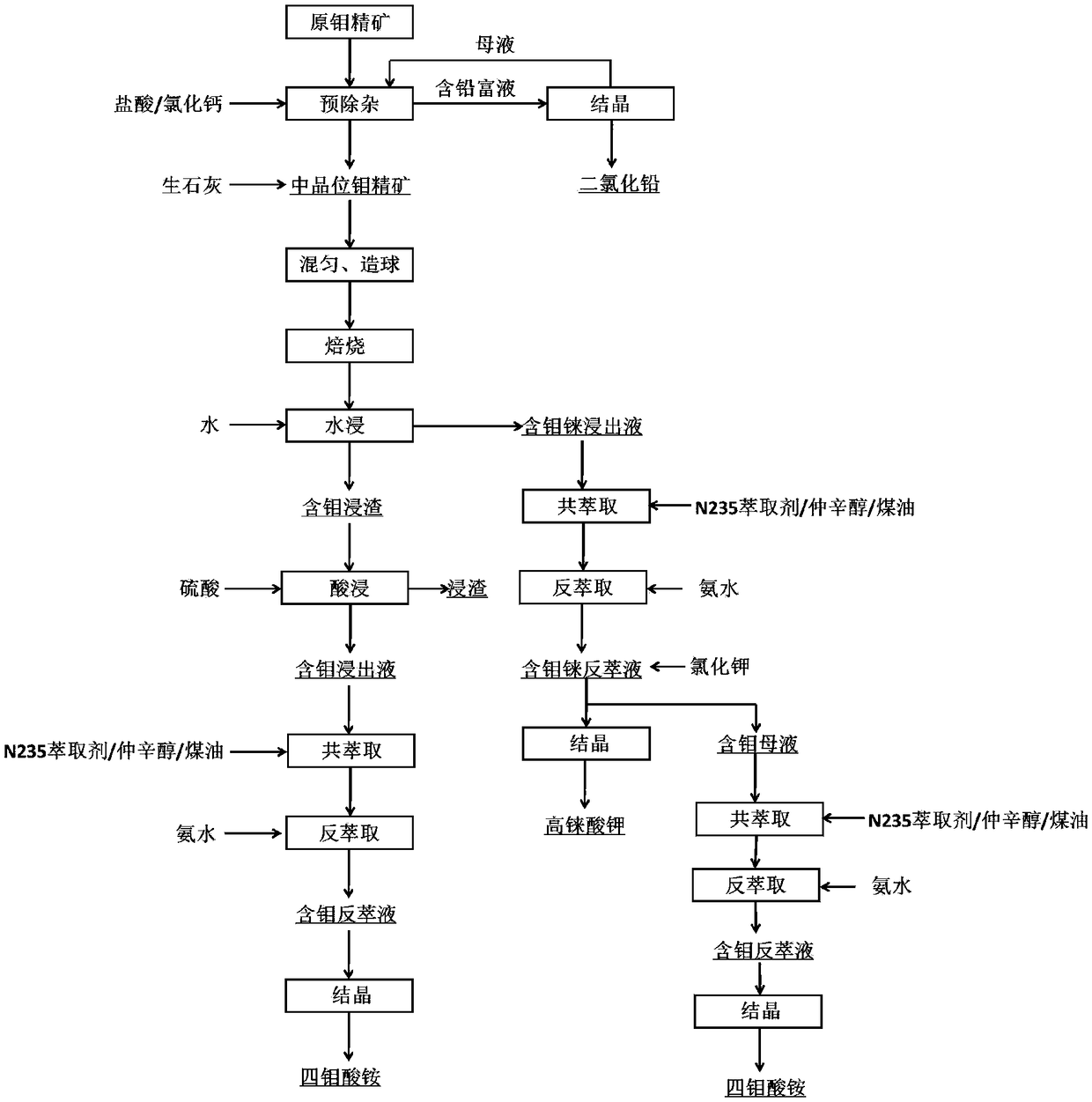
The invention discloses a method for comprehensive recovery of molybdenum and rhenium from high molybdenum-rhenium concentrate. The method comprises the following steps: mixing, pelletizing and roasting molybdenum-rhenium concentrate and lime to obtain a calcined material; leaching molybdenum and rhenium from the calcined material by using a sulfuric acid solution; neutralizing lixivium by adopting the calcined material to obtain a neutralization solution; purifying the neutralization solution, removing phosphorus, arsenic and silicon, and then acidifying until the pH of the solution is 2.0-3.0; synchronously extracting molybdenum and rhenium from the purified neutralization solution by adopting the solvent, and carrying out acidic precipitation on strip liquor to obtain an ammonium molybdate product; synchronously extracting molybdenum and rhenium from precipitated molybdenum mother liquor to obtain ammonium rhenate containing molybdenum; removing molybdenum from the ammonium rhenate containing molybdenum by adopting calcium chloride to obtain a purified ammonium rhenate solution; concentrating the purified ammonium rhenate solution and then adding potassium chloride to carry out rhenium precipitation, thereby obtaining a potassium perrhenate product. The method has the advantages of short process, low cost, simplicity in operation and high recovery rate, and ammonium molybdate and potassium perrhenate product can be obtained.
Owner:陕西炼石矿业有限公司 +1
Cationic organic polymer as well as preparation method and application thereof
ActiveCN108102092AHigh yieldThe synthesis method is simpleIon-exchange process apparatusOther chemical processesPerrhenic acidHalohydrocarbon
The invention relates to a cationic organic polymer which is obtained by performing a polymerization reaction on a compound containing imidazole groups or pyridine groups and halohydrocarbon in an organic solvent at the temperature of 0-300 DEG C for 0-24h, wherein the ratio of the imidazole or pyridine groups to halogens is 1: 1; the compound containing the imidazole groups or pyridine groups isselected from the following chemical formulas; R1 is an imidazole group or pyridine group, and m, n, p and r are independently selected from integers that are greater than 0; the halohydrocarbon is selected from the following chemical formulas; X is independently selected from F, Cl, Br or I, and m is any integer which is 0 or greater. The invention also discloses application of the cationic organic polymer prepared by the preparation method, which serves as an HTcO4 radical and / or a perrhenic acid radical, in an adsorbent.
Owner:SUZHOU UNIV
Catalyst for preparing chlorotrifluoroethene from trichlorotrifluoroethane through catalytic hydrodechlorination and preparation method of catalyst
ActiveCN105944734AHigh activityImprove stabilityPreparation by dehalogenationCatalyst activation/preparationPerrhenic acidPotassium
The invention discloses a catalyst for preparing chlorotrifluoroethene from trichlorotrifluoroethane through catalytic hydrodechlorination and a preparation method of the catalyst. The method comprises steps as follows: SiO2 particles are roasted at a temperature of 400 DEG C; a mixed solution containing CoC12.6H2O or RhCl3.3H2O, CrCl3.6H2O or MnCl2.4H2O, KCl.6H2O or perrhenic acid and Ni (H2PO2).6H2O is prepared; the SiO2 particles are subjected to isometric impregnation and dried at a temperature of 60-80 DEG C for 12-16 h; dried substances are put into a tube furnace, nitrogen is introduced, the temperature is raised to 240-300 DEG C at the speed of 6-8 DEG C / min, the reaction pressure is 0-2 MPa, and the reaction time is 4 h; products are naturally cooled to the room temperature, and O2 containing 2% of N2 is introduced for passivating treatment for 4 h. The prepared catalyst contains 0.1%-15% of Co or Rh, 0.5%-22% of Cr or Mn and 0.1%-5% of alkali metal K or rare-earth metal Re. The catalyst has the advantages of high activity, good stability, good reaction selectivity, mild reaction condition and the like.
Owner:CHANGSHU 3F FLUOROCHEM IND CO LTD
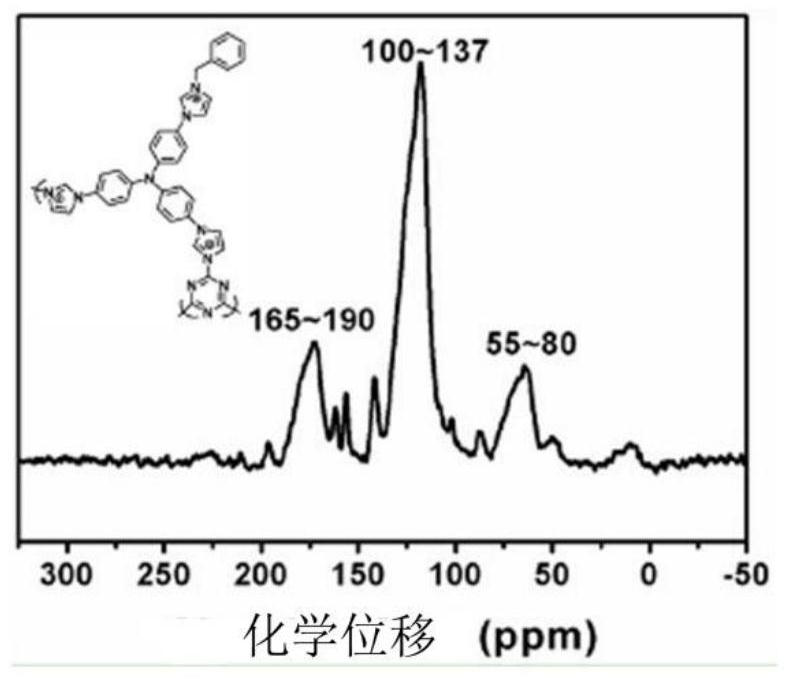
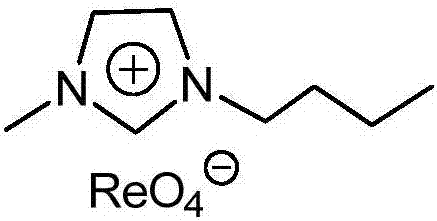
Imidazolium perrhenate ionic liquid with amino groups as well as preparation method and application of imidazolium perrhenate ionic liquid
ActiveCN106045913ALow costHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsHydrobromidePerrhenic acid
The invention relates to an imidazolium perrhenate ionic liquid with amino groups as well as a preparation method and an application of the imidazolium perrhenate ionic liquid. The structural formula of the imidazolium perrhenate ionic liquid with the amino groups is shown as the formula (I). According to the preparation method, 3-bromopropylamine hydrobromide or 2-bromoethylamine hydrobromide is mixed with imidazolium under nitrogen protection, then the mixture is dissolved in absolute ethyl alcohol and subjected to a reflux reaction at 85-95 DEG C for 20-25 h, and an imidazolium bromide ionic liquid is obtained; the imidazolium bromide ionic liquid with amino groups and NH4ReO4 are subjected to heating reflux until no ammonia gas is emitted, cooled to the room temperature and filtered, solids are subjected to rotary evaporation and vacuum drying, and the imidazolium perrhenate ionic liquid with the amino groups is obtained. When used for catalyzing an epoxy compound to synthesize cyclic carbonate, the ionic liquid has the advantages that the yield and the activity are high, a promoter or other solvents are not needed, reaction conditions are mild, the stability is high, repeated recycling is realized and the like.
Owner:LIAONING UNIVERSITY
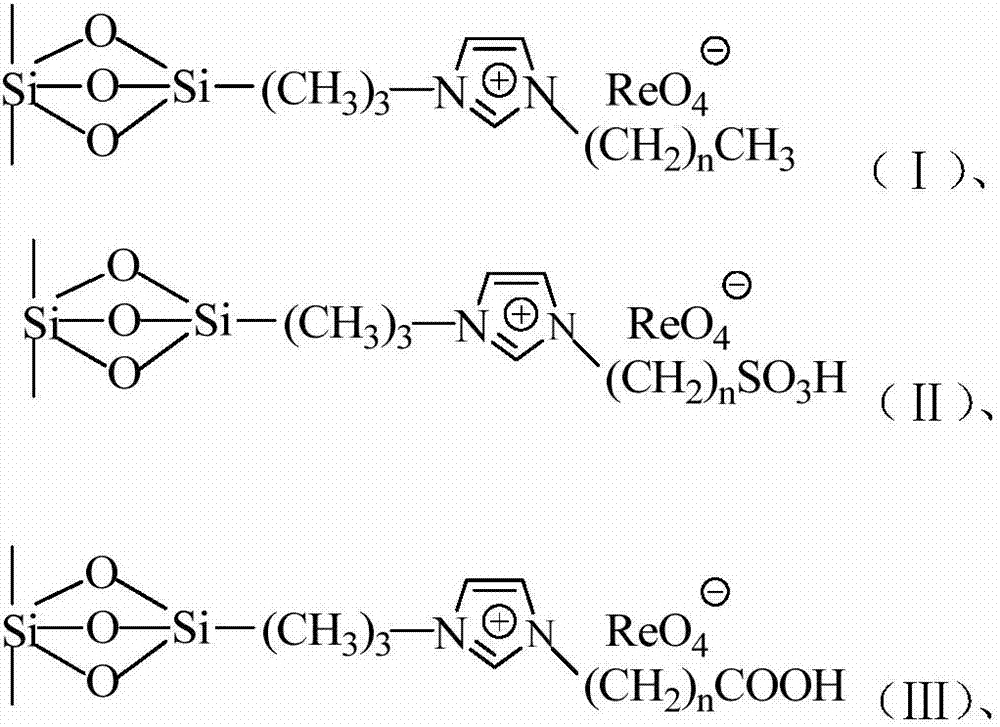
Supported perrhenate ionic liquid as well as preparation method thereof
ActiveCN103464200AReduce dosageEfficient separationOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPerrhenic acidIonic liquid
The invention relates to a supported perrhenate ionic liquid as well as a preparation method thereof. The technical scheme adopted is as follows: the preparation method comprises the following steps: selecting MCM-41 (Mobil Composition of Matters) as a carrier material; firstly, carrying out a reaction on chloropropyl triethoxysilane and the MCM-41; grafting a chloropropyl silicon structure to the surface of the MCM-41, and removing the byproduct ethanol; then, adding N-methyl imidazole so as to quaternize to obtain MCM-41 supported alkyl imidazole chlorine salt; and further adding ammonium perrhenate, and converting the chlorine salt into perrhenate through double-decompose reaction to obtain the MCM-41 supported alkyl imidazole perrhenate ionic liquid. The molar ratio of the reaction materials chloropropyl triethoxysilane, N-methyl imidazole, the MCM-41 and perrhenate is 1:(4-1):(3-1):(10-0.1). The method is applicable to support of various different types of alkyl imidazole perrhenate ionic liquids.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
Method for preparing ammonium perrhenate
ActiveCN104176784AAchieve recyclingImprove economyRhenium compoundsPerrhenic acidAmmonium perrhenate
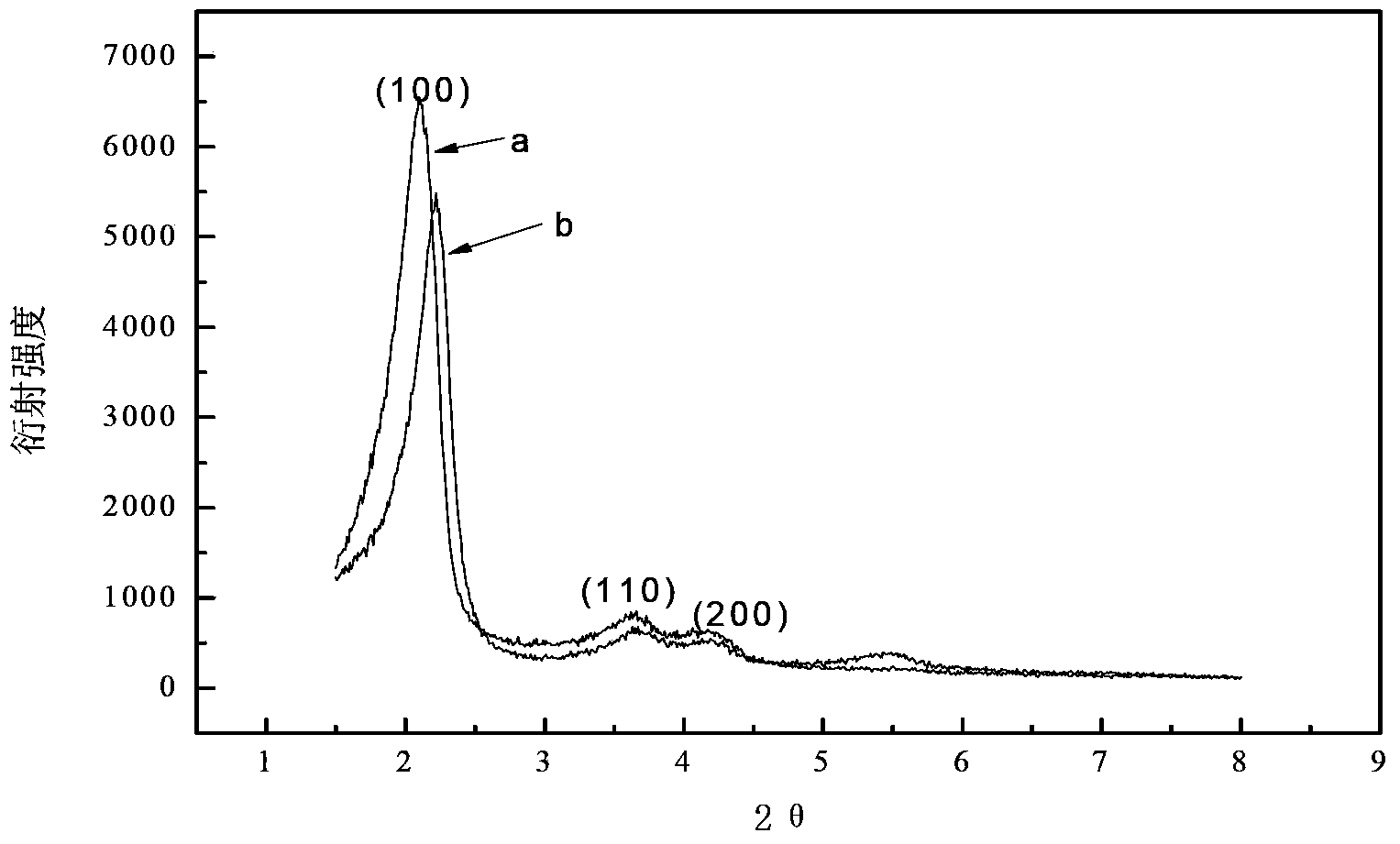
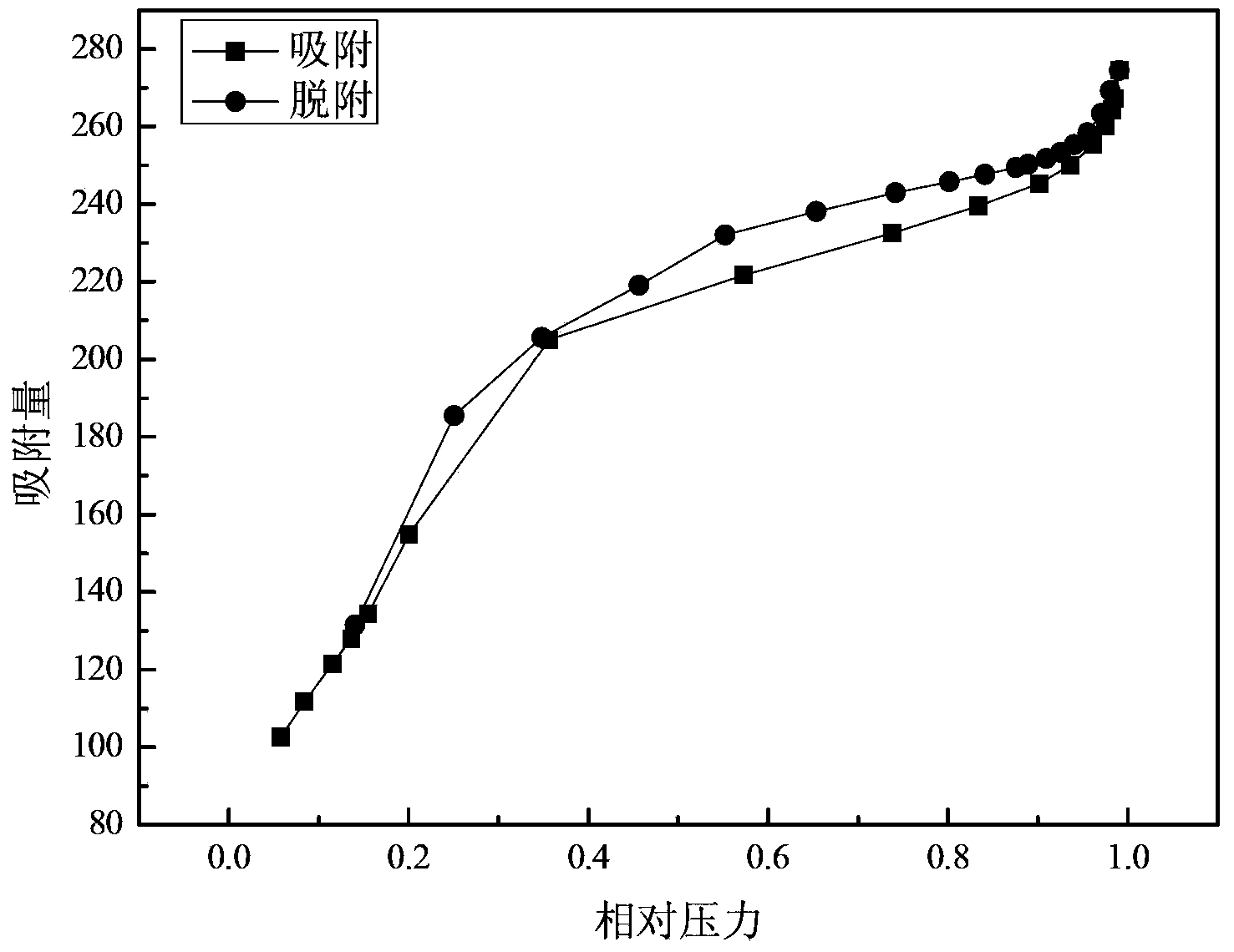
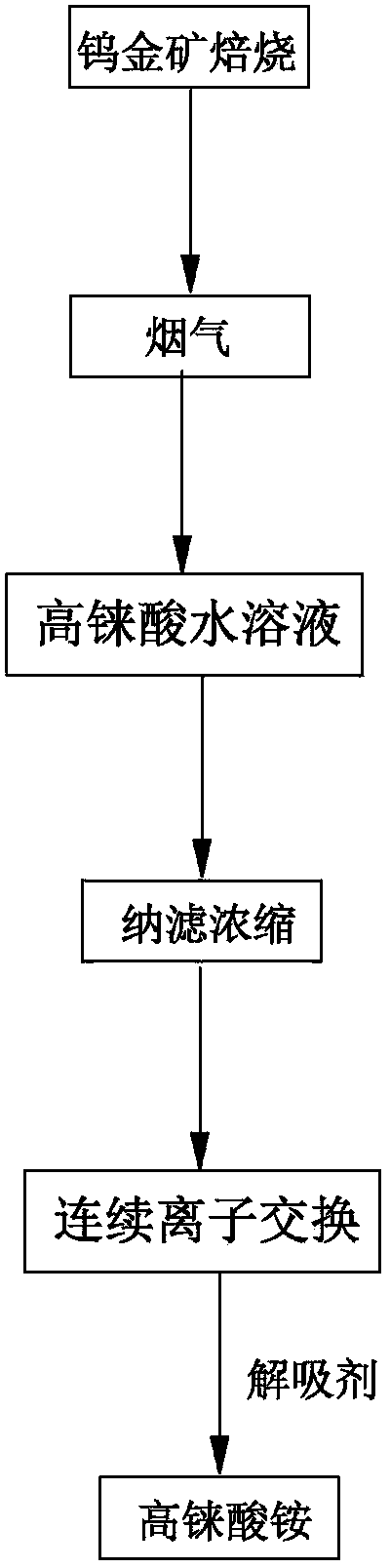
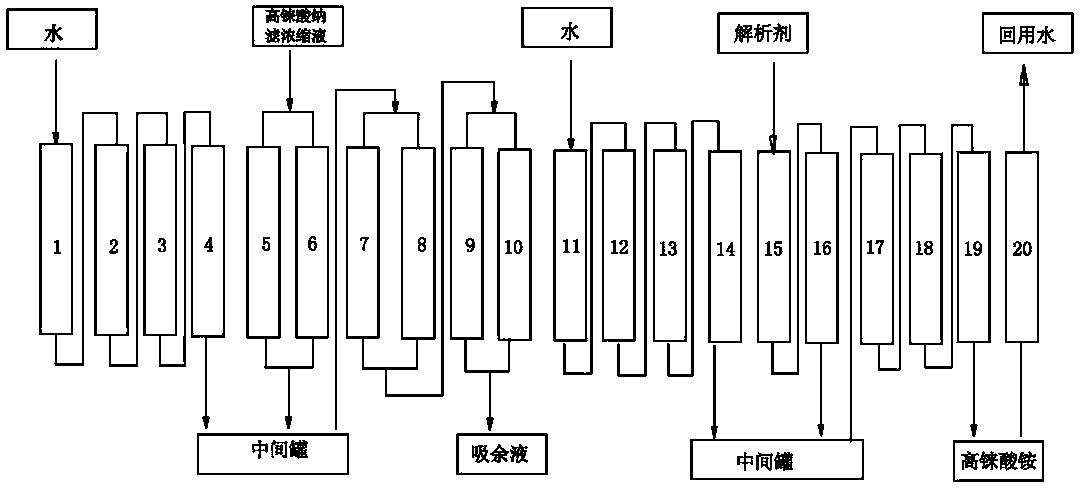
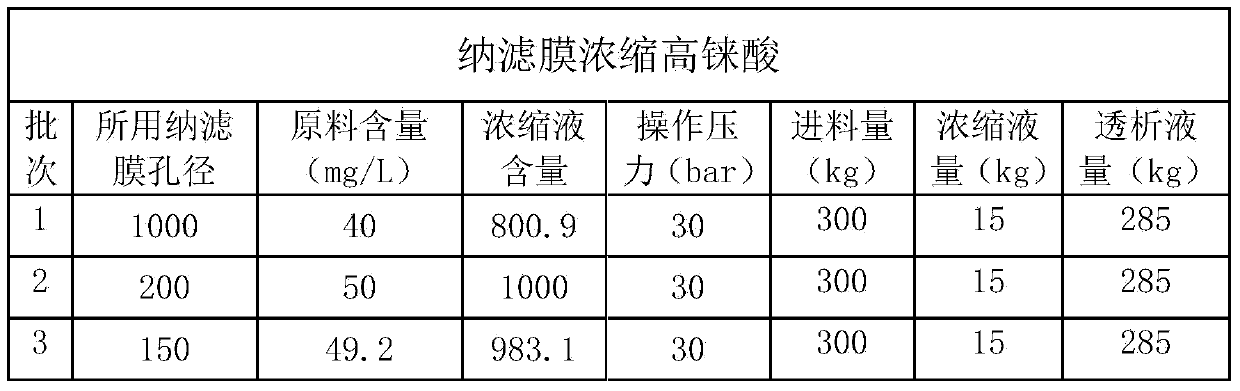
The invention discloses a method for preparing ammonium perrhenate, which is characterized by comprising the following steps: 1. washing and cooling flue gas generated by tungsten gold ore roasting through an eluting tower, so that perrhenic acid contained in the flue gas is dissolved in water, and the water solution containing the perrhenic acid is used for circularly eluting the roasting flue gas to increase the concentration; 2. collecting the perrhenic acid water solution, carrying out rough filtration, and carrying out nanofiltration concentration on the filtrate with nanofiltration membrane equipment to obtain a perrhenic acid nanofiltration concentrated solution; and 3. passing the perrhenic acid nanofiltration concentrated solution through continuous ion exchange equipment, eluting rhenium with a resolving agent, heating to concentrate the collected eluate at 100 DEG C, cooling, and crystallizing to obtain the ammonium perrhenate. By using the flue gas generated by tungsten gold ore roasting as the raw material, the nanofiltration membrane equipment and continuous ion exchange equipment are utilized to recover the ammonium perrhenate, thereby achieving the goals of resource recovery and environment protection; and thus, the method has favorable economic value and environmental benefit.
Owner:XIAMEN STARMEM TECH
Method for extracting molybdenum and rhenium from rhenium and molybdenum containing concentrate
ActiveCN108588446AHarm reductionLow impurity contentProcess efficiency improvementPerrhenic acidSulfide
The invention belongs to the molybdenum metallurgical industry of the field of non-ferrous metallurgy and relates to a method for extracting molybdenum and rhenium from rhenium and molybdenum containing concentrate. The method specifically comprises the following eight steps of: a pretreatment process, a blending and granulation process, a fix-roasting process, a water leaching process, a precipitation process, a crystallization process, an acid leaching process, a coextraction-reextraction process and an acid precipitation process, wherein the obtained molybdenum is separated out in the formof ammonium tetramolybdate crystals; and the rhenium is separated out in the form of potassium perrhenate crystals. According to the method provided by the invention, the molybdenum concentrate is pretreated and deleaded so that lead is recycled in the form of lead dichloride; the harms of the lead are greatly reduced; quick lime is added to transform the sulfide in the concentrate into sulfate sothat the problem about environmental pollution caused by the SO2 gas generated in and oxidation and roasting process is prevented; besides, the method provided by the invention can hopefully shortenthe technological process, reduce the equipment investment, increase the recycle rate of the molybdenum and the rhenium, improve the product quality and provide conveniences for producing ammonium molybdate products.
Owner:西安鑫城投资有限公司
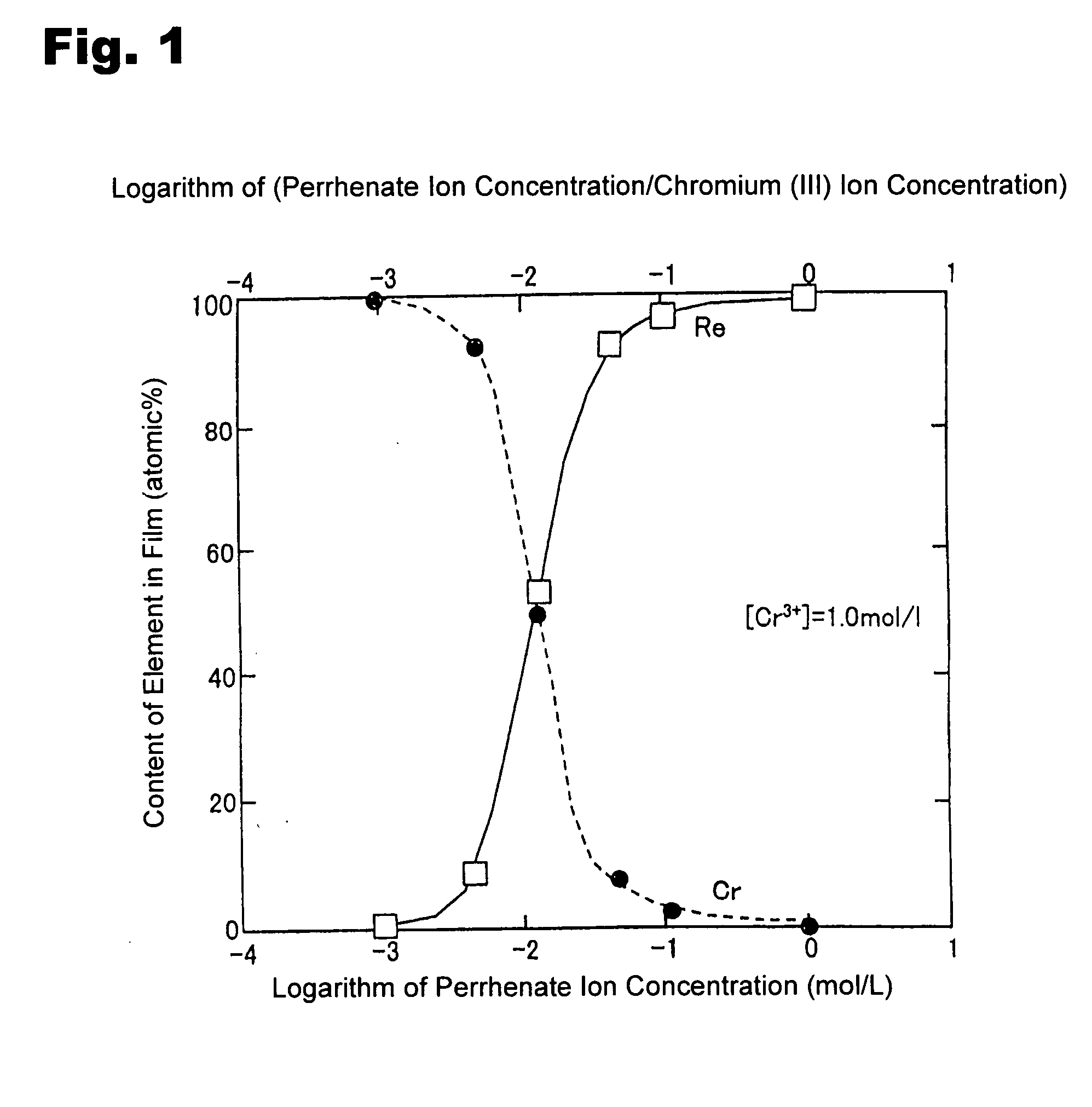
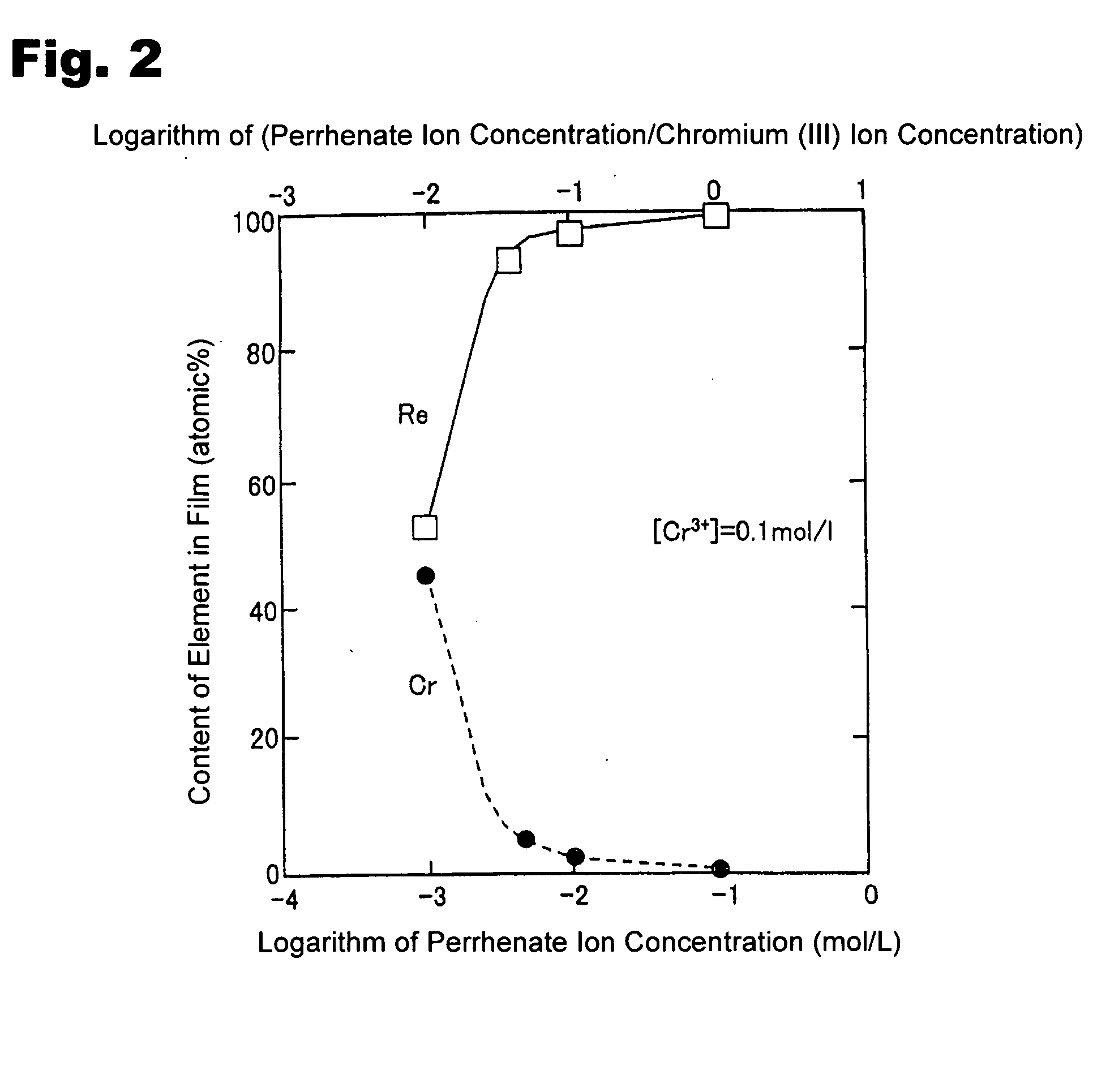
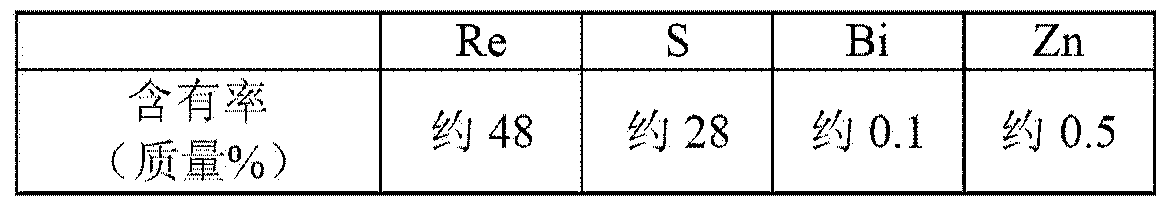
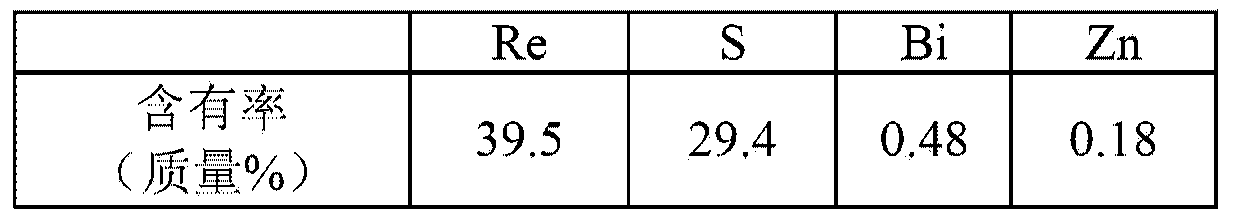
Method for forming re coating film or re-cr alloy coating film through electroplating
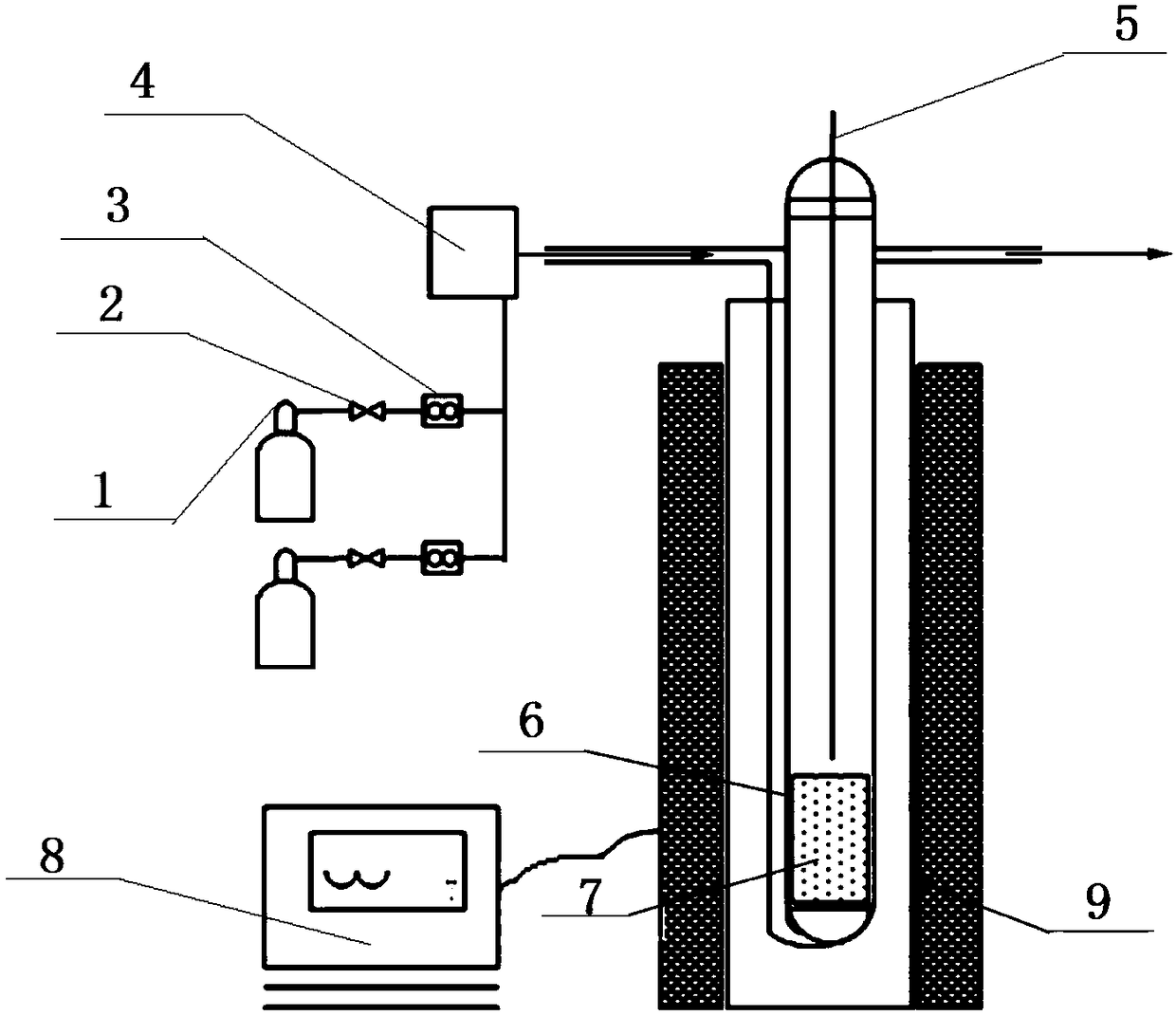
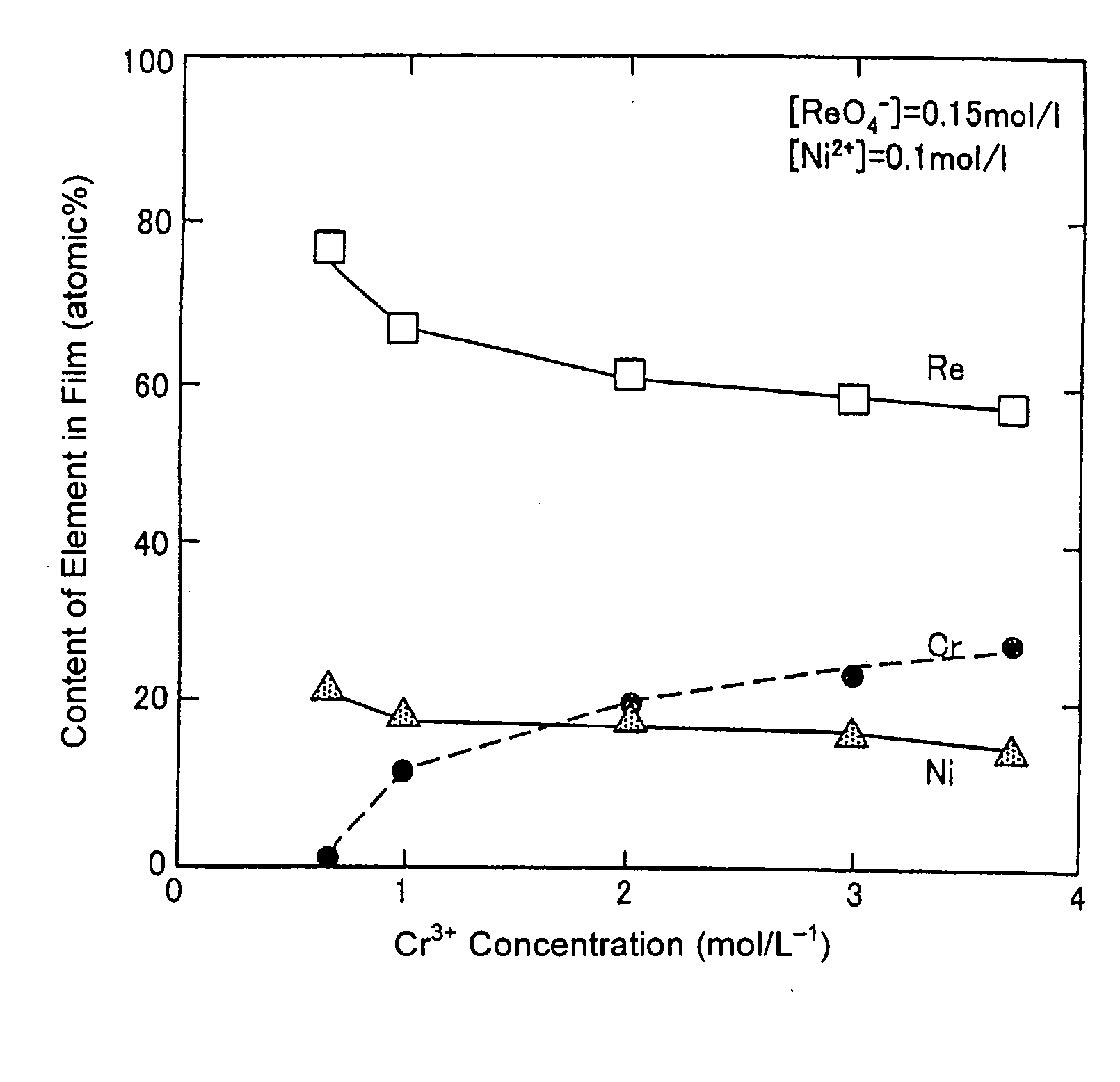
Disclosed is a method for forming: a Re—Cr alloy film consisting of Re in the range of greater than 0 (zero) to less than 98% by atomic composition, and the remainder being Cr except inevitable impurities; a Re-based film consisting of 98% or more, by atomic composition, of Re, with the remainder being Cr and inevitable impurities; or a Re—Cr—Ni alloy film consisting of Re in the range of 50 to less than 98% by atomic composition, Cr in the range of 2 to less than 45% by atomic composition, and the remainder being Ni except inevitable impurities. The method comprises performing an electroplating process using an electroplating bath containing an aqueous solution which includes a perrhenate ion and a chromium (III) ion. The present invention allows a Re—Cr alloy, Re-based or Re—Cr—Ni alloy film usable as a corrosion-resistant alloy coating for a high-temperature component or the like to be formed through an electroplating process using an aqueous solution, so as to provide heat / corrosion resistances to the component, even if it has a complicated shape, in a simplified manner at a low cost.
Owner:TOSHIO NARITA +1
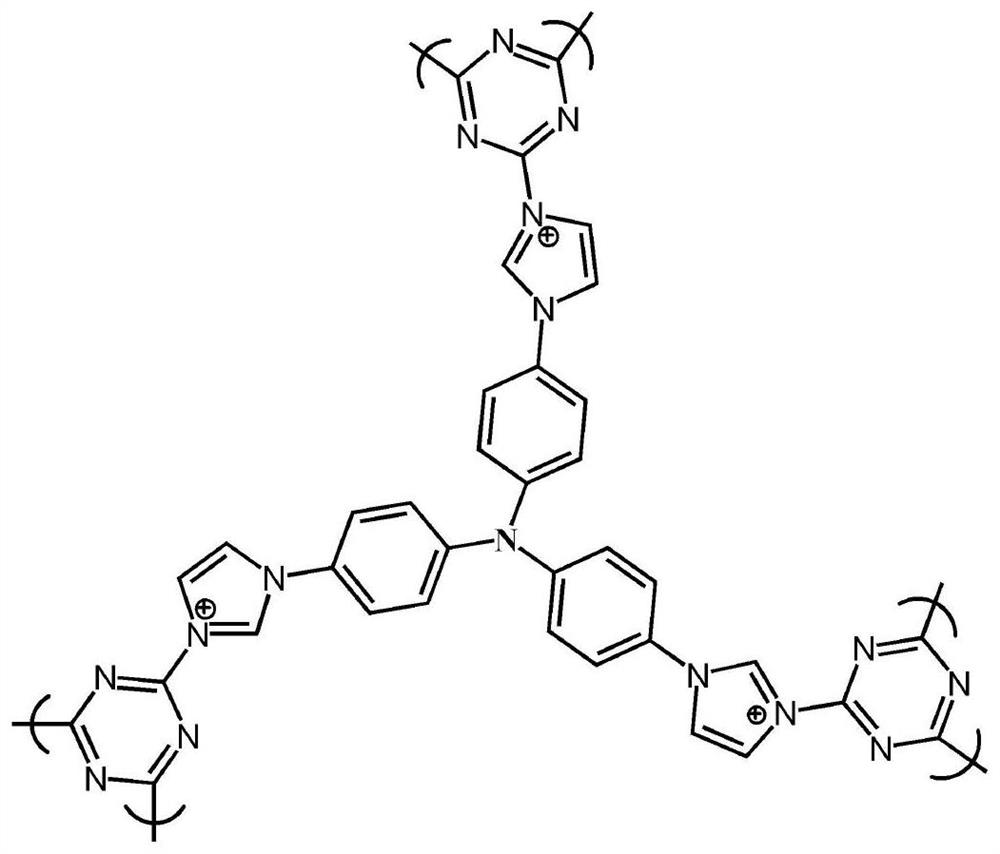
Porous organic framework material and preparation method thereof, and application in selective separation of perrhenate radicals
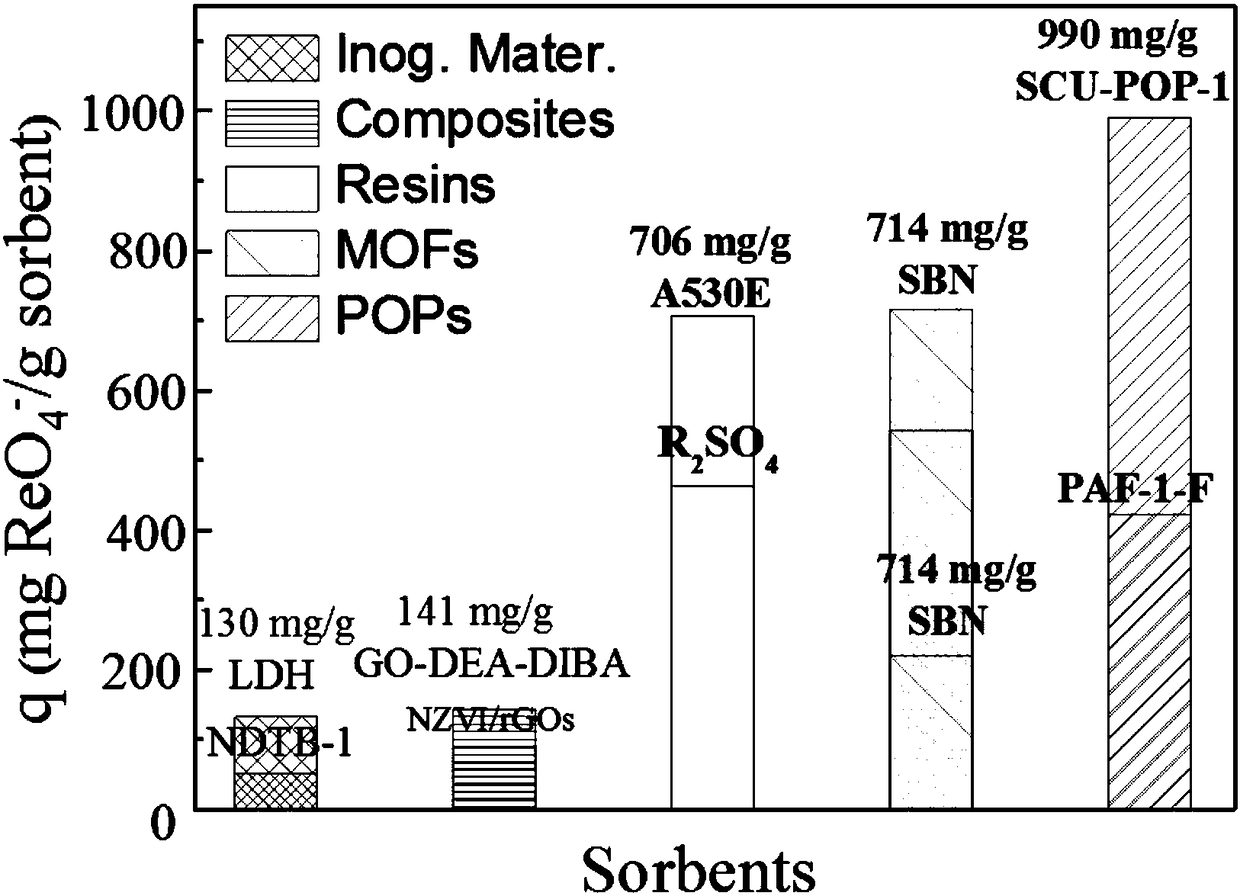
The invention provides a preparation method of a porous organic framework material. The method comprises the following steps: mixing tris(4-imidazolylphenyl)amine, cyanuric chloride and a solvent, andcarrying out a quaternization reaction to obtain the porous organic framework material. According to the preparation method, tris(4-imidazolylphenyl)amine TIPA and cyanuric chloride are used as raw materials for quaternization reaction, so that the prepared porous material has a special structure of a cation skeleton; and after free anions such as chlorine ions and the like exist in pore channelsare mixed with a solution containing perrhenate radicals, and selective separation of perrhenate radicals is carried out through anion exchange in the pore channels. According to the invention, experimental results show that the specific surface area of the porous organic framework material provided by the invention is 274 m<2>, the adsorption rate is as high as 100%, and the maximum adsorption capacity is 442 mg / g.
Owner:潍坊光华精细化工有限公司 +1
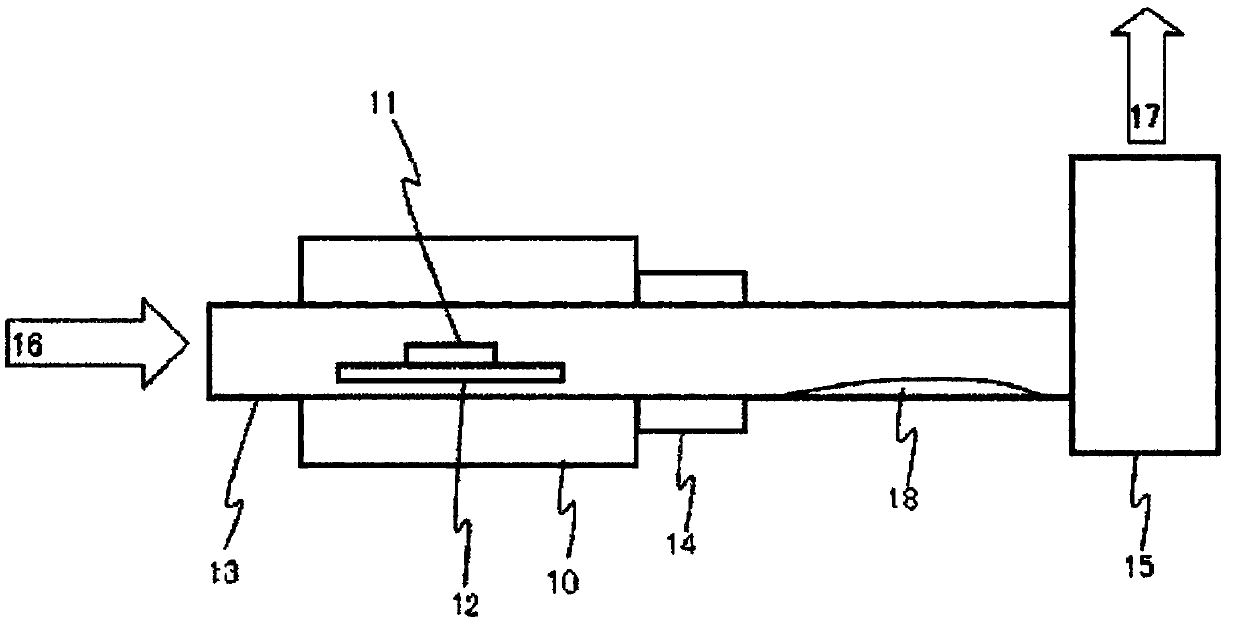
Method for producing aqueous solution of perrhenic acid from rhenium sulfide
Provided is a method that enables high-purity perrhenic acid to be produced from crude rhenium sulfide using a dry process. This method for producing an aqueous solution of perrhenic acid includes: 1) a step in which roasting is performed on rhenium sulfide in the presence of an oxygen-containing gas, and gasified rhenium oxide is collected; 2) a step in which the rhenium oxide is cooled and solidified, while keeping sulfur oxides associated with the gasified rhenium oxide in a gaseous state, and then the purity of the rhenium oxide is increased by performing solid-gas separation; and 3) a step in which an aqueous solution of perrhenic acid is obtained by dissolving the solidified rhenium oxide in water, or by dissolving the solidified rhenium oxide in water after heating and gasifying the solidified rhenium oxide.
Owner:PAN PACIFIC COPPER CO LTD
Epoxy plasticizer synthesis method based on perrhenate ionic liquid
ActiveCN107325890AHigh activityReduce dosageOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEpoxyPerrhenic acid
The invention relates to an epoxy plasticizer synthesis method based on perrhenate ionic liquid. The method is characterized in that the perrhenate ionic liquid with the negative ions being ReO4<-> is used as a catalyst; H2O2 is directly used as an oxidizing agent; a solvent-free method is used for catalyzing soybean oil or fatty acid methyl ester to perform epoxidation reaction, and the epoxy plasticizer is synthesized. The reaction temperature is 30 to 60 DEG C; the reaction time is 2 to 6h. After the reaction is completed, centrifugal separation is performed to separate the catalyst; an oil phase is washed to a neutral state by de-ionized water; distillation is performed, thus an epoxy product can be obtained. The used catalyst is insoluble in raw material oil; the recovery and the use are easy; the environment pollution is avoided; the quality of the obtained epoxy plasticizer is higher.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
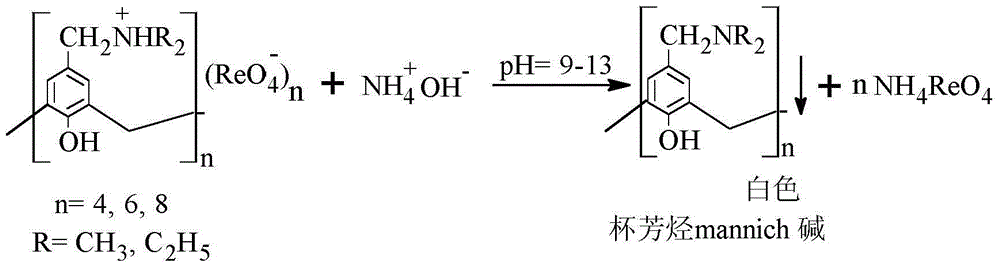
Method for separating and recovering rhenium
InactiveCN103553139AAchieve separationRealize the purpose of recycling rheniumRhenium compoundsPerrhenic acidOrganic solvent
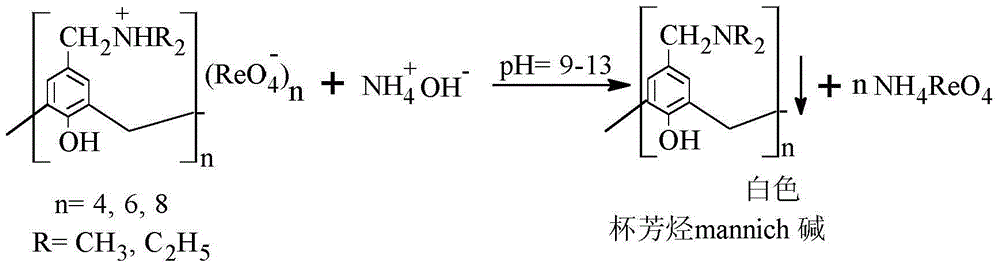
The invention belongs to the field of separation and reclamation of dispersed elements, and particularly relates to a method for separating and recovering a rhenium element. The method comprises the following steps: transforming a sample containing rhenium into a perrhenic acid ReO4<-> solution; enabling mannich alkali of calixarene to react with perrhenic acid ReO4<-> under an acid condition, so as to generate a white precipitate of perrhenic acid calixarenes ammonium salt; enabling the white precipitate of the perrhenic acid calixarenes ammonium salt to react under an alkaline condition; degrading the product into the perrhenic acid ReO4<-> and the mannich alkali of calixarene; concentrating the perrhenic acid ReO4<-> dissolved into the alkali; finally separating out perrhenate; recycling the calixarene mannich alkali after filtering. The method disclosed by the invention is simple, low in cost, free of pollution, and high in yield; no organic solvent is used in an operation process, and the pH value is controlled, so as to achieve the targets of separation and recovery. The reagent calixarene mannich alkali for recovery can be recycled.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
Manufacturing method of perrhenic acid aqueous solution from rhenium sulfide
To provide a method which can manufacture highly-pure perrhenic acid using a dry process from crude rhenium sulfide. A manufacturing method of perrhenic acid aqueous solution contains a process of gasifying and discharging sulfur oxide and getting rhenium oxide as burning residual by performing furst burning of rhenium sulfide in presence of oxygen content gas and producing the rhenium oxide and the sulfur oxide; a process of collecting gasified rhenium oxide by performing second burning of the burning residual in presence of the oxygen content gas; a process of cooling and solidifying the gasified rhenium oxide or a process getting a perrhenic acid aqueous solution by dissolving the gasified rhenium oxide in water while cooling with water; and a process of getting the perrhenic acid aqueous solution by dissolving the solidified rhenium oxide in water, or heating and gasifying the solidified rhenium oxide and dissolving it in water, when the rhenium oxide is solidified.
Owner:PAN PACIFIC COPPER CO LTD
Preparation method of catalyst for catalyzing and cracking naphtha to prepare propylene
The invention discloses a preparation method of a catalyst for catalyzing and cracking naphtha to prepare propylene. The preparation method comprises the following steps of firstly, using a hydrothermal synthesis method to prepare an EU-1 / ZSM-5 composite molecular sieve; then, adding gamma-Al2O3 (aluminum oxide), uniformly mixing, forming, and loading perrhenic acid and tetrabutylammonium perrhenate by an impregnating method, so as to obtain the catalyst for catalyzing and cracking naphtha to prepare propylene. The prepared catalyst for catalyzing and cracking naphtha to prepare propylene canbe used for catalyzing and cracking the naphtha to obtain more propylene products. In the preferable implementing type, the amount of ethylene, propylene and butane is equal to 94% or above of the total weight of cracked product, and the yield rate of the propylene is high.
Owner:北京燧火科技有限公司
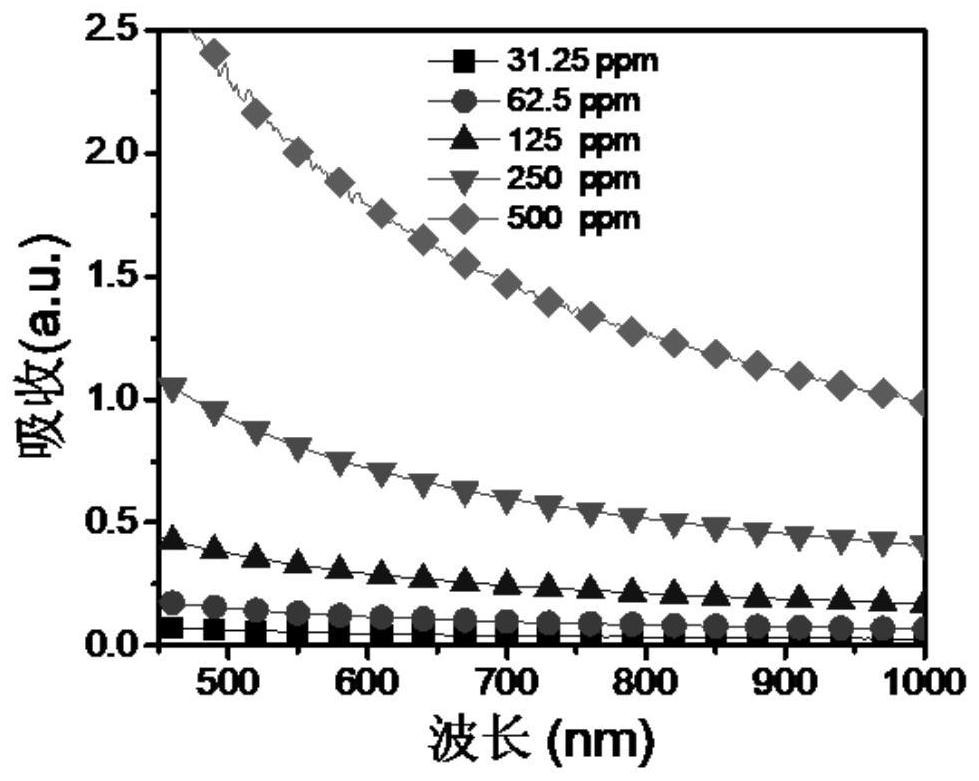
Spectrophotometric method for measuring concentration of perrhenate in aqueous solution simply and conveniently
InactiveCN105372197AThe operation method is simple and convenientLow costColor/spectral properties measurementsPerrhenic acidAqueous solution
The invention provides a spectrophotometric method for measuring concentration of perrhenate in an aqueous solution simply and conveniently. The spectrophotometric method is characterized in that with stannous chloride taken as a reduction agent, rhenium and dimethylglyoxime form a stable orange-yellow complex in an acid solution, the solution is diluted to reach a certain volume, and then an ultraviolet-visible spectrophotometer is used for measurement. Compared with the prior art, the detection method has the advantages that pretreatment of a sample solution is not required, operation is simple and convenient, the concentration of a hydrochloric acid medium is low, few hydrochloric acid media are consumed and the like.
Owner:FUZHOU UNIV
Application of titanium trichloride and treatment method of rhenium-containing wastewater
ActiveCN113247998AStrong reductionLow toxicityRadioactive contaminantsProcess efficiency improvementTitanium chloridePerrhenic acid
The invention provides an application of titanium trichloride and a treatment method of rhenium-containing wastewater, and relates to the technical field of wastewater treatment. The invention relates to the application of titanium trichloride, in particular to the application of titanium trichloride as a coagulant in wastewater. The rhenium-containing wastewater treatment method comprises the following steps: taking wastewater containing perrhenate ions, adding a titanium trichloride solution, and adding a sodium hydroxide solution to adjust the pH value to 3.2-9.2; and S2, stirring the wastewater of which the pH value is adjusted in two stages, standing for layering after the stirring is finished, and filtering. In the wastewater after the pH value is adjusted, the molar ratio of titanium trichloride to perrhenate ions is (5-30): 1. The invention provides a new application of titanium trichloride, and solves the problems that in the existing rhenium-containing wastewater, the removal effect of perrhenate is not ideal, and the technological process is complex.
Owner:LOGISTICAL ENGINEERING UNIVERSITY OF PLA
Method of preparing rhenium complex using borohydride exchange resin
ActiveUS6888019B1Reduce decreaseIn-vivo radioactive preparationsGroup 7/17 organic compounds without C-metal linkagesRheniumEthylenediamine tetraacetate
Disclosed is a method of preparing a rhenium complex by use of a borohydride exchange resin, including dissolving perrhenic acid in a solution containing ethylenediamine tetraacetate, mannitol and stannous chloride to obtain a perrhenic acid solution (step 1), which is then mixed with disulfide in the presence of a borohydride exchange resin and reacts at room temperature, to obtain a rhenium complex (step 2). The preparation method of the current invention is characterized in that rhenium is reduced to have a desired oxidation number through the dissolving of the step 1, whereby the reaction of the step 2 can occur at room temperature. In addition, use of the borohydride exchange resin as a reducing agent results in direct formation of the rhenium complex of sulfide from disulfide, without the need of synthesis of thiol-protected S-precursor. Therefore, radioactive pharmaceuticals having high values can be economically and efficiently produced.
Owner:KOREA ATOMIC ENERGY RES INST
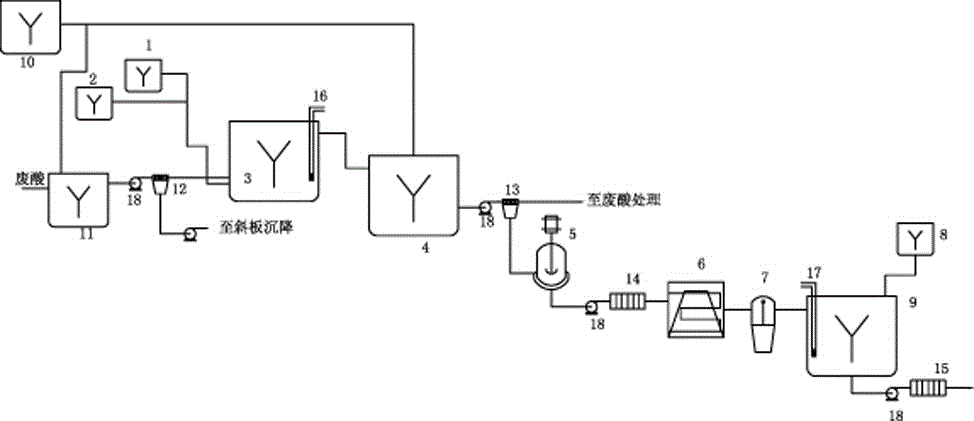
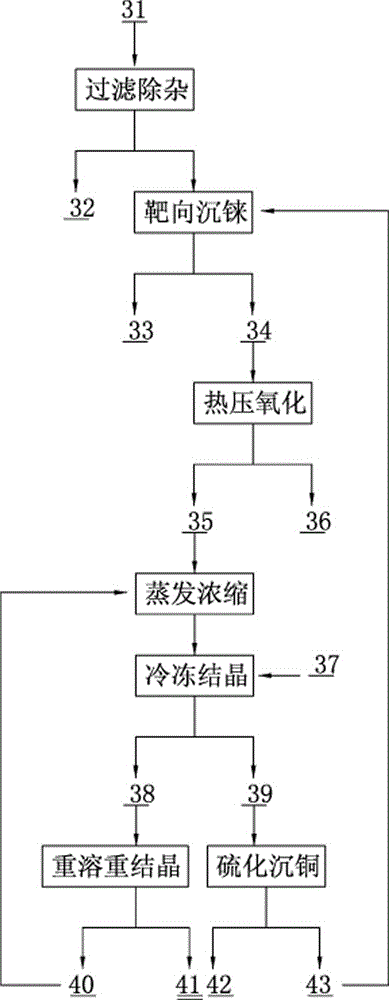
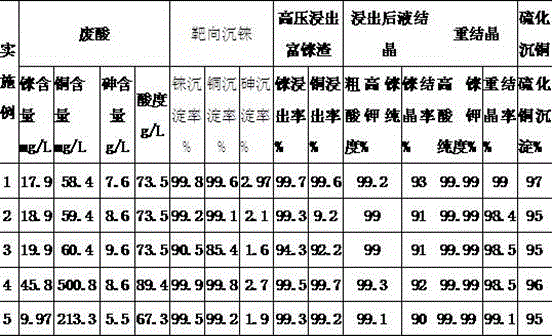
Method for rapidly recovering rhenium, copper and lead from copper smelting waste acid and device for implementing method
ActiveCN105543491AReduce processingImprove protectionProcess efficiency improvementEnvironmental resistanceAnti-gravity
The invention discloses a method for rapidly recovering rhenium, copper and lead from copper smelting waste acid and a device for implementing the method. According to the method, the copper smelting waste acid is subjected to lead removing through filtering, lead residues with the lead content higher than 55% are obtained and taken as lead extraction raw materials, the lead extraction raw materials are subjected to targeted rhenium precipitation, rhenium-rich residues are obtained and are subjected to hot-pressing oxidizing leaching, a rhenium-rich leaching agent is obtained, the rhenium-rich leaching agent after evaporation and concentration is subjected to KCl rhenium precipitation and recrystallization, potassium perrhenate is prepared, and a liquid obtained after rhenium precipitation is vulcanized for copper deposition. The device comprises a rhenium and copper precipitant storage tank, a precipitation tank, a flocculating agent storage tank, a flocculation reaction tank, a reaction kettle, an evaporation-crystallization device, a centrifugal separator, a waste acid anti-gravity-osmosis filter, a rhenium-rich copper residue anti-gravity-osmosis filter, a filter press, a rhenium and copper precipitation tank steam heating system, a copper precipitation tank steam heating system and a slurry pump. The method and the device have the characteristics of low investment, rapid and short procedures, simple and efficient operation, and high recovery rate, and are clean and environment-friendly.
Owner:ZIJIN MINING GROUP
Load type perrhenate ionic liquid based cellulose degradation method
ActiveCN107267687AHigh activityPromote degradationOrganic-compounds/hydrides/coordination-complexes catalystsGlucose productionCellulosePerrhenic acid
The invention relates to a load type perrhenate ionic liquid based cellulose degradation method. According to the technical scheme, the method is characterized in that cellulose is dissolved in an ionic liquid solvent, and load type perrhenate ionic liquid in which ReO4<-> is used as negative ion is used as a catalyst for degrading; the degrading reaction is carried out for 10-60min at the temperature of 120-170 DEG C; and water is added to dilute reaction liquid after the reaction is finished, and then the dilute is filtered and separated. The method is simple; the catalyst is green, environment-friendly and easily recovered; the cellulose degrading effect is good.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
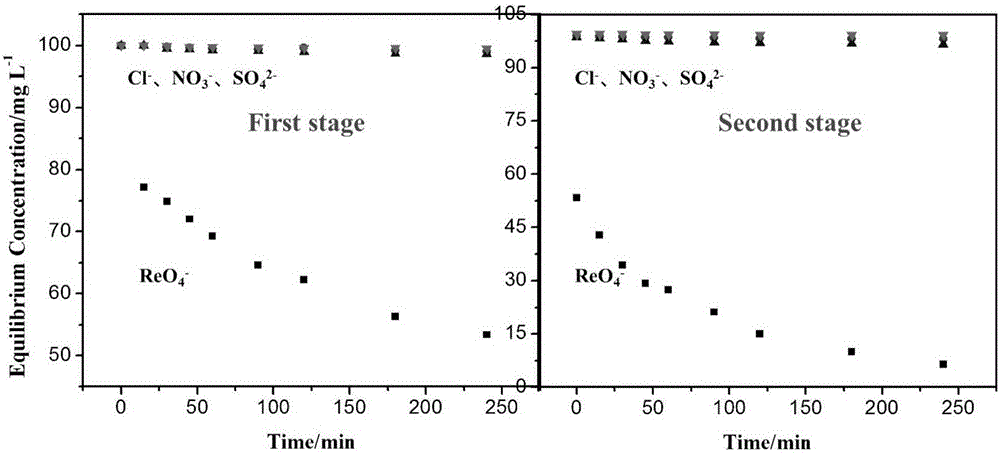
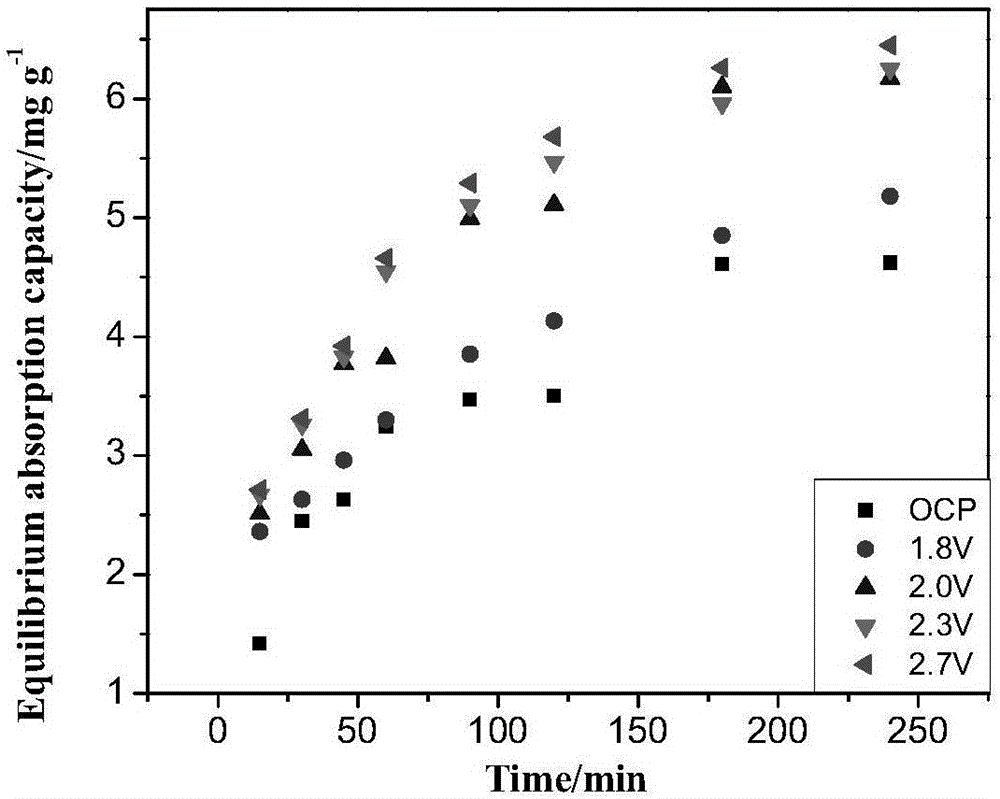
Method for selectively separating perrhenic acid radical through two-section capacitive deionization process
The invention discloses a method for selectively separating a perrhenic acid radical through a two-section capacitive deionization process. The method comprises the following steps of: introducing a to-be-adsorbed solution containing the perrhenic acid radical into a first-section capacitor, and adsorbing the to-be-adsorbed solution by active carbon in an anode region under slot voltage of 1.8-3.0V; and introducing the to-be-adsorbed solution into a second-section capacitor, and adsorbing the to-be-adsorbed solution by active carbon in anode region under slot voltage of 1.8-3.0V. The invention further discloses a device for selectively separating the perrhenic acid radical through the two-section capacitive deionization process. The method for selectively separating the perrhenic acid radical through the two-section capacitive deionization process is good in selectivity, has a selective adsorption rate of 93% or more on ReO4<->, and has adsorption smaller than 1% on other ions such as SO4<2->, Cl<-> and NO3<->. The adopted adsorption material is low in price, the active carbon is a common carbon material, is low in price, is simple to manufacture, and is convenient to purchase; and relative to other carbon materials, implementation cost of the technical scheme is reduced.
Owner:CENT SOUTH UNIV
A kind of preparation method of high-purity ammonium rhenate
ActiveCN108408785BLow Tl contentMeet the requirements of low Tl contentRhenium compoundsPerrhenic acidPhysical chemistry
The invention discloses a preparation method of high purity ammonium rhenate, the method comprises the following steps of S1, dissolving ammonium rhenate in pure water to obtain an ammonium rhenate solution and adjusting pH value to 7 to 10, then performing oxidation and precipitation in sequence, and centrifuging to obtain an ammonium rhenate solution without containing TI; S2, after performing concentration and crystallization on the ammonium rhenate solution without containing TI, performing oxidation volatilization to obtain a perrhenic acid solution; S3, exchanging the perrhenic acid solution by adopting a cation exchange resin for removing impurities; S4, adding ammonia water into the perrhenic acid solution subjected to adsorption and impurities removal and performing concentrationand crystallization to obtain the high purity ammonium rhenate. According to the preparation method of the high purity ammonium rhenate disclosed by the invention, TI<+> in the ammonium rhenate solution is oxidized into TI<3+>, a harmful impurity TI is removed through a synergistic effect of hydrolytic precipitation of TI<3+> and adsorption packaging of a flocculating agent, then trace TI and other impurity elements are removed through oxidation volatilization, ion exchange and concentration crystallization to obtain the high purity ammonium rhenate with low TI content, the high purity ammonium rhenate meets the requirements of rhenium with low TI content in aeronautical material.
Owner:NORTHWEST INSTITUTE FOR NON-FERROUS METAL RESEARCH
Process for Separating and Recovering Rhenium
The invention belongs to the field of separation and reclamation of dispersed elements, and particularly relates to a method for separating and recovering a rhenium element. The method comprises the following steps: transforming a sample containing rhenium into a perrhenic acid ReO4<-> solution; enabling mannich alkali of calixarene to react with perrhenic acid ReO4<-> under an acid condition, so as to generate a white precipitate of perrhenic acid calixarenes ammonium salt; enabling the white precipitate of the perrhenic acid calixarenes ammonium salt to react under an alkaline condition; degrading the product into the perrhenic acid ReO4<-> and the mannich alkali of calixarene; concentrating the perrhenic acid ReO4<-> dissolved into the alkali; finally separating out perrhenate; recycling the calixarene mannich alkali after filtering. The method disclosed by the invention is simple, low in cost, free of pollution, and high in yield; no organic solvent is used in an operation process, and the pH value is controlled, so as to achieve the targets of separation and recovery. The reagent calixarene mannich alkali for recovery can be recycled.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
A kind of porous organic framework material, preparation method and application in selective separation of perrhenate
ActiveCN112111060BCation exchanger materialsOrganic anion exchangersPerrhenic acidPhysical chemistry
The invention provides a preparation method of a porous organic framework material, comprising the following steps: mixing tris(4-imidazolylphenyl)amine, cyanuric chloride and a solvent, and performing a quaternization reaction to obtain a porous organic framework material. In the present invention, tris(4-imidazolylphenyl)amine TIPA and cyanuric chloride are used as raw materials to carry out quaternization reaction, so that the prepared porous material has a special structure of cationic skeleton, and there are free anions such as chloride ions in the pores etc., after mixing with a solution containing perrhenate, the selective separation of perrhenate is carried out by anion exchange in the pores. The experimental results show that the specific surface area of the porous organic framework material provided by this application is 274m 2 , the adsorption rate is as high as 100%, and the maximum adsorption capacity is 442 mg / g.
Owner:潍坊光华精细化工有限公司 +1
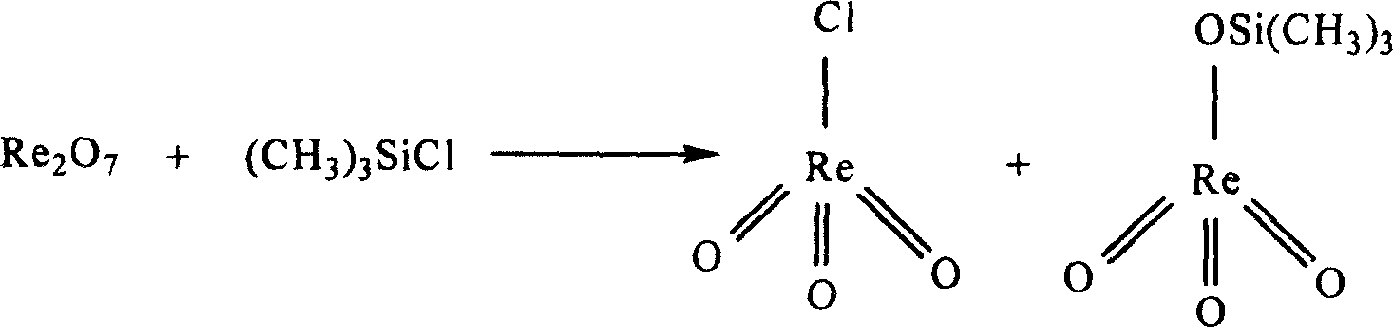
Method for synthesizing methyl rhenium trioxide from perrhenate
InactiveCN100371339CThe synthesis process is simpleShort synthesis cycleGroup 7/17 element organic compoundsMethylrhenium trioxidePerrhenic acid
Owner:LIAONING UNIVERSITY
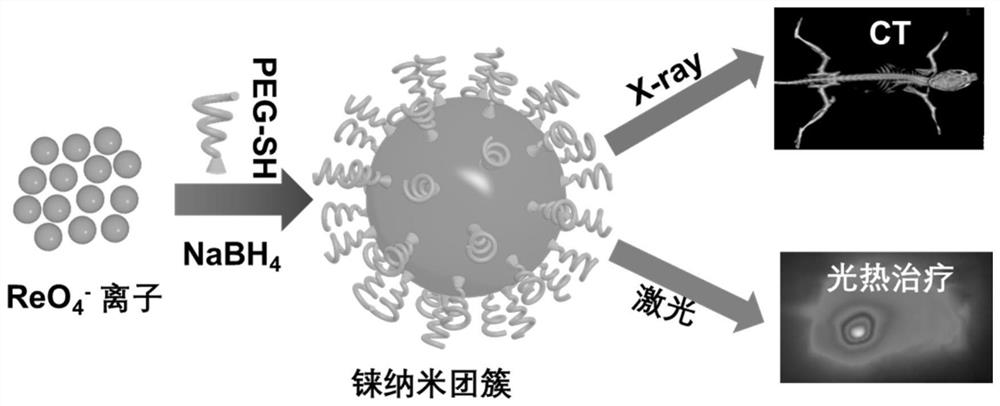
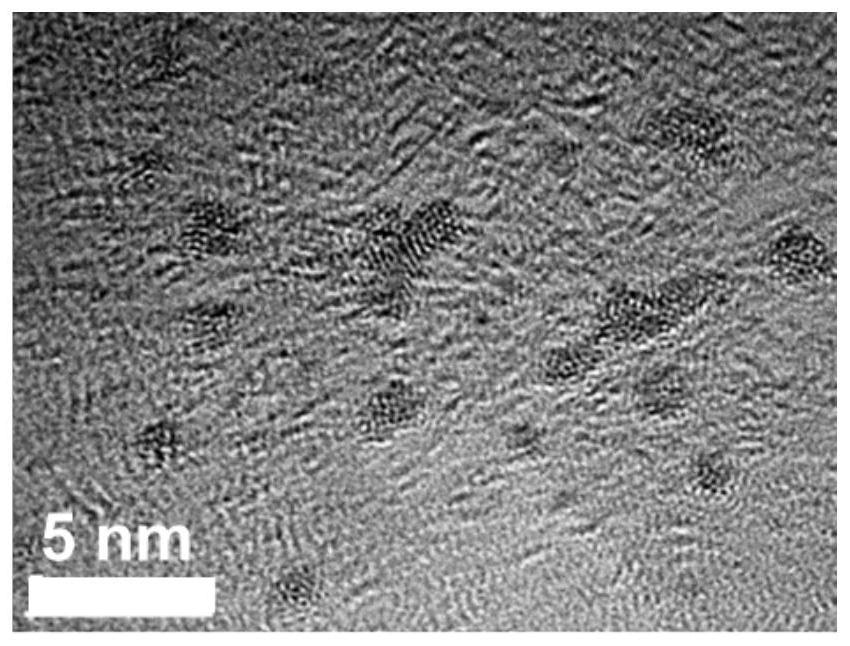
A degradable rhenium nanocluster for ct imaging and photothermal therapy and its preparation method and application
ActiveCN109833477BBiodegradableImprove securityEnergy modified materialsAntipyreticDiseasePerrhenic acid
The invention discloses a degradable rhenium nanocluster used for CT imaging and photothermal treatment and its preparation method and application. The component is an ultra-small rhenium nanocluster modified by a stabilizer with a size of 1-3nm. The metal nano-preparation of the present invention is biodegradable, and can be converted into water-soluble perrhenate ions under the action of hydrogen peroxide molecules in the body, and excreted through the kidneys, which improves the safety of clinical application; and because at the same time With strong near-infrared absorption and X-ray attenuation capabilities, the preparation can be used for photothermal therapy and CT contrast imaging respectively, realizing the effective combination of disease diagnosis and treatment.
Owner:HEFEI UNIV OF TECH
Method for producing aqueous solution of perrhenic acid from rhenium sulfide
Provided is a method that enables high-purity perrhenic acid to be produced from crude rhenium sulfide using a dry process. This method for producing an aqueous solution of perrhenic acid includes: 1) a step in which a first roasting is performed on rhenium sulfide in the presence of an oxygen-containing gas, generating rhenium oxide and sulfur oxide, and then gasifying and discharging the sulfur oxide to obtain rhenium oxide as a roasting residue; 2) a step in which a second roasting is performed on the roasting residue in the presence of an oxygen-containing gas, and gasified rhenium oxide is collected; 3) a step in which the gasified rhenium oxide is solidified by cooling, or a step in which an aqueous solution of perrhenic acid is obtained by dissolving the gasified rhenium oxide in water while cooling the gasified rhenium oxide; and 4) and a step in which, if the rhenium oxide was solidified, an aqueous solution of perrhenic acid is obtained either by dissolving the solidified rhenium oxide in water, or by dissolving the solidified rhenium oxide in water after heating and gasifying the solidified rhenium oxide.
Owner:PAN PACIFIC COPPER CO LTD
A method for extracting molybdenum and rhenium from rhenium-containing molybdenum concentrate
ActiveCN108588446BHarm reductionLow impurity contentProcess efficiency improvementPerrhenic acidSulfide
The invention belongs to the molybdenum metallurgy industry in the field of nonferrous metallurgy, and relates to a method for extracting molybdenum and rhenium from rhenium-containing molybdenum concentrate, which specifically includes 8 steps, which are pretreatment process, mixing and granulation process, solidification and roasting process, Water leaching process, precipitation, crystallization process, acid leaching process, co-extraction-reextraction process, acid precipitation process, the obtained molybdenum is crystallized in the form of ammonium tetramolybdate, and rhenium is crystallized in the form of potassium perrhenate. The present invention pretreats the molybdenum concentrate to remove lead, so that the lead is recovered in the form of lead dichloride, and the harm of lead is greatly reduced. Quicklime is added to convert the sulfide in the concentrate into sulfate, and eliminate the sulfide produced in the oxidation roasting process. SO 2 In addition, the process of the present invention is expected to shorten the process flow, reduce equipment investment, improve the recovery rate of molybdenum and rhenium and the quality of products, and facilitate the production of ammonium molybdate products.
Owner:西安鑫城投资有限公司
A mofs material for fluorescence recognition of pertechnetate or perrhenate, its preparation method and application
ActiveCN112322282BHigh charge densityIncreased site of actionOther chemical processesLuminescent compositionsPtru catalystPerrhenic acid
Owner:ZHEJIANG UNIV
Imprinted polymer for separation of perrhenate ions, preparation method and application thereof
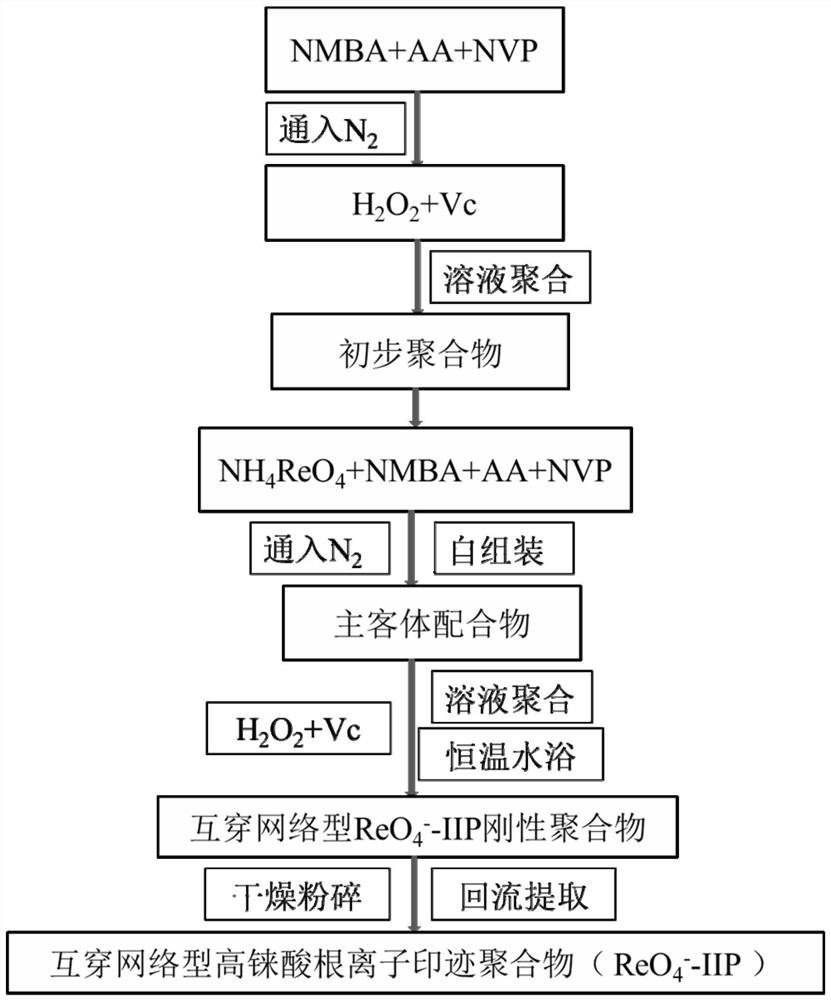
ActiveCN110092869BEasy to prepareThe recycling process is simple and controllableOther chemical processesProcess efficiency improvementFunctional monomerMeth-
The invention discloses an imprinted polymer for separating perrhenate ions, a preparation method and application thereof, and belongs to the field of hydrometallurgy. In this method, perrhenate (ReO 4 ‑ ) is a template ion, N-vinylpyrrolidone (NVP) and acrylic acid (AA) are functional monomers, N'N-methylenebisacrylamide (NMBA) is a crosslinking agent, H 2 o 2 ‑Vc is the initiator, water is the solvent, and the perrhenate ion-imprinted polymer (ReO 4 ‑ ‑IIP), and use it as an adsorption adsorbent to separate and recover the rhenium (Re) element in the rhenium-containing waste solution, which improves the separation effect and recovery efficiency of Re. The ion imprinted polymer prepared by the invention has high selectivity, large adsorption capacity, high desorption rate and high reuse rate, and makes up for the shortcomings of traditional separation materials in the field of alloy wet recovery.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com