Cationic organic polymer as well as preparation method and application thereof
A cationic, polymer technology, applied in the field of chemistry, which can solve problems such as poor selectivity, large impact, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
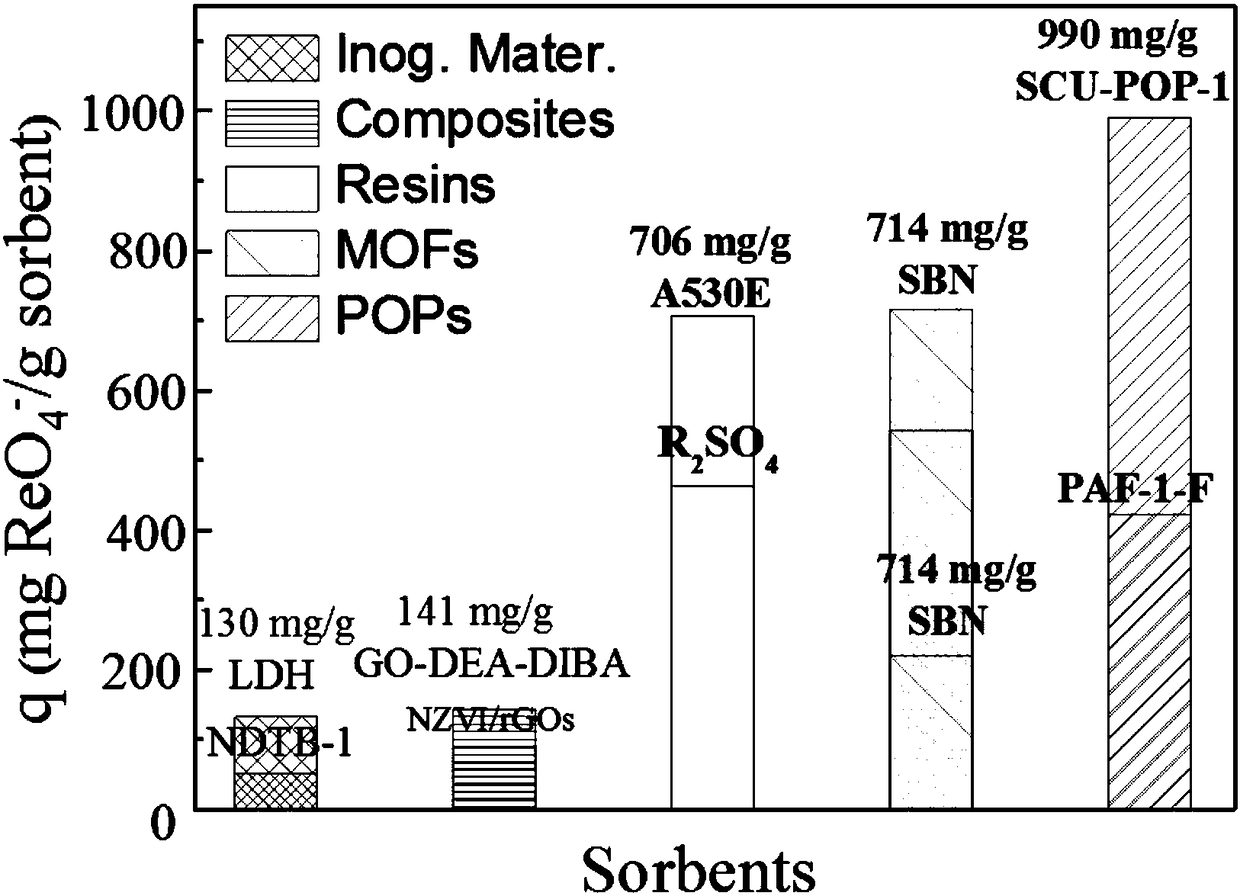
Image
Examples
Embodiment 1
[0041] With tetrakis (1-imidazolylphenyl) ethylene and α, α-dibromo-p-xylene as raw materials, the reaction is carried out according to the following reaction scheme, and the specific method is as follows:
[0042]
[0043] Take a 30 mL microwave tube, add 0.134 g (0.225 mmol) of substance A (tetrakis(1-imidazolylphenyl)ethylene) and 16 mL of N,N'-dimethylformamide (DMF), stir until uniformly dispersed , then 0.119 g (0.45 mmol) of substance B (α,α-dibromo-p-xylene) was added and stirred until dissolved. Put the microwave tube containing the above mixture into a microwave reactor, and react at 100° C. for 4 hours. After centrifugation, a yellow solid product was obtained (yield: 61%).
[0044] The above yellow solid was washed 3 times with 20 ml of DMF, 2-3 times with ethanol, and 3 times with water. The washed solid was freeze-dried to obtain a yellow powder, which was named SCU-POP-1-Br.
[0045] Soak SCU-POP-1-Br in a saturated sodium chloride solution, the chloride i...
Embodiment 2
[0048] Take a 30 mL microwave tube, add 0.134 g (0.225 mmol) of tetrakis(1-imidazolylphenyl)ethylene and 16 mL of N,N-dimethylformamide (DMF), stir until uniformly dispersed, then add 0.1071 g (0.3 mmol) of 1,3,5-tris(bromomethyl)benzene was stirred until dissolved, the microwave tube containing the above mixture was put into a microwave reactor, and reacted at 100° C. for 4 hours. After centrifugation, a yellow solid product was obtained (yield: 75%).
[0049] The above yellow solid was washed 3 times with 20 ml of DMF, 2-3 times with ethanol, and 3 times with water. The obtained solid was freeze-dried to obtain a yellow powder, which was named SCU-POP-2-Br.
[0050] Soak SCU-POP-2-Br in a saturated sodium chloride solution, the chloride ions in the solution undergo an ion exchange reaction with the bromide ions in SCU-POP-2-Br, centrifuge, wash 3 times with water and freeze-dry to obtain Obtain cationic organic polymer, it is named as SCU-POP-2-Cl, hereinafter referred to ...
Embodiment 3
[0052] Take a 30 mL microwave tube, add 0.134 g (0.225 mmol) of tetrakis(1-imidazolylphenyl)ethylene and 16 mL of N,N-dimethylformamide (DMF), stir until uniformly dispersed, then add 0.1026 g (0.225 mmol) of 1,2,4,5-tetrakis(bromomethyl)benzene was stirred until dissolved, the microwave tube containing the above mixture was put into a microwave reactor, and reacted at 100° C. for 4 hours. After centrifugation, a yellow solid product was obtained (yield: 88%).
[0053] The above yellow solid was washed 3 times with 20 ml of DMF, 2-3 times with ethanol, and 3 times with water. The yellow powder obtained after freeze-drying the obtained solid was named SCU-POP-3-Br.
[0054] Soak SCU-POP-3-Br in saturated sodium chloride solution, centrifuge, wash 3 times with water and then freeze-dry to obtain a cationic organic polymer, which is named SCU-POP-3-Cl, hereinafter referred to as SCU-POP -3, its NMR spectrum is as follows Figure 10 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com