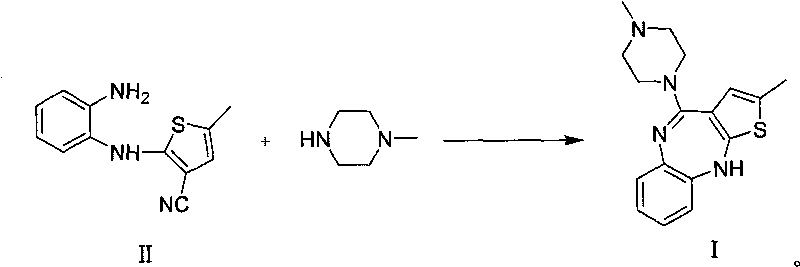
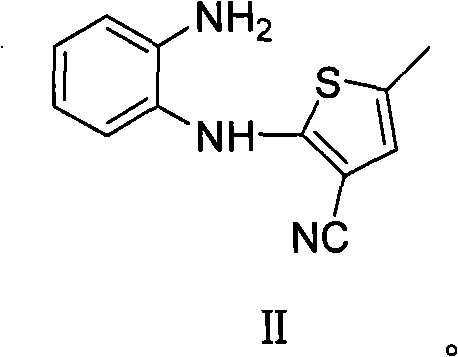
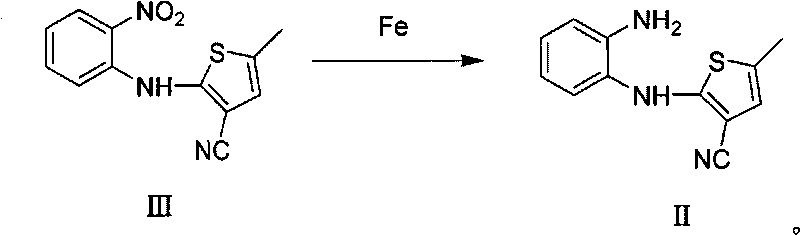
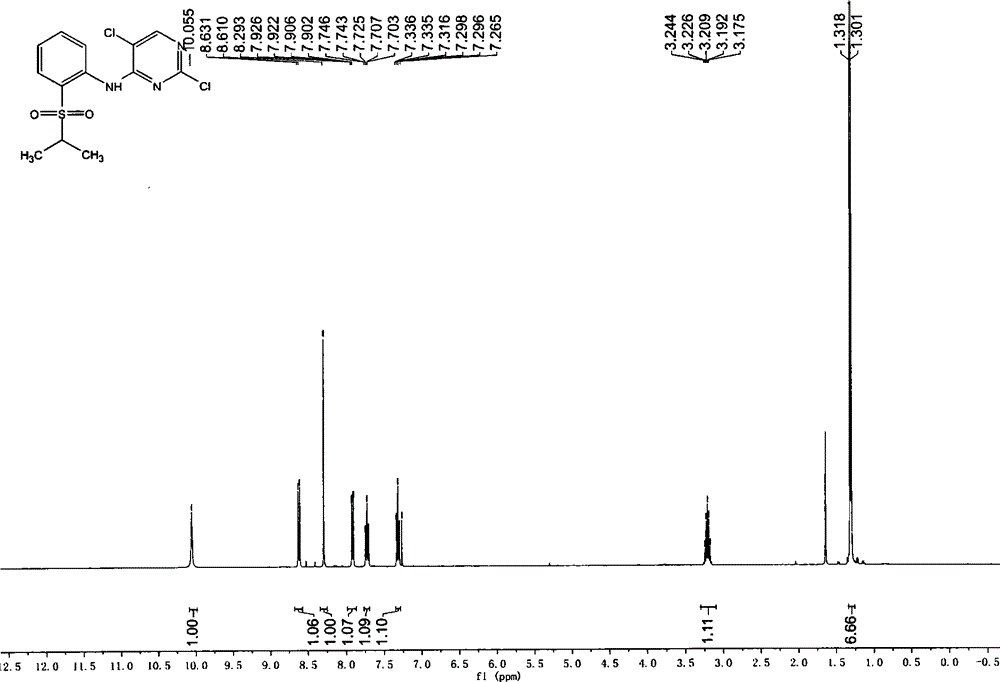
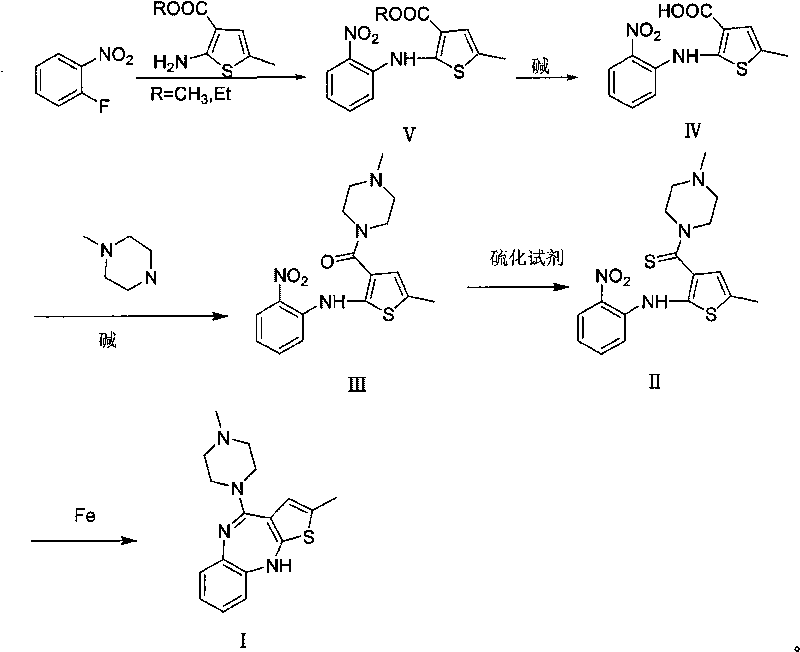
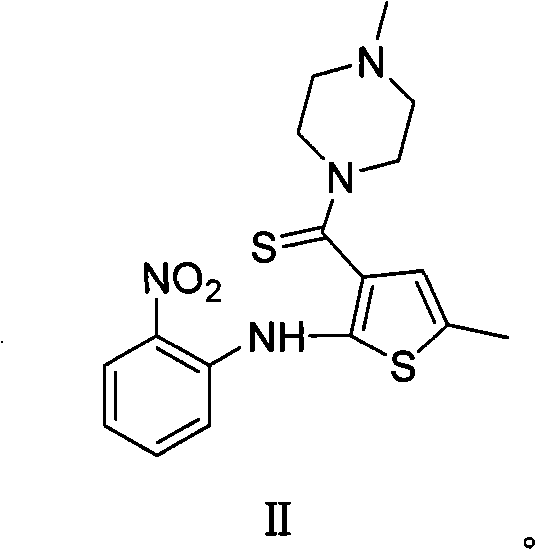
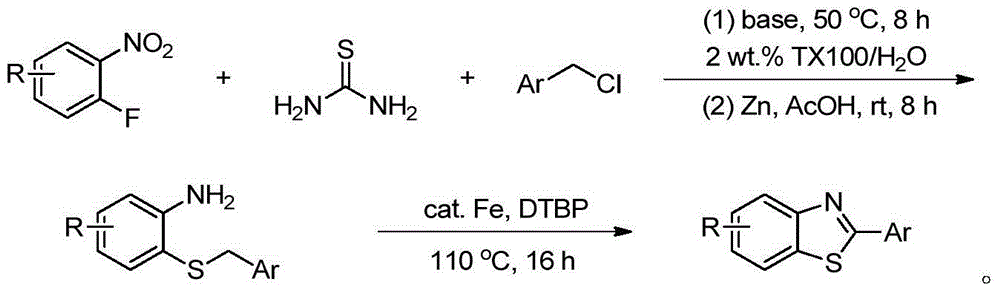
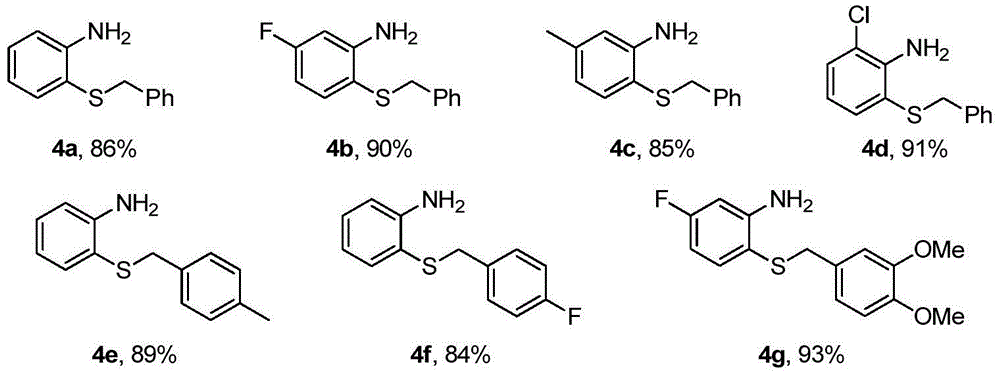
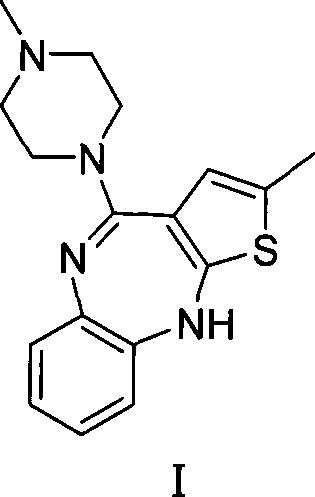
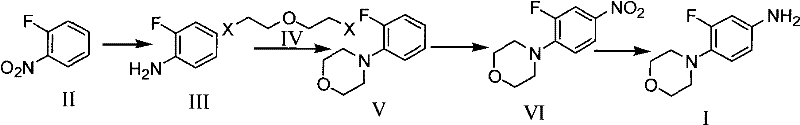
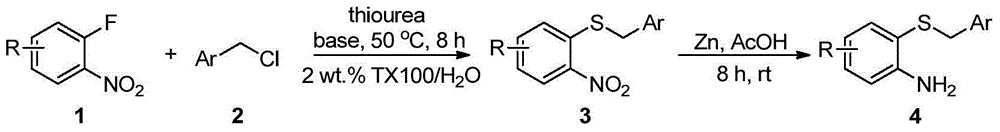Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "O-fluoronitrobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Catalyst for o-Fluoro nitrobenzene hydrogenation and its preparation and application
InactiveCN1631524AHigh yieldLower ground contentOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsRare-earth elementBenzene
The invention concerns the reaction of benzene catalyst adding hydrogen, specific speaking, it's a kind of catalyst to produce 4-ammonia base-3-F benzene fen and its application. The catalyst is made of carrier, active parts and helping dose. The main active parts are: dear metal Pt, Pd or Rh, whose weight is of catalyst 0.1%--20%. Help doses are IA, ó�A, ó¾B, ó° or Sparse soil chemical element. The catalyst has the feature of high active in reactivation, good choosing ability and many reuses. The catalyst can also be used in other replacing nitric unit benzene adding hydrogen and catalyst process.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
The preparation method of 2-methoxy-5-bromoaniline
InactiveCN102276482AWide range of usesCheap methodOrganic compound preparationAmino-hyroxy compound preparationPharmaceutical industryOrganic synthesis
The present invention relates to the field of organic synthesis, in particular to a preparation method of 2-methoxy-5-bromoaniline, which uses o-fluoronitrobenzene as a raw material, and undergoes three-step finishing synthesis of bromination, etherification and reduction reactions to obtain a medicine Intermediate 2-methoxy-5-bromoaniline. 2-Methoxy-5-bromoaniline is an important fine chemical intermediate and has a wide range of uses in the pharmaceutical industry. At present, there are few reports on related synthesis methods, and none of them are suitable for industrial production. The invention relates to a preparation method of 2-methoxy-5-bromoaniline. The method uses o-fluoronitrobenzene as a raw material, and undergoes three steps of bromination, etherification and reduction to obtain the pharmaceutical intermediate 2-methoxy-5-bromoaniline, with a total yield of 67.7%. The method of the invention has cheap raw materials and mild reaction conditions, and opens up a process for synthesizing 2-methoxy-5-bromoaniline.
Owner:CHANGZHOU UNIV
Catalyst for preparing o-fluoroaniline from o-fluoronitrobenzene by hydrogenation and preparation method of catalyst
ActiveCN104117353AImprove stabilitySolution to short lifeOrganic compound preparationAmino compound preparationO-fluoronitrobenzeneMetal
The invention discloses a catalyst for preparing o-fluoroaniline from o-fluoronitrobenzene by hydrogenation. The catalyst contains an Al2O3 supporter and the following components in percentage by mass: 0.05%-1% of Pt supported on the Al2O3 supporter, 0.05%-3% of metal M1 and 0.01%-3% of metal M2, wherein the metal M1 is Pd, Sn or Zn; the metal M2 is K, Co, Ga, In, Mn, Ag or Ce. Besides, the invention also provides a preparation method of the catalyst. When the catalyst prepared by the preparation method is used for catalyzing o-fluoronitrobenzene hydrogenation, the mole conversion rate of o-fluoronitrobenzene is up to 100%, the mole percentage of a defluorination product is smaller than 0.1%, the amount of o-fluoronitrobenzene treated by the catalyst of unit mass is large, the use amount of the catalyst is small, and the catalyst can be reused after being regenerated.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Method for preparing o-fluoroaniline by hydrogenating o-fluoronitrobenzene
ActiveCN104130129AEfficient hydrogenationHigh catalytic activityOrganic compound preparationAmino compound preparationHydrogenLiquid state
The invention discloses a method for preparing o-fluoroaniline by hydrogenating o-fluoronitrobenzene. The method comprises the following steps: 1, filling a fixed bed reactor with a catalyst, introducing air and heating; 2, uniformly mixing o-fluoronitrobenzene steam and preheated hydrogen to obtain mixed gas, introducing the mixed gas into the fixed bed reactor, and performing a catalytic hydrogenation reaction in the presence of the catalyst; and 3, delivering the material subjected to the catalytic hydrogenation reaction into a condenser, condensing the material to convert the o-fluoronitrobenzene steam in the material into a liquid state, and separating the liquid-state o-fluoronitrobenzene in an oil-water separator to obtain oil-state o-fluoroaniline. The o-fluoronitrobenzene is catalytically hydrogenated by adopting the method, the treatment amount is large, the o-fluoronitrobenzene is completely converted, the molar conversion rate of the o-fluoronitrobenzene is 100 percent, and the molar percentage of a defluorinated product is less than 0.1 percent; and the product separating and purifying operation is simple at low energy consumption, and no toxic or harmful ions are doped in the product.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Method for separating and comprehensively utilizing nitrobenzene chlorides
InactiveCN101613286ASolve pollutionHigh economic valueOrganic compound preparationHalogenated hydrocarbon preparationChemical reactionEconomic benefits
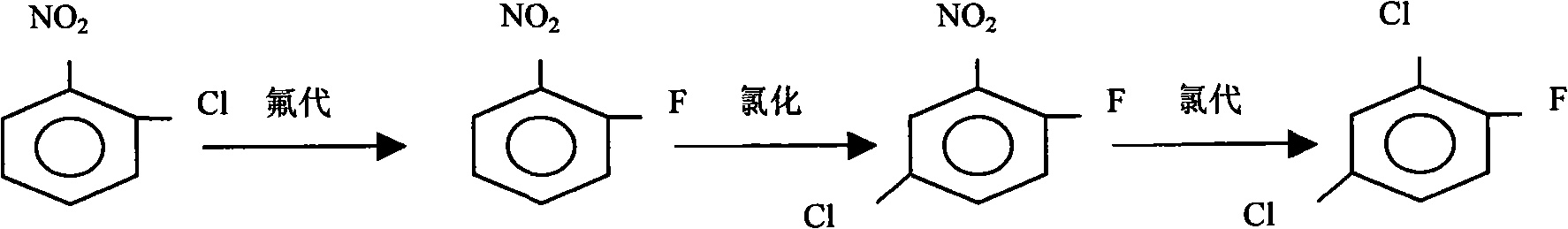
The invention discloses a method for separating and comprehensively utilizing nitrobenzene chlorides, which comprises the separation and comprehensive utilization of monochloride and the comprehensive utilization of dichloride, wherein the monochloride is subjected to fluorine substituted reaction to separate m-nitrochlorobenzene, o-fluoronitrobenzene and dinitro-fluorobenzene, and the o-fluoronitrobenzene and the dinitro-fluorobenzene are taken as products for sale, or a mixture is subjected to chlorination to generate 2,4-dichlor fluorbenzene with higher economic value; and the dichloride is subjected to chlorination to generate the 2,4-dichlor fluorbenzene with higher economic value. The method improves the yield of the m-nitrochlorobenzene on the one hand, and utilizes the resources with low economic value to produce the product with higher economic value by steps of reasonable chemical reaction and separation, brings economic benefit to enterprises and solves environmental pollution problem on the other hand.
Owner:淮安嘉诚高新化工股份有限公司
1,3-dibromo-4-fluorobenzene preparation method
InactiveCN109354569AOrganic compound preparationAmino compound preparationBromineO-fluoronitrobenzene
The invention discloses an industrial preparation method of 1,3-dibromo-4-fluorobenzene. The industrial preparation method of 1,3-dibromo-4-fluorobenzene comprises the steps that o-fluoronitrobenzeneserves as an initial raw material, and 1,3-dibromo-4-fluorobenzene is synthesized through bromine applying, reduction and diazotization-Sandmeyer three step reactions. The obtained 1,3-dibromo-4-fluorobenzene is yellow oily liquid, the purity is 97.5%, the raw material conversion rates of all steps each reach 100%, and the total recovery of the whole process reaches 52.7%.
Owner:CHANGZHOU UNIV
Preparation method of antipsychotic drug olanzapine
Owner:宁波人健化学制药有限公司
Method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction
ActiveCN101585771ATake advantage ofAvoid direct separationOrganic chemistryOrganic compound preparationChlorobenzenePotassium fluoride
The invention discloses a method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction, comprising steps of: generating a mixture mainly composed of parachloronitrobenzene, o-chloronitrobenzene and m-chloronitrobenzene in process of parachloronitrobenzene, o-chloronitrobenzene by chlorobenzene nitration method; removing low-boiling-point substances by evaporation; then adding anhydrous potassium fluoride and catalyst to the mixture for fluoro-reaction at 150 DEG. C to 250 DEG. C; after reaction, filtering to remove potassium chloride, after rectifying to acquire parachloronitrobenzene, o-chloronitrobenzene, the residual portion via recrystallization acquires m-chloronitrobenzene; the catalyst is quaternary ammonium salt or calixarene; charge rate of chloronitrobenzene mixture and mixture is 1:0.01 to 1. The method of the invention is simple to operate, in scale and changes waste material into things of value, which realizes zero discharge. The method is characterizedin sustainable development, energy saving, consumption reduction and environmentally friendly property.
Owner:浙江省常山长盛化工有限公司
Method for hydrogenation preparation of 4-amino-3-fluorophenol from o-fluoro-nitrobenzene and device therefor
InactiveCN1634867AEasy to separateLower reaction costOrganic compound preparationAmino-hyroxy compound preparationNitrobenzeneP-Aminophenol
The invention relates to hydrogenation preparation of 4-aminophenol from nitrobenzene, especially a method for hydrogenation preparation of 4-amino-3-fluorophenol from o-fluoro-nitrobenzene and device therefore. The reaction is carried out in autoclave with acid medium at 50í½300C.,0.1í½10.0MPa, and the stirring rate is 100í½900 r / min, and the catalytically hydrogenated product is separated, distilled, filtered, and dried to prepare the product. O-fluoro nitrobenzene can be converted into 4-amino-3-fluobenzene by said method with conversion approaching 100%. 4-amino-3-fluobenzene is colourless crystallisate with selective around 92% and purity up to 99%. The catalytic process in the invention can be used for heterogeneous catalysis process of hydrogenation preparation of aminophenol from o-fluoro nitrobenzene and its substituted nitrobenzene.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing N-(4-chlorphenyl)-1,2-phenylenediamine as key clofazimine intermediate
InactiveCN107445845AOrganic compound preparationAmino compound preparation by condensation/addition reactionsP-chloroanilineOrganic solvent
The invention provides a method for synthesizing N-(4-chlorphenyl)-1,2-phenylenediamine as a key clofazimine intermediate. The method specifically comprises the following steps: 1) by taking o-fluoronitrobenzene and p-chloroaniline as raw materials and utilizing organic base as a catalyst for condensation reaction, reacting in an organic solvent and performing conventional treatment after ending the reaction, thereby acquiring a condensation intermediate N-(4-chlorphenyl)-2-nitryl-1-aniline; 2) by utilizing metallic nickel as the catalyst, performing catalytic hydrogenation reaction on the acquired condensation intermediate in the organic solvent and performing conventional treatment after reducing, thereby acquiring a rough product of N-(4-chlorphenyl)-1,2-phenylenediamine; and 3) re-crystallizing the acquired rough product with the organic solvent, thereby acquiring the end product.
Owner:CHONGQING WERLCHEM FINE CHEM
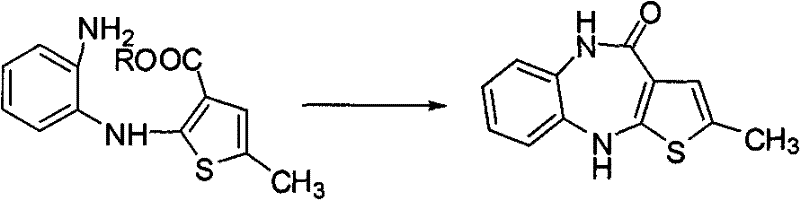
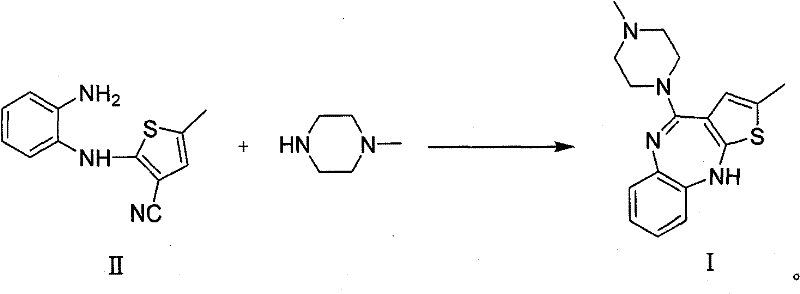
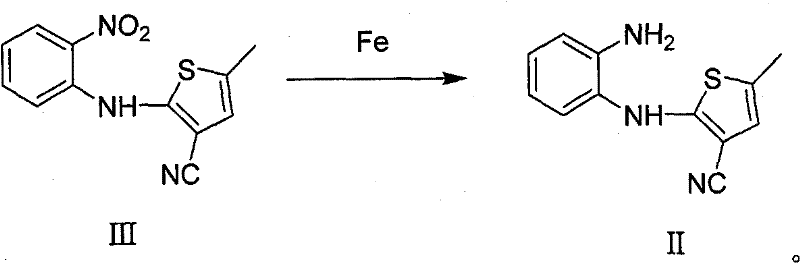
Method for preparing olanzapine
The invention relates to a synthetic route for preparing olanzapine by using o-fluoro-nitrobenzene as a raw material. In the method, the o-fluoro-nitrobenzene is used as a starting material to prepare the olanzapine by steps of coupling, reduction and ring closing.
Owner:ZHEJIANG HISUN PHARMA CO LTD
New intermediate of non-small-cell lung carcinoma treating drug Ceritinib, and preparation method thereof
The present invention relates to the field of pharmaceutical chemistry, to a preparation method of a new intermediate of 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidine-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (Ceritinib). According to the present invention, o-fluoronitrobenzene is adopted as a starting raw material, substitution, reduction and condensation are performed to obtain the new intermediate 2-X-5-chloro-N-(2-(isopropyl sulfide)phenyl)pyrimidine-4-amine (X is halogen, p-methyl benzene sulfonyloxy, methyl sulfonyloxy or trifluoromethylsulfonyloxy), the new intermediate can be oxidized to obtain a sulfonyl derivative, and the sulfonyl derivative and 2-isopropoxy-5-methyl-4-(piperidine-4-yl)aniline are subjected to condensation to finally obtain 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidine-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (Ceritinib); and the synthesis method has characteristics of readily available raw materials, high yield, mild reaction, simple operation and low production cost, and is suitable for industrial production.
Owner:SHANGHAI SCIENPHARM CO LTD
Process for 4- amino-3-fluorophenol
InactiveCN101274898ASuitable for industrial productionSimple production processOrganic compound preparationMetal/metal-oxides/metal-hydroxide catalystsPlatinumOrganic solvent
The invention relates to a new process for producing 4-amino-3-fluorophenol, which takes o-fluoronitrolbenzene as raw material and platinum-carbon or palladium-charcoal as a catalyst and adopts hydrogen pressure reaction to produce the 4-amino-3-fluorophenol in an acidic aqueous solution which contains organic solvents. The production technology is simpler, the production cost is more reasonable and the method is more suitable for industrial production.
Owner:SHANDONG UNIV AT WEIHAI
Technique for preparing olanzapine
ActiveCN101723954AReduce usageSynthetic raw materials are readily availableNervous disorderOrganic chemistrySulfurCoupling
The invention relates to a synthetic route which takes o-fluoro-nitrolbenzene as starting raw material to prepare olanzapine, which comprises five steps: coupling, saponification, esterification, sulfur substitution and deoxidation.
Owner:ZHEJIANG HISUN PHARMA CO LTD
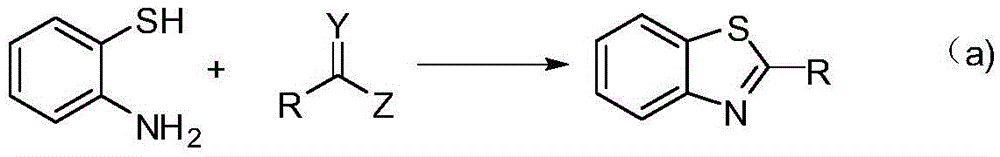
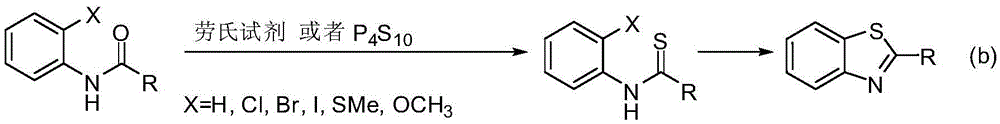
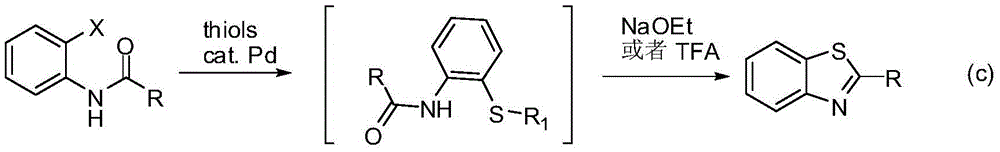
Method for synthesizing poly-substituted 2-aryl benzothiazoles by utilizing thiourea as sulphur source
InactiveCN104892545AShort reaction stepsMild reaction conditionsOrganic chemistryAntineoplastic agentsThioureaBenzyl chloride
The invention discloses a method for synthesizing poly-substituted 2-aryl benzothiazoles by utilizing thiourea as a sulphur source. According to the method, thiourea reacts with benzyl chloride to generate an S-benzylisothiourea salt in situ. After that, through the aromatic nucleophilic substitution reaction of the obtained S-benzylisothiourea salt with 2-fluoronitrobenzene and then the one-step reduction (one-pot reaction) process, o-amino phenyl benzyl thioether as an intermediate product can be obtained. Finally, through the iron-catalyzed cross-dehydrogenative-coupling reaction of the o-amino phenyl benzyl thioether, a target product can be obtained. Compared with the traditional synthetic method, the method has the significant advantages of (1) short reaction step, wherein the target product can be synthesized through only three steps of simple reactions by utilizing simple chemical raw materials; (2) mild reaction condition, high atom economy, and relatively safe and cheap reaction reagents; (3) high reaction yield, good substrate tolerance and free of any dangerous or high-toxicity reagent. Therefore, the method might be applied to the large-scale production.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of 4-chlorine-3-fluorine benzene ether
InactiveCN107721832AHigh yieldHigh purityOrganic compound preparationEther preparation by ester reactionsN dimethylformamideOil phase
The invention discloses a preparation method of 4-chlorine-3-fluorine benzene ether. The preparation method comprises the following steps: firstly, by taking o-fluoronitrobenzene as a raw material, preparing 4-amino-3-fluorophenol; taking N,N-dimethylformamide as a reaction medium, carrying out acylating chlorination reaction between the 4-amino-3-fluorophenol and an acyl chloride reagent, thus preparing 4-chlorine-3-fluorophenol, mixing sodium phenoxide with a sodium hydroxide aqueous solution, then adding the 4-chlorine-3-fluorophenol, and uniformly stirring and mixing, thus preparing a mixed solution; adding the prepared mixed solution into a reactor, then adding diethyl sulfate, stirring for 3 min, and heating to 80 to 120 DEG C for reaction; at the end of reaction, adjusting the pH ofa reaction system to 2 to 5, allowing the reaction system to stand still for layering, collecting an oil phase, washing the oil phase with the sodium hydroxide aqueous solution, and collecting the oil phase, namely the 4-chlorine-3-fluorine benzene ether. The method disclosed by the invention is easy to operate, low in cost, high in target product purity and high in yield.
Owner:DONGGUAN LIANZHOU INTPROP OPERATION MANAGEMENT CO LTD
Synthesis method of flurbiprofen
InactiveCN108218667AWide variety of sourcesShort reaction pathOxygen-containing compound preparationOrganic compound preparationSynthesis methodsO-fluoronitrobenzene
The invention relates to a synthesis method of flurbiprofen. The synthesis method of the flurbiprofen belongs to the technical field of drug synthesis, and comprises the steps of adopting o-fluoronitrobenzene as a starting material; carrying out substitution reaction, coupled reaction and hydrolysis reaction in an organic solvent to obtain the target product flurbiprofen. According to the synthesis method of the flurbiprofen provided by the invention, the raw material source is wide, the used organic solvent has no need to be subjected to nonaqueous treatment, the reaction safety coefficient is high, the process operation is simple, three wastes are less, and the obtained flurbiprofen has high yield and high purity.
Owner:HEBEI YIPIN PHARMA
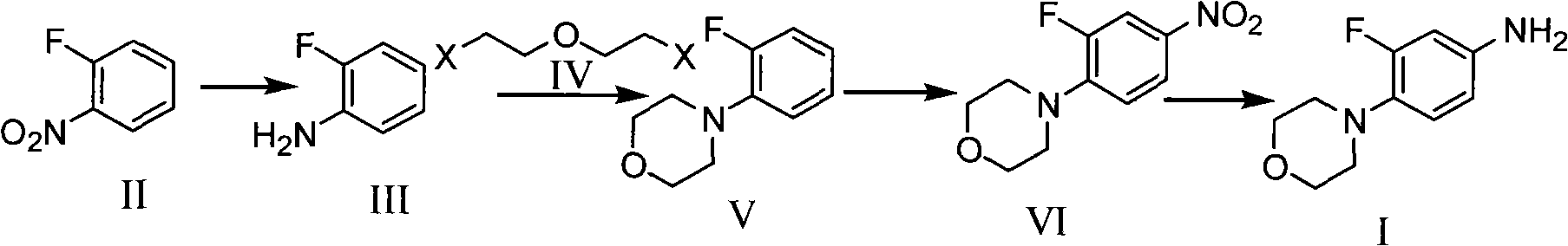
Method for preparing 3-fluorine-4 morpholinyl phenylamine
The invention belongs to a method for preparing pharmaceutical intermediate, and discloses a method for preparing 3-fluorine-4 morpholinyl phenylamine, comprising the steps: (1) reducing o-fluoro-nitrobenzene to obtain o-fluoroaniline; (2) adding the o-fluoroaniline and deacidifying agent into organic solvent, and slowly adding disubstituted ethyl ether into a reaction system at the temperature of100-150 DEG C and then stirring at the temperature of 100-200 DEG C to react for preparing o-fluoro-morpholinyl benzene; (3) taking nitric acid with the mass percent of 65-98% as nitrating agent andacetic acid as solvent, and leading the o-fluoro-morpholinyl benzene obtained in the step (2) to have nitration reaction to obtain 3-fluorine-4 morpholinyl nitrobenzene; (4) reducing the 3-fluorine-4morpholinyl nitrobenzene obtained in the step (3) and obtaining the 3-fluorine-4 morpholinyl phenylamine. As the method takes the o-fluoro-nitrobenzene as initial reactant, the price of the raw materials is low, and the production cost can be reduced. Meanwhile, no waste water containing fluorine is generated in the preparation process of the method, so that the method has little environmental pollution and is environment-friendly.
Owner:SUZHOU JINGYE MEDICINE & CHEM
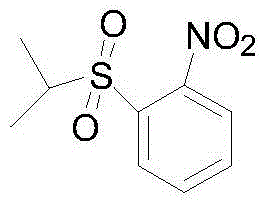
One-pot synthesis method of anticancer drug ceritinib intermediate 1-(isopropylsulfonyl)-2-nitrobenzene
InactiveCN104592068ALow reaction temperatureReduce manufacturing costOrganic chemistryOrganic compound preparationN dimethylformamideReaction rate
Owner:常州百敖威生物科技有限公司
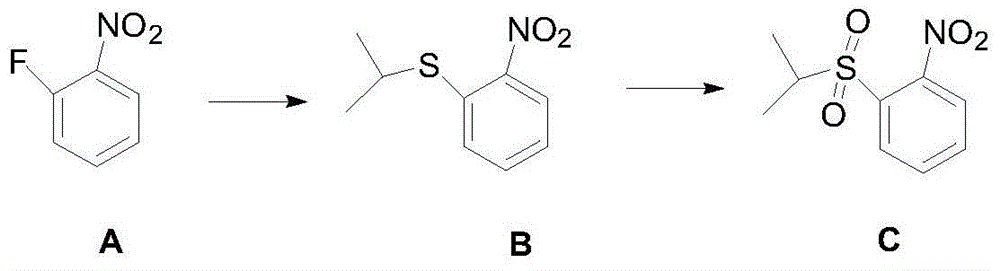
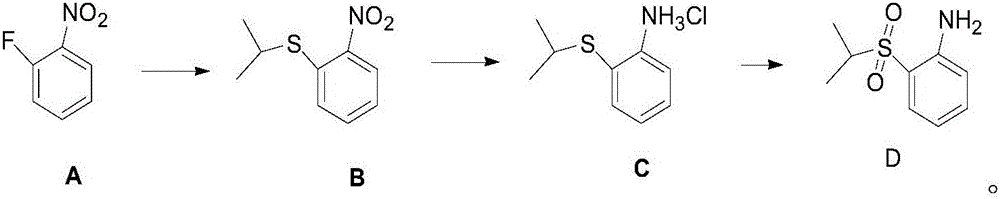
Anti-cancer drug ceritinib intermediate 1-amino-2-(isopropyl sulfonyl)benzene synthetic method
InactiveCN106083670ALow reaction temperatureReduce energy consumptionOrganic compound preparationSulfide preparationSulfideAniline
The invention belongs to the technical field of medicine synthesis, and especially relates to a synthetic method of an anti-cancer drug ceritinib intermediate 1-amino-2-(isopropyl sulfonyl)benzene. The synthetic method comprises the following steps: employing o-fluoronitrobenzene, sodium hydride and isopropyl mercaptan to synthesize a 2-(isopropyl sulfide) nitrobenzene crude product, then directly reducing the crude product to iron powder, forming salt to obtain the 2-(isopropyl sulfide) aniline hydrochloride, and finally dropping hydrogen peroxide for oxidation in 2-(isopropyl sulfide) aniline hydrochloride for oxidation, forming salt, and dissociating to obtain a target product. The synthetic method has the advantages of simpleness, little sewage amount, low energy consumption, low production cost, and the product has the advantages of high purity, high yield, and easy industrial production, and is suitable for further popularization and application.
Owner:常州安迪沃克医药科技有限公司
Preparation method of o-fluoroaniline
InactiveCN107118109AHigh yieldHigh purityOrganic compound preparationAmino compound preparationO-fluoronitrobenzeneOrganic synthesis
The invention discloses a preparation method of o-fluoroaniline, which relates to the field of organic synthesis, and specifically comprises the following steps: in a continuous reactor, the system is filled with water, and after three times of nitrogen replacement, the temperature is raised to 80°C, and the system is simultaneously pumped Put in o-fluoronitrobenzene, catalyst, inhibitory dehalogenation agent, water, (catalyst, inhibitory dehalogenation agent and water are mixed, stir evenly and pour in together) and hydrogen gas is introduced at the same time to ensure a pressure of 2.0MPa. Keep feeding continuously, the product is stratified through the stratified tank, and the product is obtained from the lower layer. Apply to the upper water layer. The preparation method disclosed by the invention is suitable for continuous large-scale production of o-fluoroaniline, has simple operation, high product purity and high yield, and the percentage content of the dehalogenated product is reduced to 0.02%.
Owner:SHANGYU XIES CHEM IND
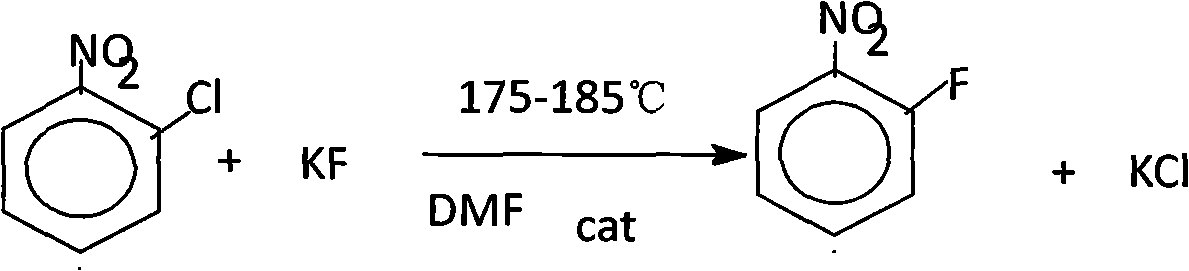
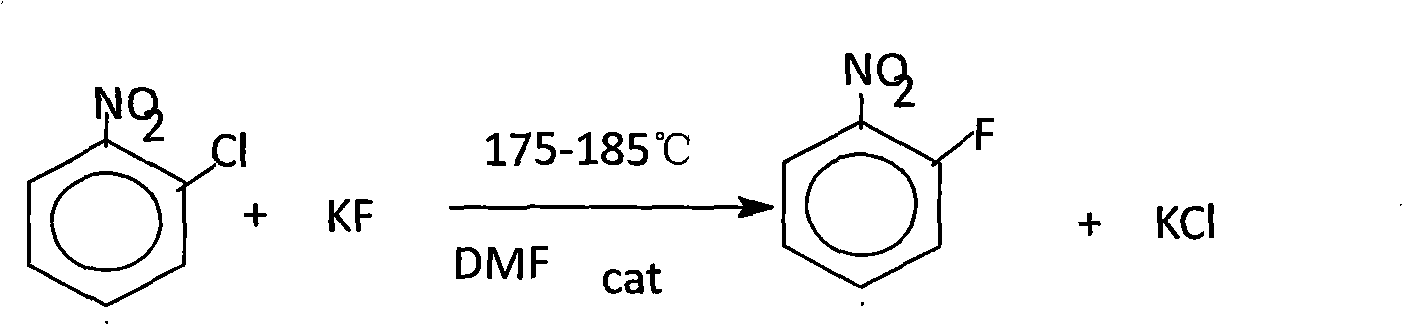
Production process of o-fluoronitrobenzene
InactiveCN101838203AHigh reactivityLow priceOrganic chemistryOrganic compound preparationPotassium fluorideTetramethylammonium chloride
The invention discloses a production process of o-fluoronitrobenzene. Fluorination is carryied out on main materials of o-chloronitrobenzene and potassium fluoride, and then after vacuum distillation and rectification, the o-fluoronitrobenzene product can be obtained. The process has scientific and reasonable design and adopts DMF as the solvent which is easy to be recycled and has high reaction efficiency; and simultaneously, tetramethylammonium chloride as the catalyst is cheap and has high reaction activity, thus saving the cost.
Owner:盘锦鸿鹤氟化学有限公司
A method for preparing o-fluoroaniline by hydrogenation of o-fluoronitrobenzene
ActiveCN104130129BEfficient hydrogenationHigh catalytic activityOrganic compound preparationAmino compound preparationHydrogenLiquid state
The invention discloses a method for preparing o-fluoroaniline by hydrogenating o-fluoronitrobenzene. The method comprises the following steps: 1, filling a fixed bed reactor with a catalyst, introducing air and heating; 2, uniformly mixing o-fluoronitrobenzene steam and preheated hydrogen to obtain mixed gas, introducing the mixed gas into the fixed bed reactor, and performing a catalytic hydrogenation reaction in the presence of the catalyst; and 3, delivering the material subjected to the catalytic hydrogenation reaction into a condenser, condensing the material to convert the o-fluoronitrobenzene steam in the material into a liquid state, and separating the liquid-state o-fluoronitrobenzene in an oil-water separator to obtain oil-state o-fluoroaniline. The o-fluoronitrobenzene is catalytically hydrogenated by adopting the method, the treatment amount is large, the o-fluoronitrobenzene is completely converted, the molar conversion rate of the o-fluoronitrobenzene is 100 percent, and the molar percentage of a defluorinated product is less than 0.1 percent; and the product separating and purifying operation is simple at low energy consumption, and no toxic or harmful ions are doped in the product.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction
ActiveCN101585771BTake advantage ofAvoid direct separationOrganic chemistryOrganic compound preparationChlorobenzeneQuaternary ammonium cation
The invention discloses a method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction, comprising steps of: generating a mixture mainly composed of parachloronitrobenzene, o-chloronitrobenzene and m-chloronitrobenzene in process of parachloronitrobenzene, o-chloronitrobenzene by chlorobenzene nitration method; removing low-boiling-point substances by evaporation; then adding anhydrous potassium fluoride and catalyst to the mixture for fluoro-reaction at 150 DEG. C to 250 DEG. C; after reaction, filtering to remove potassium chloride, after rectifying to acquire parachloronitrobenzene, o-chloronitrobenzene, the residual portion via recrystallization acquires m-chloronitrobenzene; the catalyst is quaternary ammonium salt or calixarene; charge rate of chloronitrobenzene mixture and mixture is 1:0.01 to 1. The method of the invention is simple to operate, in scale and changes waste material into things of value, which realizes zero discharge. The method is characterizedin sustainable development, energy saving, consumption reduction and environmentally friendly property.
Owner:浙江省常山长盛化工有限公司
Comprehensive development separation method of m-chloronitrobenzene, p-chloronitrobenzene, and o-chloronitrobenzene mixture
ActiveCN110437073AReduce coke contentReduce pollutionOrganic chemistryOrganic compound preparationPotassium fluorideP-fluoronitrobenzene
The invention provides a comprehensive development separation method of m-chloronitrobenzene, p-chloronitrobenzene, and o-chloronitrobenzene mixture. The comprehensive development separation method ofm-chloronitrobenzene, p-chloronitrobenzene, and o-chloronitrobenzene mixture comprises following steps: the m-chloronitrobenzene, p-chloronitrobenzene, and o-chloronitrobenzene mixture, potassium fluoride, and tetrabutylammonium chloride are introduced into a distiller, under vacuum conditions, water vapour in the system is discharged, the temperature is increased to 140 to 145 DEG C, ultrasonicreaction is carried out, the temperature is reduced to 73 to 78 DEG C, water is added, and an oil layer and a water layer are obtained through separation; the oil layer is introduced into an ice waterbath for stirring crystallization, filtering is carried out to obtain filtrate o-fluoronitrobenzene; a crystal product obtained through separation is introduced into a rectifying tower, crystal melting is carried out, tower top temperature is controlled to be 205 to 210 DEG C, tower bottom temperature is controlled to be 235 to 240 DEG C, a non-condensing collector is adopted for rectification, and a p-fluoronitrobenzene crude product and a m-fluoronitrobenzene crude product are obtained; the p-fluoronitrobenzene crude product is heated to 150 to 180 DEG C, is cooled to 50 to 55 DEG C, and iscooled to 25 to 30 DEG C slowly for separation, a filtrate is collected, and purified p-fluoronitrobenzene is obtained; the m-fluoronitrobenzene crude product is cooled to 10 to 15 DEG C, is heated to 30 to 40 DEG C slowly, separation is carried out, and a crystal product is collected to obtain purified m-fluoronitrobenzene.
Owner:SHANGYU XIES CHEM IND
Catalyst and preparation method for preparing o-fluoroaniline by hydrogenation of o-fluoronitrobenzene
ActiveCN104117353BImprove stabilitySolution to short lifeOrganic compound preparationAmino compound preparationO-fluoronitrobenzeneMetal
The invention discloses a catalyst for preparing o-fluoroaniline from o-fluoronitrobenzene by hydrogenation. The catalyst contains an Al2O3 supporter and the following components in percentage by mass: 0.05%-1% of Pt supported on the Al2O3 supporter, 0.05%-3% of metal M1 and 0.01%-3% of metal M2, wherein the metal M1 is Pd, Sn or Zn; the metal M2 is K, Co, Ga, In, Mn, Ag or Ce. Besides, the invention also provides a preparation method of the catalyst. When the catalyst prepared by the preparation method is used for catalyzing o-fluoronitrobenzene hydrogenation, the mole conversion rate of o-fluoronitrobenzene is up to 100%, the mole percentage of a defluorination product is smaller than 0.1%, the amount of o-fluoronitrobenzene treated by the catalyst of unit mass is large, the use amount of the catalyst is small, and the catalyst can be reused after being regenerated.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Method for synthesizing benzothiazole derivatives
InactiveCN105646396AObvious cost advantageProductive timeOrganic chemistryThioureaTrifluoroacetic acid
The invention discloses a method for synthesizing benzothiazole derivatives. Thiourea is used as a sulfur source, and the thiourea and o-fluoronitrobenzene derivatives are continuously converted into the benzothiazole derivatives by means of three-step conversion. The method includes enabling 2-benzylthio-benzamide derivatives to react to trifluoroacetic acid and the thiourea to obtain reaction products, extracting the reaction products by the aid of n-hexane, concentrating n-hexane layers to obtain the benzothiazole derivatives, and adding the o-fluoronitrobenzene derivatives and sodium hydroxide solution into lower-layer water phases to obtain 2-benzylthio-nitrobenzene derivatives; reducing tin dichloride to obtain 2-benzylthio-aminobenzene derivatives; ultimately acylating the 2-benzylthio-aminobenzene derivatives to obtain 2-benzylthio-substituted benzamide analogs. The method has the advantages of simplicity, environmental protection and low cost.
Owner:SHANGHAI INST OF TECH
Method for preparing olanzapine
The invention relates to a synthetic route for preparing olanzapine by using o-fluoro-nitrobenzene as a raw material. In the method, the o-fluoro-nitrobenzene is used as a starting material to prepare the olanzapine by steps of coupling, reduction and ring closing.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Method for preparing 3-fluorine-4 morpholinyl phenylamine
The invention belongs to a method for preparing pharmaceutical intermediate, and discloses a method for preparing 3-fluorine-4 morpholinyl phenylamine, comprising the steps: (1) reducing o-fluoro-nitrobenzene to obtain o-fluoroaniline; (2) adding the o-fluoroaniline and deacidifying agent into organic solvent, and slowly adding disubstituted ethyl ether into a reaction system at the temperature of 100-150 DEG C and then stirring at the temperature of 100-200 DEG C to react for preparing o-fluoro-morpholinyl benzene; (3) taking nitric acid with the mass percent of 65-98% as nitrating agent andacetic acid as solvent, and leading the o-fluoro-morpholinyl benzene obtained in the step (2) to have nitration reaction to obtain 3-fluorine-4 morpholinyl nitrobenzene; (4) reducing the 3-fluorine-4morpholinyl nitrobenzene obtained in the step (3) and obtaining the 3-fluorine-4 morpholinyl phenylamine. As the method takes the o-fluoro-nitrobenzene as initial reactant, the price of the raw materials is low, and the production cost can be reduced. Meanwhile, no waste water containing fluorine is generated in the preparation process of the method, so that the method has little environmental pollution and is environment-friendly.
Owner:SUZHOU JINGYE MEDICINE & CHEM
A kind of synthetic method of 3-amino-4-fluorophenylboronic acid
ActiveCN104530107BHigh purityHigh yieldGroup 3/13 element organic compoundsO-fluoronitrobenzeneAmino acid
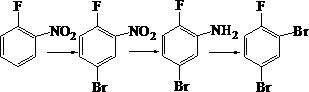
The invention discloses a synthetic method for 3-amino-4-fluorophenylboronic acid. The method includes the steps of conducting bromination on o-fluoronitrobenzene, conducting reduction to generate 5-bromo-2-fluoroanil, making 5-bromo-2-fluoroanil and tetrahydroxydiboron react in a coupled mode to generate the product, namely, 3-amino-4-fluorophenylboronic acid. According to the method, raw materials can be easily obtained, and operation is easy and convenient. The method is an appropriate route for preparing 3-amino-4-fluorophenylboronic acid.
Owner:DALIAN NETCHEM CHIRAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com