Method for preparing olanzapine
A technology based on organic solvents and cyano groups, applied in the field of synthetic routes for the preparation of olanzapine, which can solve the problems of low yield and low quality of final products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
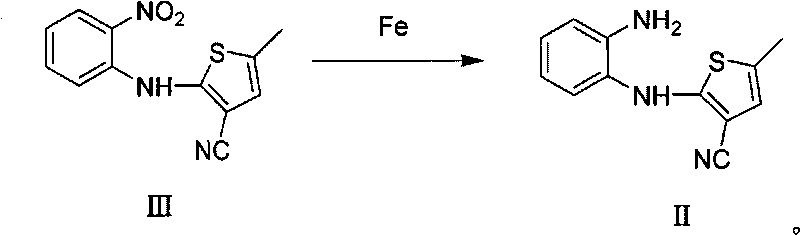
[0031] Synthesis of 2-(2-nitroanilino)-3-cyano-5-methylthiophene III:
[0032] Add 65mL of o-fluoronitrobenzene, 375mL of dimethyl sulfoxide, 75g of 2-amino-3-cyano-5-methylthiophene and 225g of potassium carbonate into a 1L three-necked flask, and heat to 60-70°C to react 4-7 Hours until the reaction of 2-amino-3-cyano-5-methylthiophene is complete. Cooled to room temperature, poured into 3L of ice water, extracted three times with dichloromethane, combined the organic phases, dried the organic phases with anhydrous magnesium sulfate, filtered, concentrated, and recrystallized from ethanol to obtain 104 g of yellow solid III (yield: 74.3%). 1 HNMR (400MHz, CDCl 3 )δ9.62(s, 1H), 8.24(d, J=8.6Hz, 1H), 7.52(dd, J=8.4Hz, 1H), 7.20(d, J=8.4Hz, 1H), 6.97(dd, J=7.3Hz, 1H), 6.78(s, 1H), 2.47(s, 3H); 13 C NMR (100MHz, CDCl 3 ): 148.9, 141.2, 136.2, 136.1, 134.1, 126.6, 123.9, 119.9, 116.1, 113.7, 104.6, 15.6; MS: 258 (M + ); IR(film, cm -1 ): 3304, 2921, 2361, 2225, 1611, 1502; ...
Embodiment 2
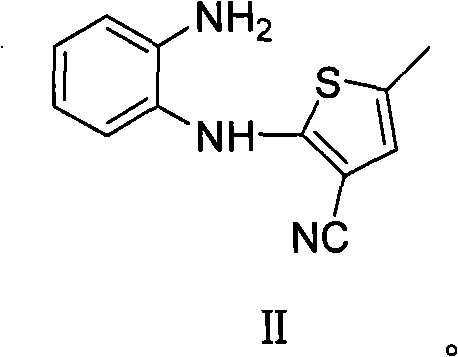
[0034] Synthesis of 2-(2-aminoanilino)-3-cyano-5-methylthiophene II:
[0035] 104g 2-(2-nitroanilino)-3-cyano-5-methylthiophene III is dissolved in 450mLN, in the mixed solvent of N-dimethylformamide and 100mL water, add 30g ammonium chloride and 66g Iron powder, heat up to 60-80°C and react for 6-9 hours. After all the raw materials have reacted, cool to ambient temperature, filter, pour the filtrate into 2.5L cold water, precipitate a light gray precipitate, filter, wash, and dry the solid. Hydro-ethanol recrystallization gave 64.8 g of crystal II (yield: 68.9%). 1 H NMR (400MHz, CDCl 3 )δ7.21(d, J=7.8Hz, 1H), 7.09(dd, J=7.6Hz, 1H), 6.77-6.84(m, 2H), 6.46(s, 1H), 6.16(s, 1H), 3.81(s, 2H), 2.27(s, 3H); 13 C NMR (100MHz, CDCl 3 ): 161.9, 141.4, 127.9, 127.7, 124.9, 122.3, 119.5, 116.9, 116.2, 86.8, 15.0; MS: 229 (M + -1); IR(film, cm -1 ): 3383, 3300, 3206, 3041, 2361, 2205, 1540; UV: 308, 217.8nm.
Embodiment 3
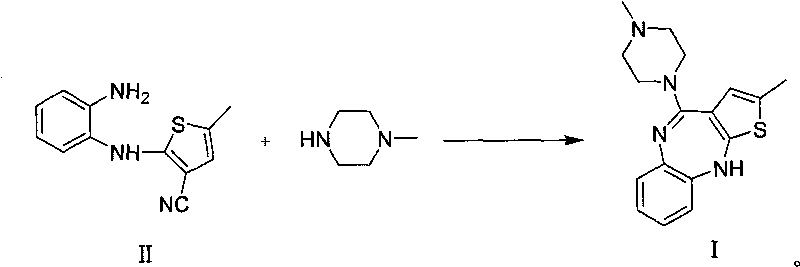
[0037] Synthesis of 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine (I):
[0038] 24.5g 2-(2-aminoanilino)-3-cyano-5-methylthiophene II and 40mL N-methylpiperazine were dissolved in a mixed solvent of 60mL dimethylsulfoxide and 60mL toluene, and 1.6 g anhydrous zinc chloride, heated to reflux for 14-16 hours until all the raw materials II have reacted. Cool to ambient temperature, pour into 400mL water, precipitate a light gray precipitate, filter, wash, dry, and recrystallize the solid with acetonitrile to obtain 26.5g of light yellow crystal I (yield: 79%), with a purity greater than 99%. 1 HNMR (400MHz, CDCl 3 )δ7.02(d, J=7.8Hz, 1H), 6.97(dd, J=7.5Hz, 1H), 6.88(dd, J=1.6, 7.6Hz, 1H), 6.60(d, J=7.7Hz, 1H), 6.30(s, 1H), 4.97(s, `H), 3.54(t, J=4Hz, 4H), 2.50(t, J=4.9Hz, 4H), 2.35(s, 3H), 2.31( s, 3H); 13 C NMR (100MHz, CDCl 3 ): 157.6, 151.7, 142.5, 141.0, 129.1, 128.2, 124.7, 123.7, 123.0, 119.7, 118.9, 55.1, 46.8, 46.2, 15.5; MS: 313 (M + +1); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com