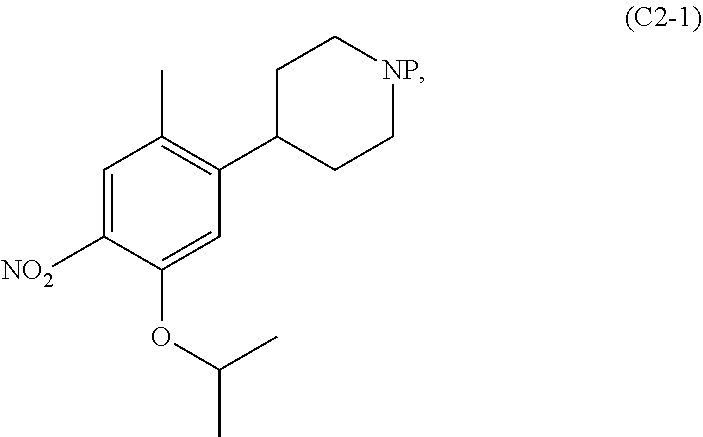
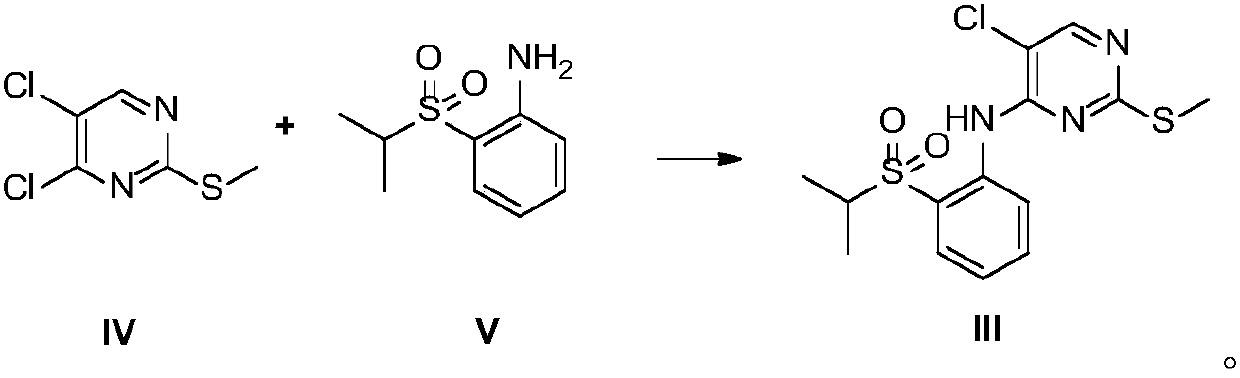
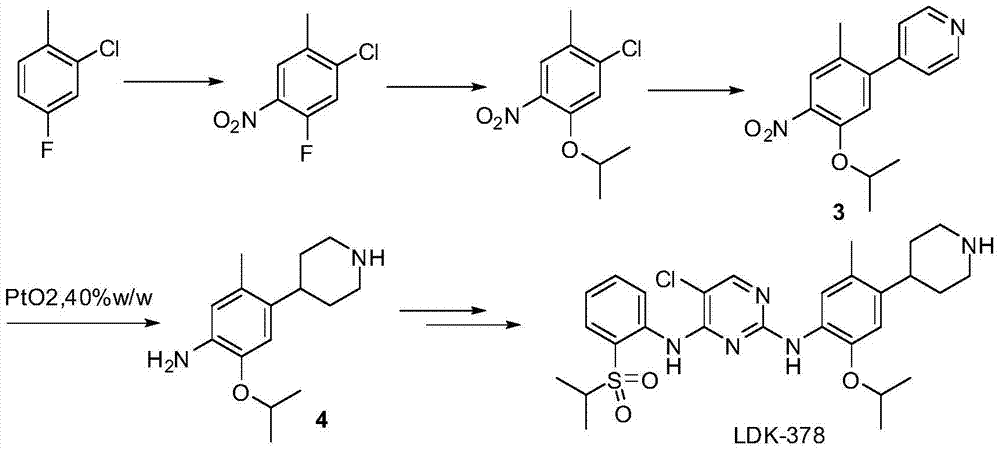
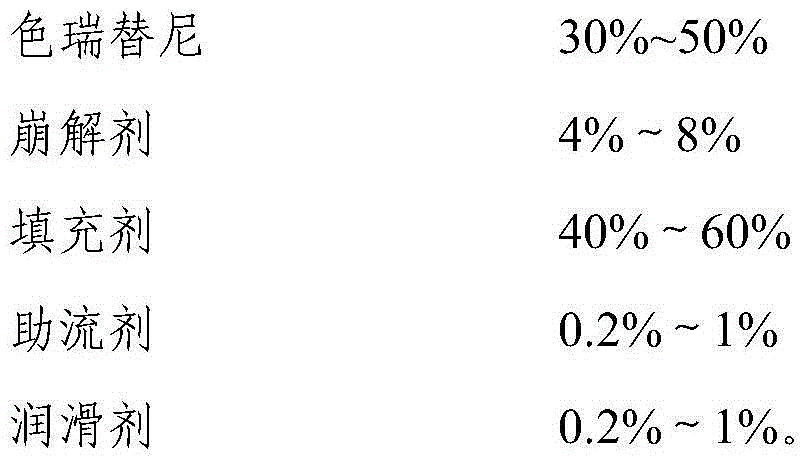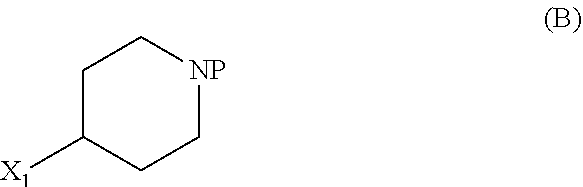Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
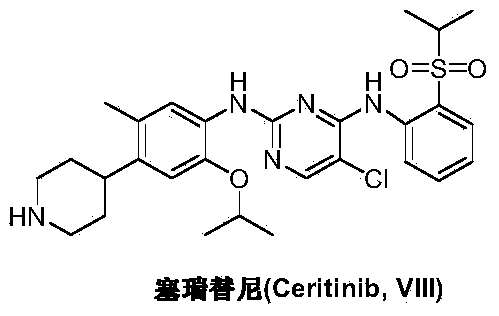
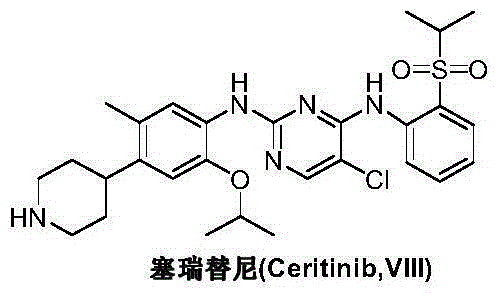
36 results about "Ceritinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
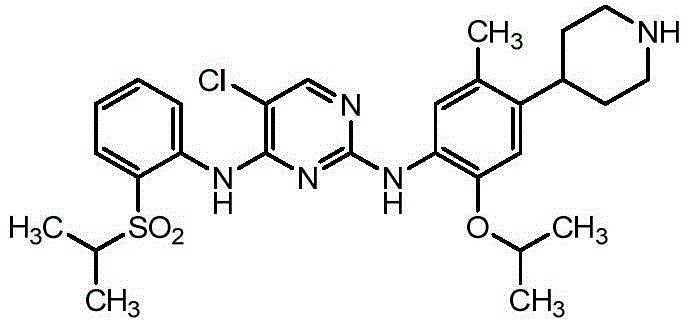
Ceritinib is used to treat lung cancer that has spread to other parts of the body. It is used for lung cancers that have a certain type of abnormal "ALK" gene.
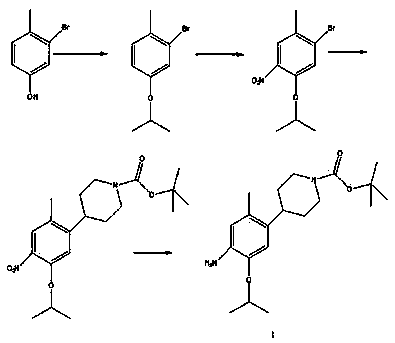
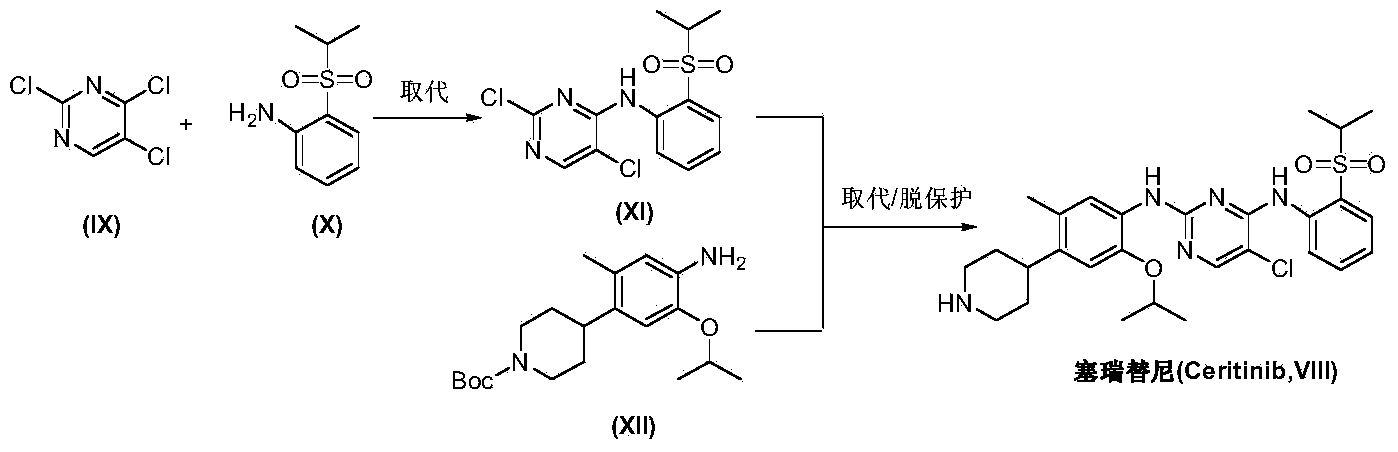
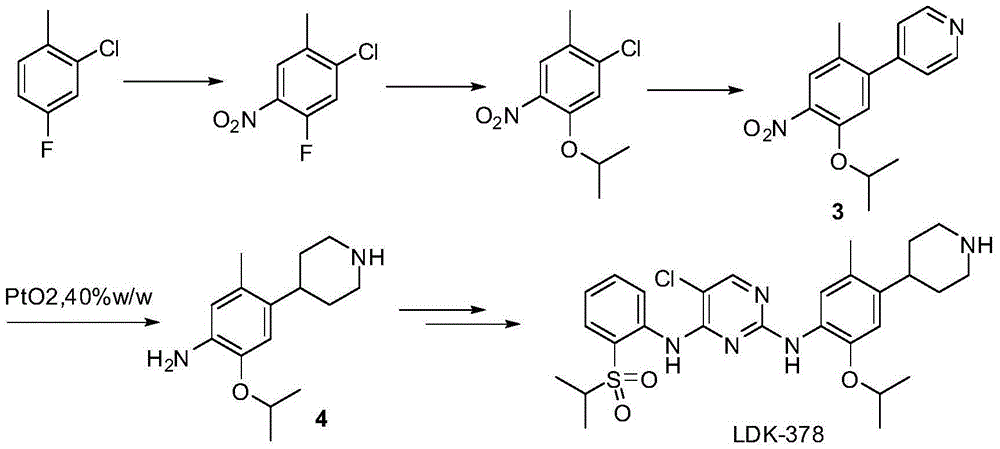
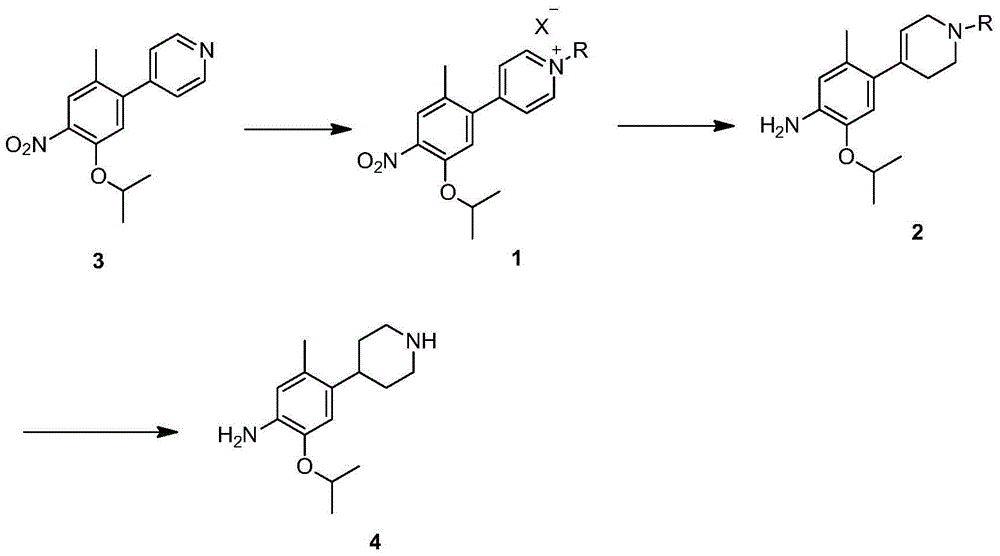
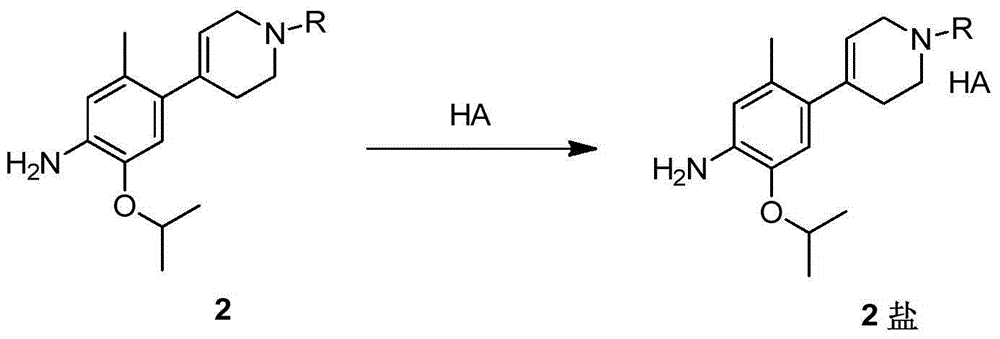
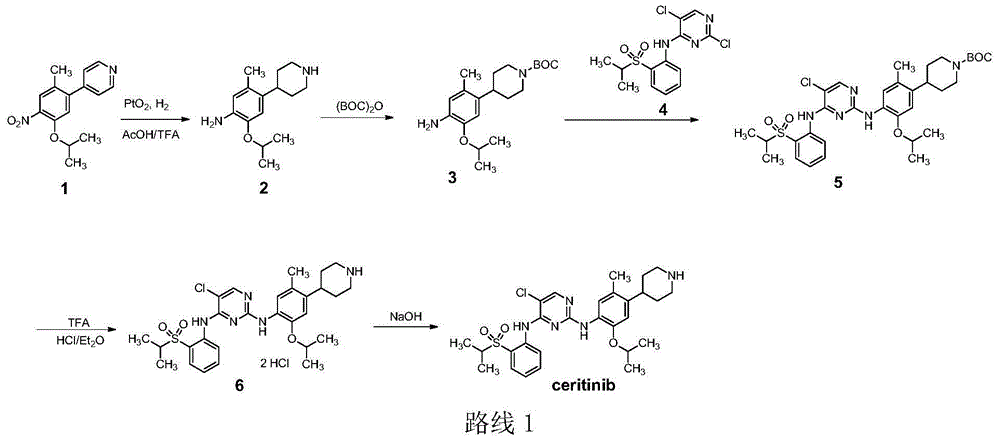
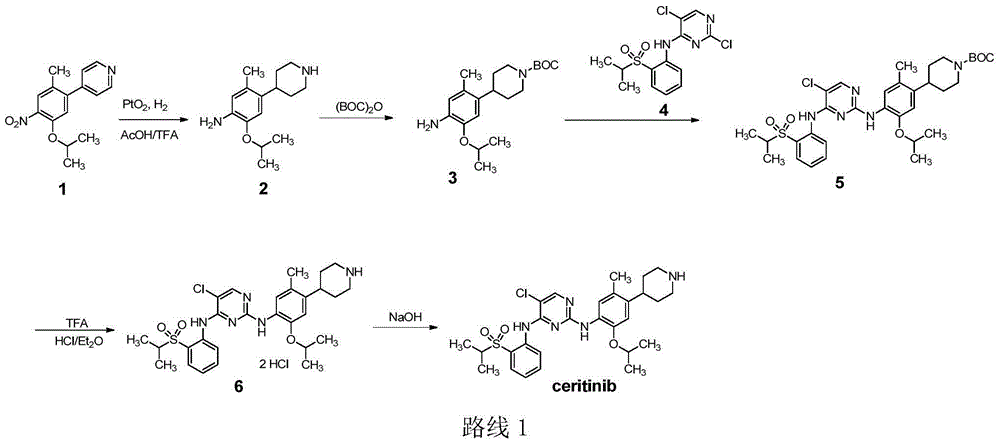
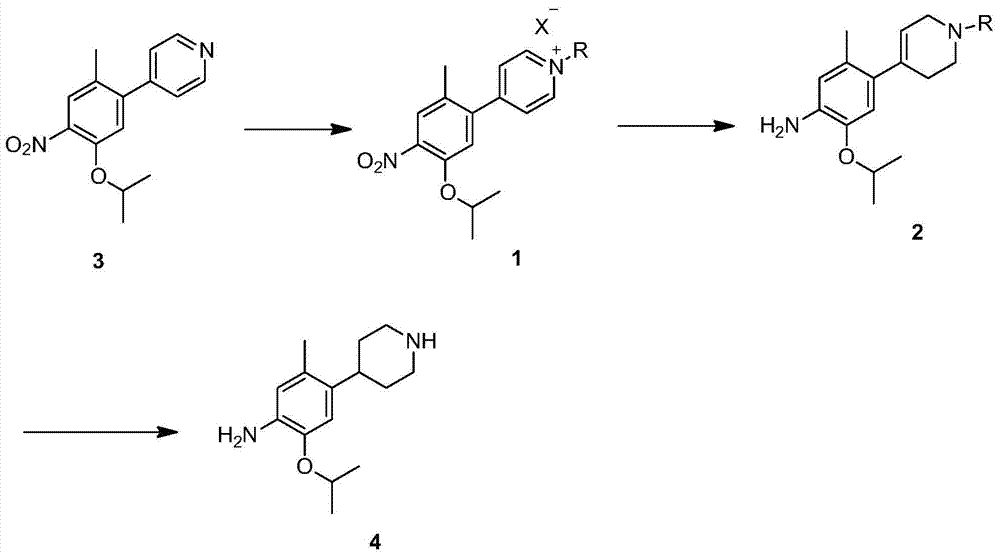
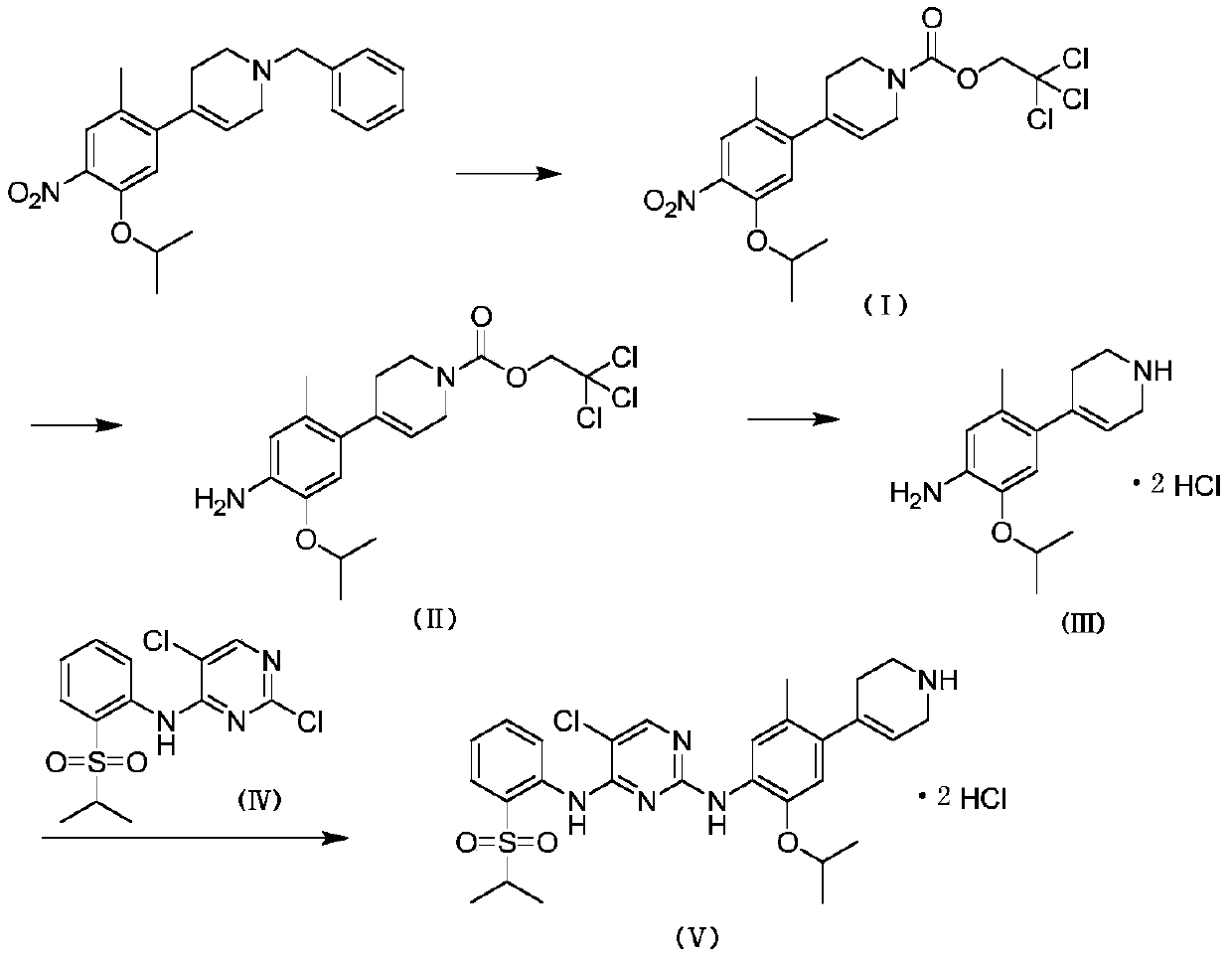
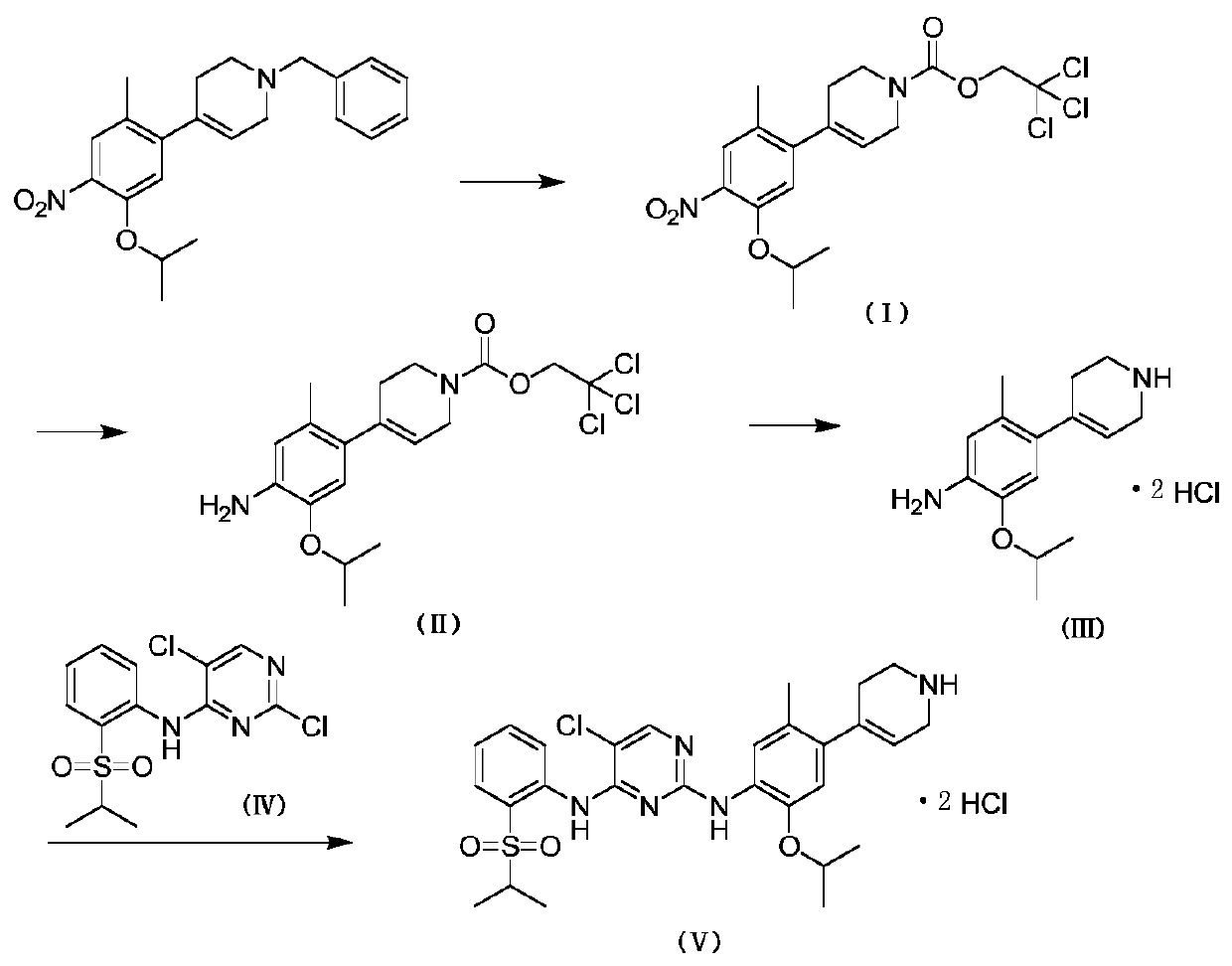
Method for preparing ceritinib
ActiveCN104356112AReduce pollutionReduce manufacturing costOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupBromine
The invention discloses a method for preparing ceritinib, belonging to the field of chemical pharmacy. The method comprises the following steps: by taking 3-bromine-4-methylphenol as an initial raw material, performing phenolic hydroxyl isopropylation, nitration, coupling and reduction reaction, obtaining a midbody 1, by further taking o-nitro fluorobenzene as another initial raw material, performing isosulfhydrylation, oxidation, reduction and pyrimidine, obtaining a midbody 2, performing coupling reduction on the midbody 1 and the midbody 1, obtaining ceritinib which is protected by BOC acid anhydride, and finally removing a t-butyloxycarboryl protecting group, and obtaining ceritinib. The method is simple and feasible to operate, relatively high in yield, small in pollution and applicable to industrial production.
Owner:安庆奇创药业有限公司
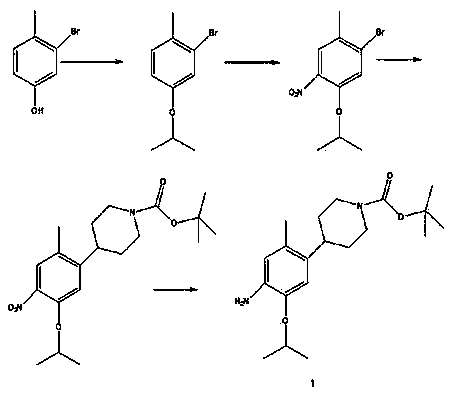
Preparation method of ceritinib and intermediate
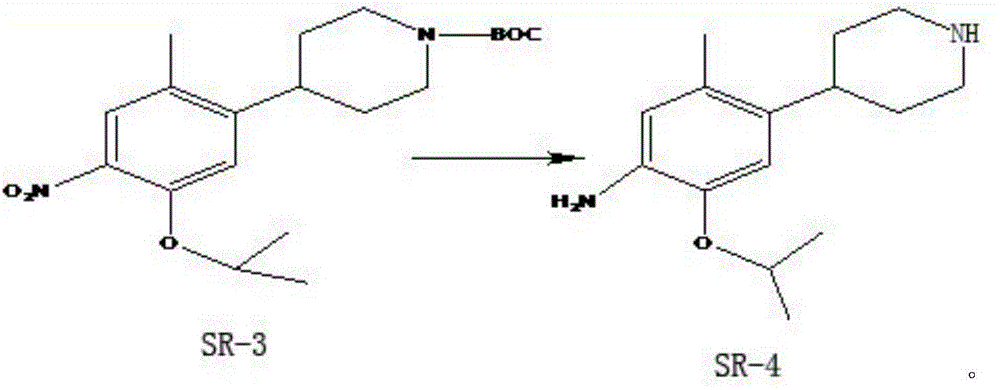
The invention discloses an intermediate 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) for preparing ceritinib and a preparation method thereof. The preparation method comprises the following steps: carrying out catalytic hydrogenation on the raw material 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine (II) to obtain 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)aniline (III); and carrying out Sandmeyer reaction on the compound (III) to obtain the 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I). The invention also discloses a preparation method of ceritinib. The 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) used as the raw material is sequentially subjected to substitution, reduction and substitution reaction to obtain the ceritinib (VIII). The preparation method has the advantages of simple technique, mild conditions and fewer side reactions, and is suitable for industrial amplification.
Owner:SCI GENERAL MATERIAL & CHEM
New intermediates for preparing ceritinib and preparation method of intermediate
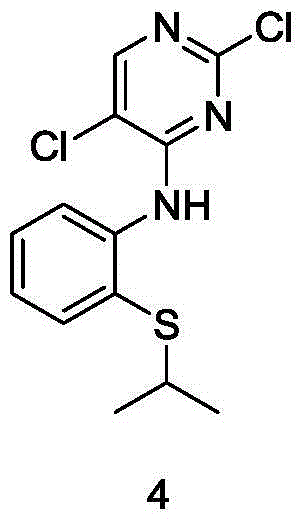
The invention relates to intermediates, namely a compound 1 of a formula (1) as shown in the specification and a compound 2 of a formula (2) as shown in the specification, for preparing ceritinib, or a chemically acceptable salt of the compound 2, wherein R represents the benzylic group of saturated or unsaturated aromatic ring methylene or heteroaromatic ring methylene, and X represents a halogen. The invention relates to a method for preparing a compound 4 by use of the new intermediates, namely the compound 1 and the compound 2, wherein the step of reduction from the compound 1 to the compound 2 is performed by use of a hydroboron or a composition thereof and an alcohol solvent; the compound 2 is reduced by use of a catalytic hydrogenation or transfer hydrogenation method to generate the compound 4. The route of preparing the compound 4 by use of the compound 1 and the compound 2 has the advantages that the chemical reduction step is combined with a catalytic hydrogenation, the use of expensive platinum dioxide is avoided and the cost of synthesizing the intermediate 4 of the ceritinib is effectively reduced.
Owner:药源生物科技(启东)有限公司
Ceritinic synthesis intermediate and preparation method thereof
The invention provides a synthesis intermediate 7 of an anti-tumor drug ceritinib. The intermediate 7 has a structural general formula as the following image. In the formula, Ar is phenyl or phenyl substituted by C1-C4 alkyl, C1-C4 alkoxyl, cyano, nitro or halogen; and X is Cl or Br. A preparation method of the intermediate 7 comprises the following step: the intermediate 7 is produced through a reaction of a compound 1 and substituted or unsubstituted benzyl halide (or ArCH2X). The invention also provides a novel route for applying an intermediate 7b in synthesizing ceritinib. With the route, a problem of using expensive platinum oxide as a reduction catalyst in an existing route 1 can be avoided. In the synthesis route 2 for synthesizing ceritinib with the compound 7b, the obtained synthesis intermediate 7b is quaternary ammonium salt. The salt can be precipitated from a solvent, and can be obtained by filtration, such that impurities can be retained in filtrate. Also, the conditions for all steps of reactions in the synthesis route 2 are mild, and post-treatment is simple. All the obtained intermediates 8b-10b do not need column chromatography purification. Therefore, the route is suitable for industrialized production.
Owner:SHANGHAI INST OF PHARMA IND +2
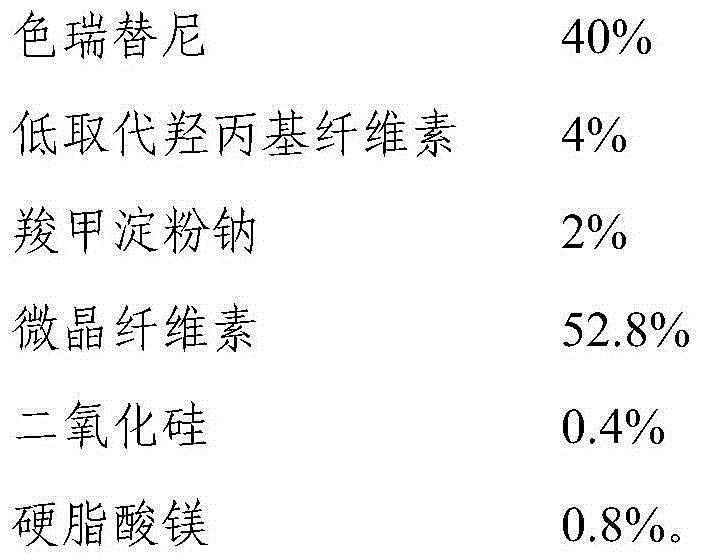
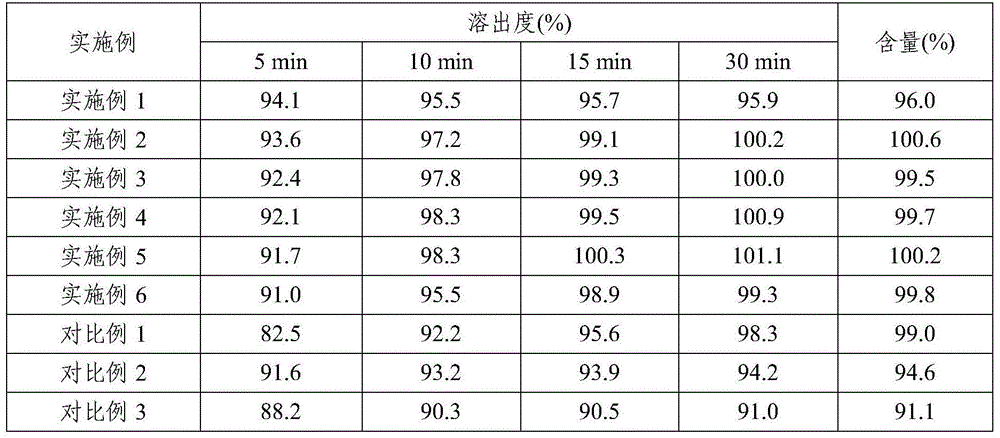
Ceritinib medicinal composition
ActiveCN106176752APromote dissolutionQuality improvementOrganic active ingredientsAntineoplastic agentsMedicineCeritinib
The invention relates to a ceritinib medicinal composition. The medicinal composition contains ceritinib and at least one pharmaceutically acceptable carrier, and the raw material particle size D90 is 20-80 [mu]m. The medicinal composition has the advantages of rapid release and stable quality. The invention also relates to a preparation method of the medicinal composition. The preparation method has the advantages of simple preparation process, and suitableness for industrial large-scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Method for preparing Ceritinib and intermediate compound of Ceritinib
InactiveCN105272921AReduce usageSimple and fast operationOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention provides a new intermediate compound for preparing Ceritinib and a preparation method for Ceritinib. The intermediate compound is shown as a formula 4 in the specification. The invention also discloses the preparation method for preparing the Ceritinib compound through the intermediate compound. The method possesses the advantages that operation is simple, yield is high, the prepared product is excellent in quality and the method is suitable for industrialized production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
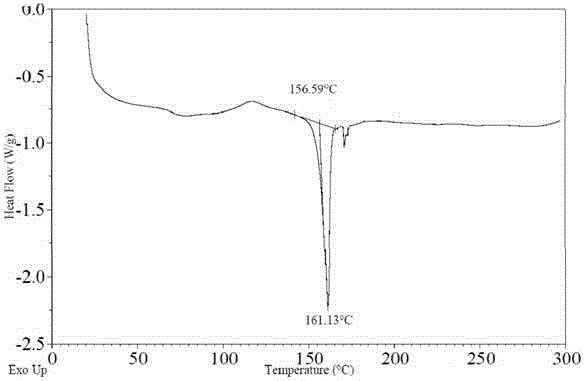
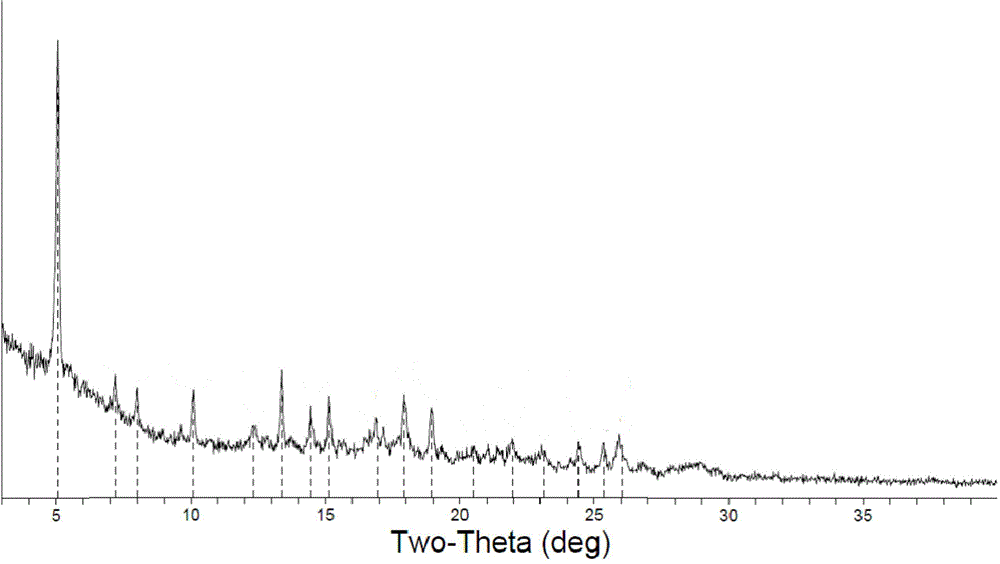
Novel crystallographic form of ceritinib and preparation method of novel crystallographic form
InactiveCN105622577ACrystal stableNo major change in purityOrganic active ingredientsOrganic chemistryDiseaseKinase
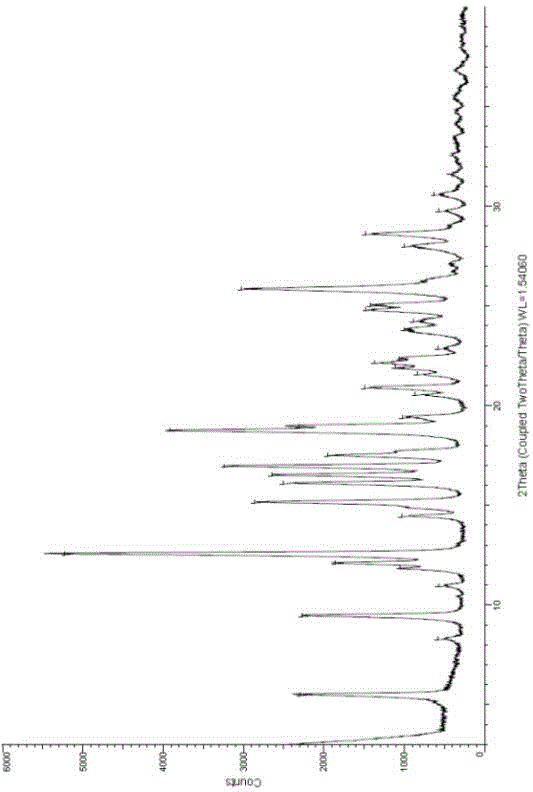
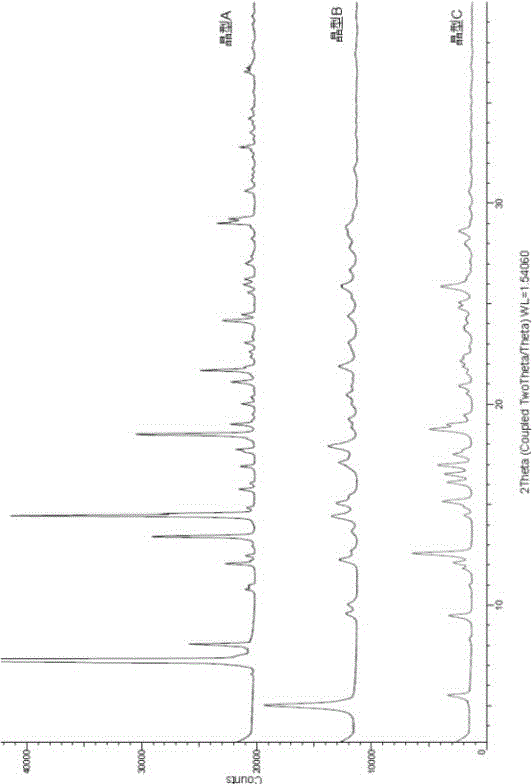
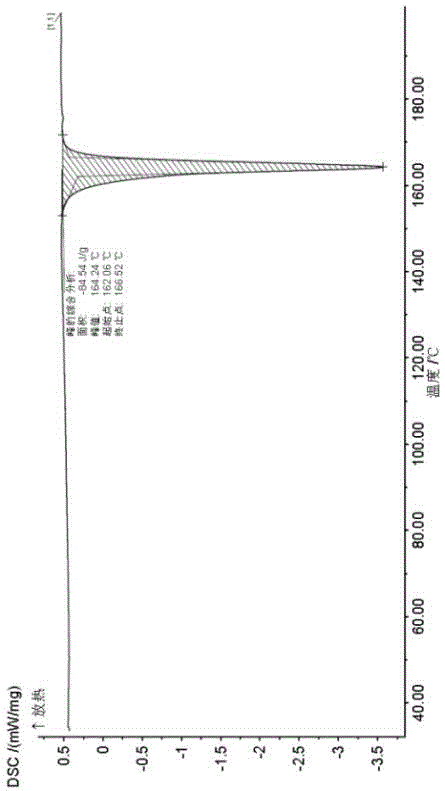
The invention relates to a novel crystallographic form of ceritinib. An X-ray powder diffraction pattern of the novel crystallographic form of ceritinib is as shown in Figure 1. The invention further relates to a method for preparing the crystallographic form, and application of the crystallographic form to preparation of a medicament for treating anaplastic lymphoma kinase mediated diseases.
Owner:HAINAN SIMCERE PHARMA CO LTD +1
Application of ceritinib in preparation of tumor chemotherapy drug sensitizer and antitumor pharmaceutical composition
InactiveCN107320474AAvoid drug resistanceGood curative effectOrganic active ingredientsAntineoplastic agentsChemotherapeutic drugsTumor chemotherapy
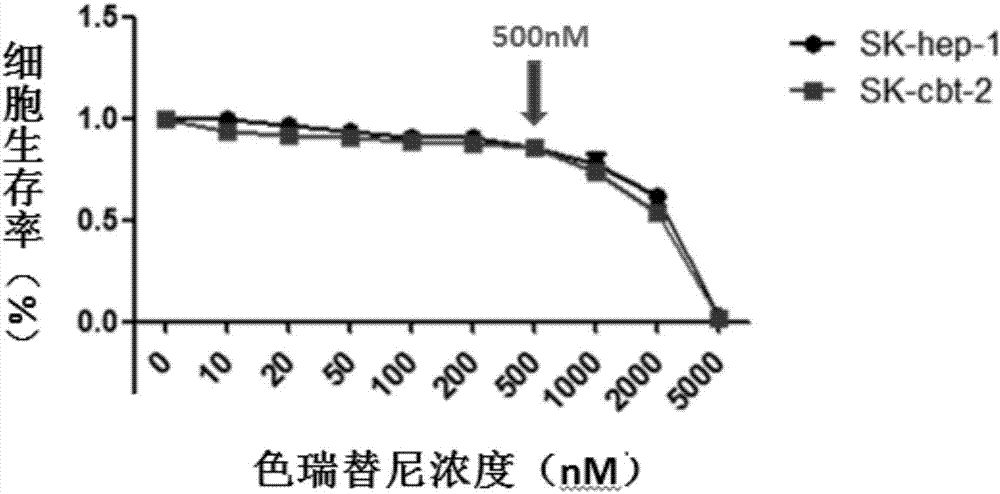
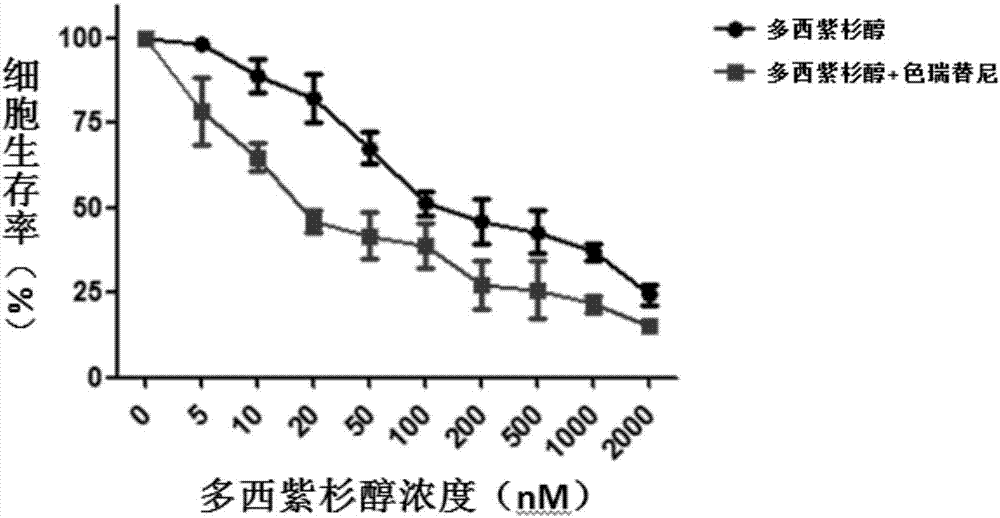
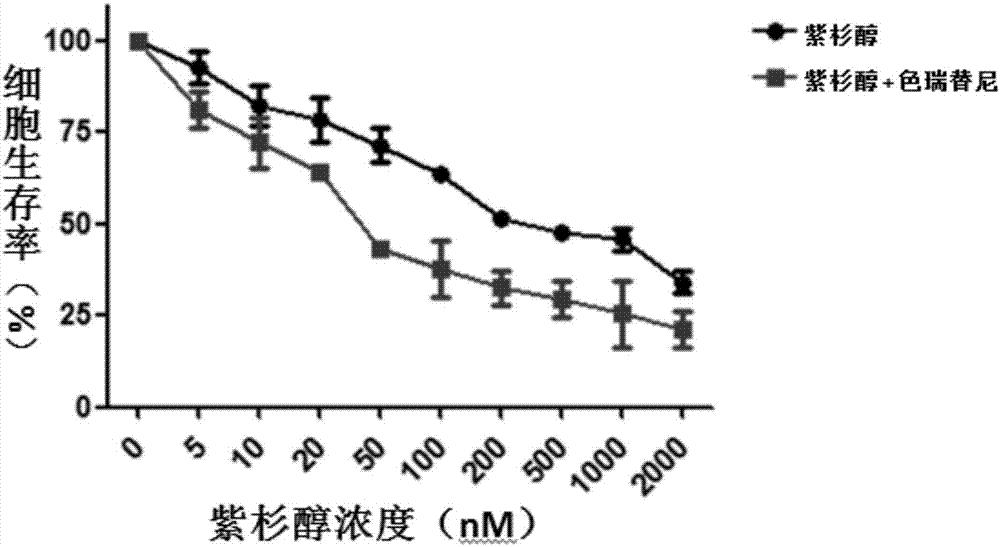
The invention discloses an application of ceritinib in the preparation of a tumor chemotherapy drug sensitizer and an antitumor pharmaceutical composition. The antitumor pharmaceutical composition contains a chemotherapy drug and a sensitizer, wherein the sensitizer is ceritinib. Researches find that the ceritinib can be used as a tumor chemotherapy drug resistance sensitizer and can be combined with the chemotherapy drug in use, so that the drug resistance of tumors to the chemotherapy drug can be overcome, the treatment effect of the chemotherapy drug to drug-resistant tumor cells can be remarkably improved, and new ways and measures are provided for the effective treatment of the tumors.
Owner:ZHEJIANG UNIV
Ceritinib compound and pharmaceutical composition thereof
InactiveCN105294650ASimple preparation processGood reproducibilityOrganic active ingredientsOrganic chemistryMedicineCrystal
The invention belongs to the field of medical chemistry, and discloses a crystal form of a ceritinib compound and a pharmaceutical composition of the ceritinib compound. The compound has the characteristic of being high in stability, and conforms to the medicinal requirements. According to the invention, the preparation technology is stable, the reproducibility is good, and the requirements of industrial mass production are met.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
New intermediate of non-small-cell lung carcinoma treating drug Ceritinib, and preparation method thereof
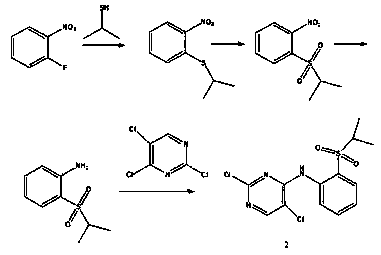
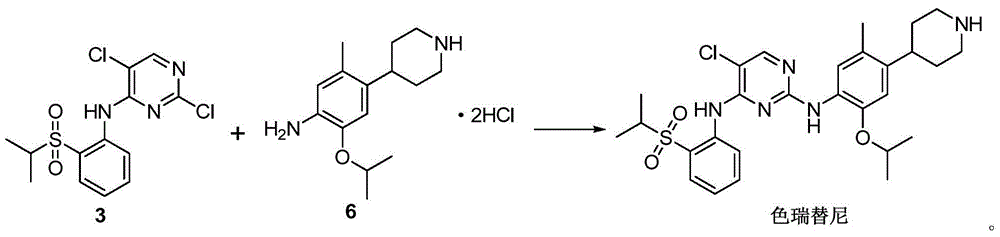
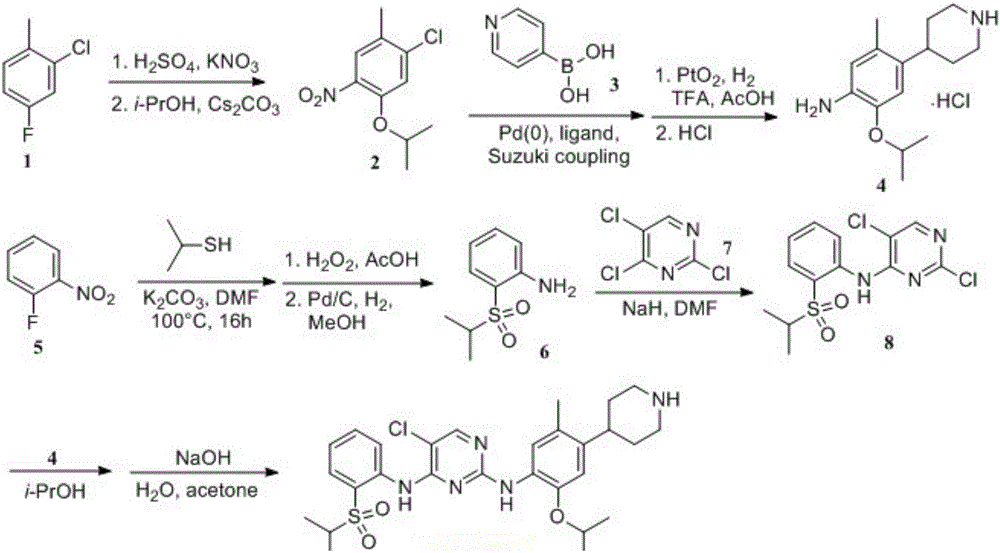
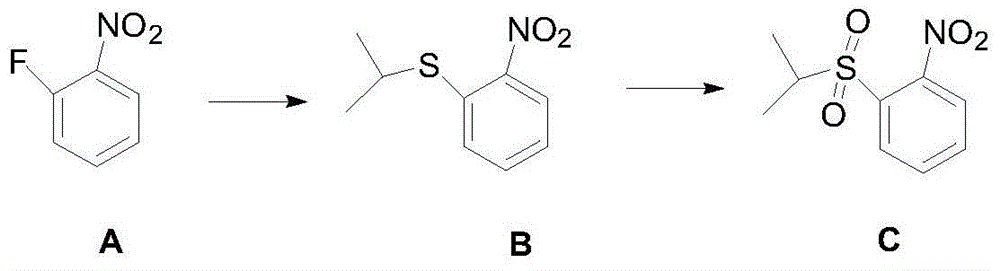
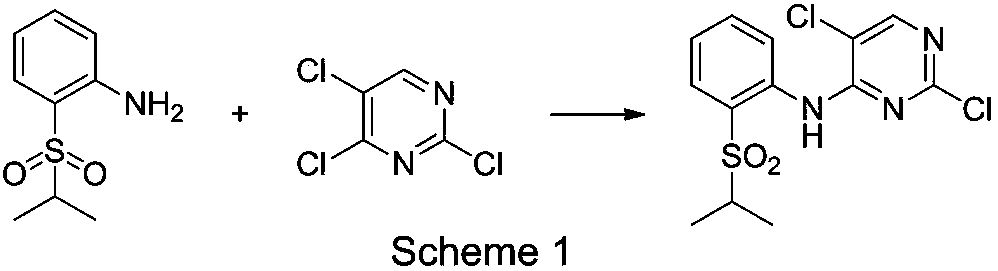
The present invention relates to the field of pharmaceutical chemistry, to a preparation method of a new intermediate of 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidine-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (Ceritinib). According to the present invention, o-fluoronitrobenzene is adopted as a starting raw material, substitution, reduction and condensation are performed to obtain the new intermediate 2-X-5-chloro-N-(2-(isopropyl sulfide)phenyl)pyrimidine-4-amine (X is halogen, p-methyl benzene sulfonyloxy, methyl sulfonyloxy or trifluoromethylsulfonyloxy), the new intermediate can be oxidized to obtain a sulfonyl derivative, and the sulfonyl derivative and 2-isopropoxy-5-methyl-4-(piperidine-4-yl)aniline are subjected to condensation to finally obtain 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidine-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (Ceritinib); and the synthesis method has characteristics of readily available raw materials, high yield, mild reaction, simple operation and low production cost, and is suitable for industrial production.
Owner:SHANGHAI SCIENPHARM CO LTD
Preparation method for ceritinib and intermediate of ceritinib
ActiveCN105985317AAvoid Heavy Metal ResiduesImprove step reaction yieldOrganic chemistryBulk chemical productionCombinatorial chemistryCeritinib
The invention specifically relates to a preparation method for ceritinib and an intermediate of ceritinib, belonging to the technical field of pharmaceutical chemistry. The method comprises the following steps: with 2,4-dichloro-5-nitropyrimidine as a raw material, carrying out a two-step condensation reaction so as to obtain a compound as shown in a formula V which is described in the specification; and subjecting the compound as shown in the formula V to reduction, chlorination and removal of a protective group R so as to obtain ceritinib. The method substantially improves reaction yield; reaction conditions are mild; the obtained product, i.e., ceritinib, does not need column chromatographic purification; and the method is suitable for industrial production and avoids heavy metal residuals.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
A ceritinib preparing method
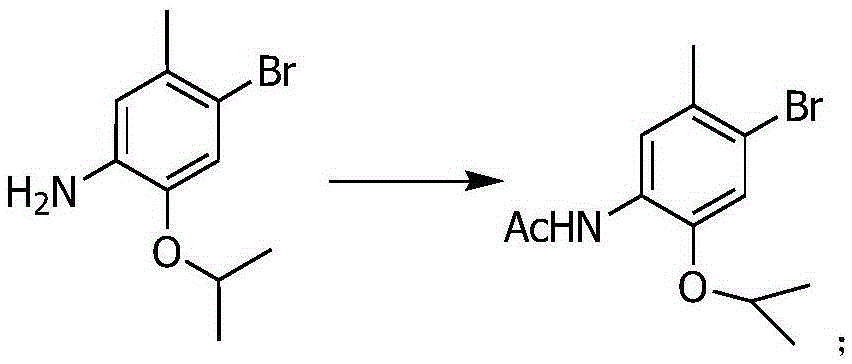
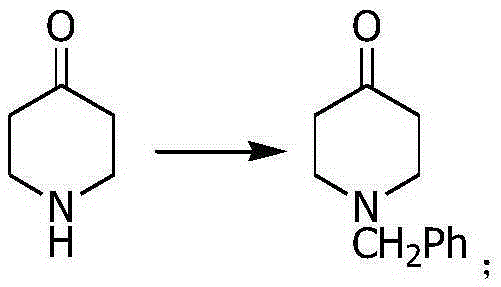
The invention relates to a ceritinib preparing method. The method includes (1) reacting 4-bromo-2-isopropoxy-5-methylaniline and acetic anhydride to generate N-(4-bromo-2-isopropoxy-5-cresyl) acetamide, (2) reacting 4-piperidone to generate N-benzyl-4-piperidone, (3) subjecting the N-(4-bromo-2-isopropoxy-5-cresyl) acetamide and the N-benzyl-4-piperidone to a docking reaction, (4) subjecting a product of the former step to dehydroxylation and (5) generating a finally product that is ceritinib from N-(4-(1-phenyl-1,2,3,6-tetrahydropyridin-4-yl)-2-isopropoxy-5-cresyl) acetamide under the existence of a catalyst. The novel ceritinib preparing method is provided. The synthesis route is low in cost, reaction conditions are mild and easy to control, and the method is suitable for large-scale industrial production.
Owner:常州市勇毅生物药业有限公司
New synthesis technology of ceritinib intermediate
InactiveCN105461616ALow costShort method stepsOrganic chemistryTert-Butyloxycarbonyl protecting groupHydrogenation reaction
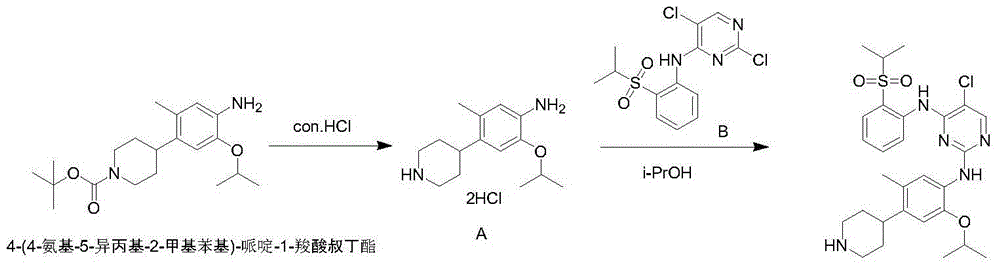
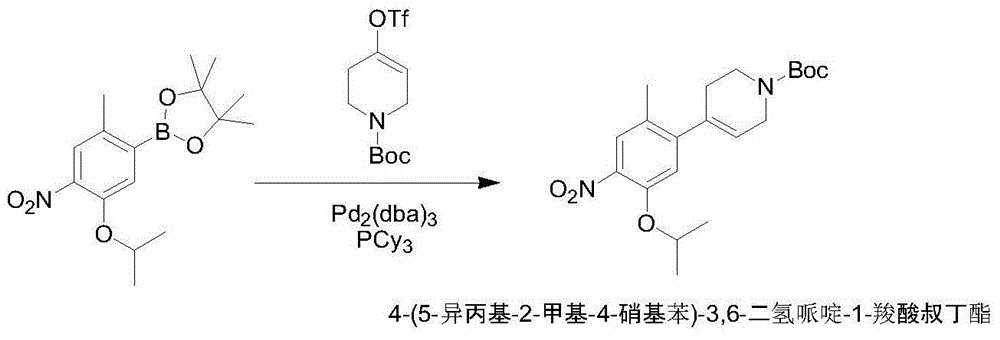
The purpose of the invention is to provide a new synthesis technology of a ceritinib intermediate. The technology is characterized in that the ceritinib intermediate tert-butyl 4-(4-amino-5-isopropyl-2-methylphenyl)-piperidyl-1-carboxylate is prepared through a Suzuki coupling and hydrogenation reaction of 1-chloro-5-isopropyl-2-methyl-4-nitrobenzene and N-tertbutyloxycarbonyl-1,2,5,6-tetrahydropyridinyl-4-boronic acid pinacol ester. The technology has the advantages of short steps, simple operation, low raw material cost, and suitableness for amplified production.
Owner:SHANGHAI STEPPHARM CO LTD
A kind of preparation method of ceritinib intermediate
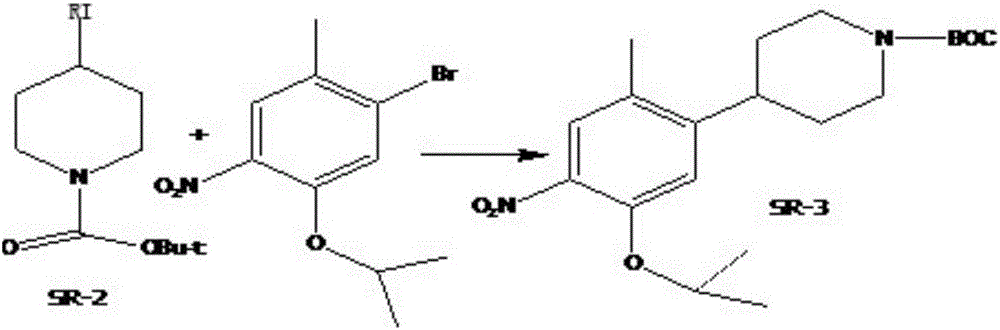
ActiveCN104356050BHigh yieldRaw materials are easy to getOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupCoupling
The invention relates to a preparation method of ceritinib. The preparation method comprises the following steps: (1) reacting metal powder with N-t-butyloxycarboryl-4 iodine piperidine with a structural formula of SR-1 to generate a metal coupling agent with a structural formula of SR-2; (2) performing a coupling reaction on the metal coupling agent SR-2 and 1-bromo-5-isopropoxy-2-methyl-4-nitrobenzene to generate a compound SR-3; (3) performing hydrogenation reduction on the compound SR-3, and removing BOC amino protection to generate the ceritinib SR-4. According to the invention, a novel preparation method of the ceritinib is developed. The synthetic route is very short; the yield is high; raw materials can be easily obtained and are low in cost; reaction conditions are very mild and easy to control; therefore, the preparation method is suitable for large-scale industrial production.
Owner:常州市勇毅生物药业有限公司
One-pot synthesis method of anticancer drug ceritinib intermediate 1-(isopropylsulfonyl)-2-nitrobenzene
InactiveCN104592068ALow reaction temperatureReduce manufacturing costOrganic chemistryOrganic compound preparationN dimethylformamideReaction rate
Owner:常州百敖威生物科技有限公司
Anti-cancer drug ceritinib intermediate 1-amino-2-(isopropyl sulfonyl)benzene synthetic method
InactiveCN106083670ALow reaction temperatureReduce energy consumptionOrganic compound preparationSulfide preparationSulfideAniline
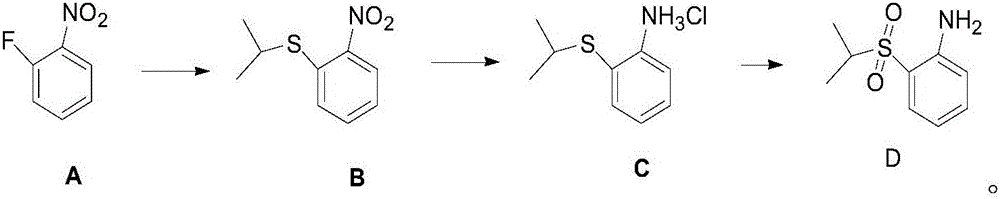
The invention belongs to the technical field of medicine synthesis, and especially relates to a synthetic method of an anti-cancer drug ceritinib intermediate 1-amino-2-(isopropyl sulfonyl)benzene. The synthetic method comprises the following steps: employing o-fluoronitrobenzene, sodium hydride and isopropyl mercaptan to synthesize a 2-(isopropyl sulfide) nitrobenzene crude product, then directly reducing the crude product to iron powder, forming salt to obtain the 2-(isopropyl sulfide) aniline hydrochloride, and finally dropping hydrogen peroxide for oxidation in 2-(isopropyl sulfide) aniline hydrochloride for oxidation, forming salt, and dissociating to obtain a target product. The synthetic method has the advantages of simpleness, little sewage amount, low energy consumption, low production cost, and the product has the advantages of high purity, high yield, and easy industrial production, and is suitable for further popularization and application.
Owner:常州安迪沃克医药科技有限公司
Anti-cancer drug ceritinib intermediate 2-chlorine(bromine)-4-fluorine-5-nitrotoluene synthetic method
InactiveCN106083595AReduce the amount of waterAvoid the problem of generating large amounts of waste waterNitro compound preparationIce waterSolvent
The invention relates to the technical field of medicine synthesis, and especially relates to an anti-cancer drug ceritinib intermediate 2-chlorine(bromine)-4-fluorine-5-nitrotoluene synthetic method. The method takes concentrated sulfuric acid as a solvent and takes concentrated nitric acid as a nitrating agent, after the reaction is complete, ice water is not added for quenching, a solvent is directly used for extracting the product, an organic layer is washed to neutrality, then the material is condensed to the crude product, the residual concentrated sulfuric acid after extraction can be repeatedly used for three times, water volume used for quenching the concentrated sulfuric acid is reduced, generation of massive waste water due to processing of the concentrated sulfuric acid can be avoided, the method has the advantages of energy saving and environmental protection, waste water processing pressure is greatly reduced, crude yield can reach 85-90%, and the method is suitable for large-scale industrial production, and is further for popularization and application.
Owner:常州安迪沃克医药科技有限公司
Preparation method of ceritinib and its intermediate
The invention discloses an intermediate 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) for preparing ceritinib and a preparation method thereof. The preparation method comprises the following steps: carrying out catalytic hydrogenation on the raw material 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine (II) to obtain 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)aniline (III); and carrying out Sandmeyer reaction on the compound (III) to obtain the 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I). The invention also discloses a preparation method of ceritinib. The 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) used as the raw material is sequentially subjected to substitution, reduction and substitution reaction to obtain the ceritinib (VIII). The preparation method has the advantages of simple technique, mild conditions and fewer side reactions, and is suitable for industrial amplification.
Owner:SCI GENERAL MATERIAL & CHEM
Ceritinib synthesis intermediate and preparation method thereof
The invention provides a synthesis intermediate 8 of an anti-tumor drug ceritinib, a preparation method thereof, and an application of the intermediate 8 in synthesizing ceritinib. The preparation method of the intermediate 8 comprises the following steps: step (1), a compound 1 and an acid HX are subjected to salt formation, such that a compound 7 is obtained; step (2), the compound 7 is reduced through sodium borohydride, such that the compound 8 is obtained. The reaction formula is as the following. With prior arts, expensive platinum oxide is needed as a hydrogenation catalyst for preparing an intermediate 2 in a next step. With the intermediate 8, the above problem is avoided. Therefore, the intermediate 8 is suitable to be used in industrialized production of ceritinib.
Owner:SHANGHAI INST OF PHARMA IND +2
Method for preparing antitumor drug ceritinib intermediate
The invention belongs to the technical field of medical chemistry, and concretely relates to a method for preparing an antitumor drug ceritinib intermediate. The method utilizes zinc salt as a catalyst under strong alkali conditions to catalyze the production of 2,5-dichloro-N-[2(isopropylsulfonyl)phenyl]pyrimidine-4-amine by using 2-(isopropylsulfonyl)aniline and 2,4,5-trichloropyrimidine as rawmaterials. The method of the invention overcomes the disadvantages of using the noble metal Pd in the prior art, does not need to use an expensive phosphorus-containing ligand, has the advantages of mild reaction conditions and high yield, and has industrial application prospects.
Owner:刘耿熙
Chemical Process for Preparing Pyrimidine Derivatives and Intermediates Thereof
The present disclosure relates to a method of synthesizing 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine (ceritinib) and / or intermediates thereof, their use as pharmaceuticals and pharmaceutical compositions and the use of intermediates for preparing ceritinib.
Owner:NOVARTIS AG
Ceritinib intermediate and preparation method thereof
The invention discloses a ceritinib intermediate and a preparation method thereof. The preparation method of the ceritinib intermediate comprises the following steps: (1) under anhydrous oxygen-free conditions, in a polar aprotic solvent and under conditions of a metal hydride, performing a N-arylation reaction on an intermediate V and an intermediate IV to obtain an intermediate III; (2) in a solvent and under an acidic condition, hydrolyzing the intermediate III to obtain an intermediate II; and (3) in a solvent, performing a chlorination reaction on the intermediate II to obtain the ceritinib intermediate. The raw materials used in the preparation method provided by the invention are safe and non-toxic, reactions are simple and easy to operate, side reactions are few, water is adopted for post-treatment and even crystallization, thus production costs are reduced, the prepared product has high purity, and the ceritinib intermediate is easy for industrialized production.
Owner:SHANGHAI VASTPRO TECH DEV CO LTD
Novel intermediate for preparing ceritinib and preparation method thereof
The invention relates to intermediates, namely a compound 1 of a formula (1) as shown in the specification and a compound 2 of a formula (2) as shown in the specification, for preparing ceritinib, or a chemically acceptable salt of the compound 2, wherein R represents the benzylic group of saturated or unsaturated aromatic ring methylene or heteroaromatic ring methylene, and X represents a halogen. The invention relates to a method for preparing a compound 4 by use of the new intermediates, namely the compound 1 and the compound 2, wherein the step of reduction from the compound 1 to the compound 2 is performed by use of a hydroboron or a composition thereof and an alcohol solvent; the compound 2 is reduced by use of a catalytic hydrogenation or transfer hydrogenation method to generate the compound 4. The route of preparing the compound 4 by use of the compound 1 and the compound 2 has the advantages that the chemical reduction step is combined with a catalytic hydrogenation, the use of expensive platinum dioxide is avoided and the cost of synthesizing the intermediate 4 of the ceritinib is effectively reduced.
Owner:药源生物科技(启东)有限公司
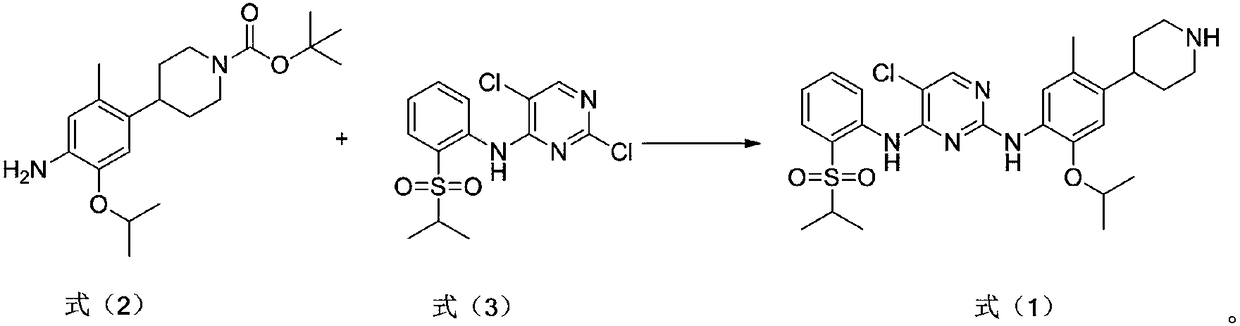
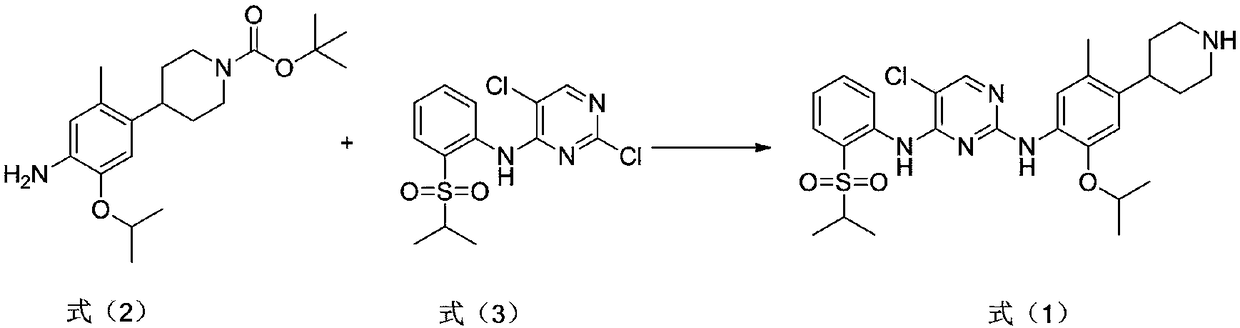
Preparation method for ceritinib
InactiveCN108689993ASimple and fast operationConditions are easy to controlOrganic chemistryOrganic baseNitrogen atmosphere
The invention discloses a preparation method for ceritinib, which comprises the following steps: under nitrogen atmosphere, a compound shown as formula (2) and a compound shown as formula (3) are added into organic base, and temperature is increased to 80 DEG C to 120 DEG C for reaction. The organic base is diisopropylethylamine, 1,8-diazabicyclo[5.4.0]undecy-7-ene, triethylene diamine or pyridine. The method disclosed by the invention is simple, environmentally friendly and easy to implement. (The reaction formula is shown in the description.).
Owner:SUZHOU BEC BIOLOGICAL TECH
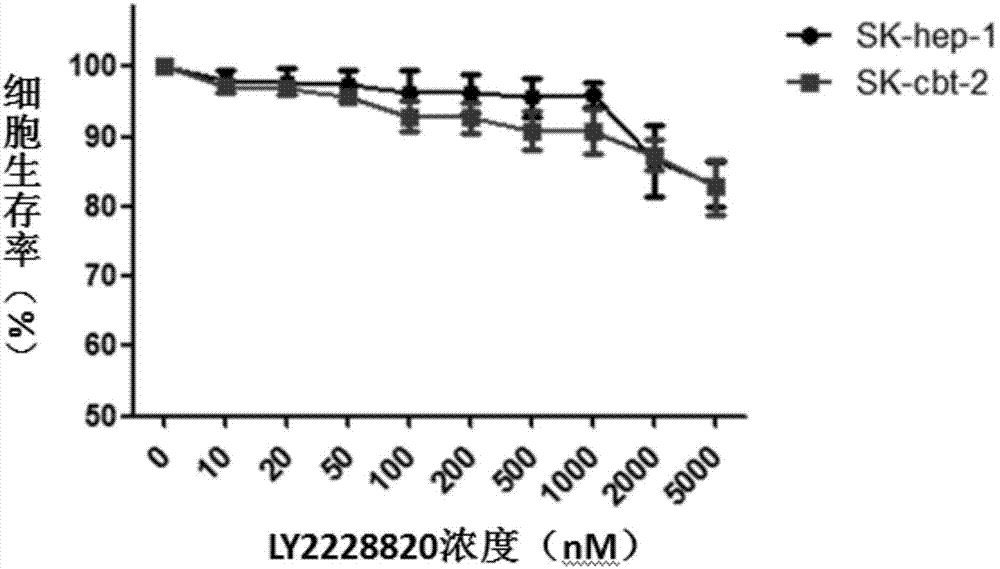
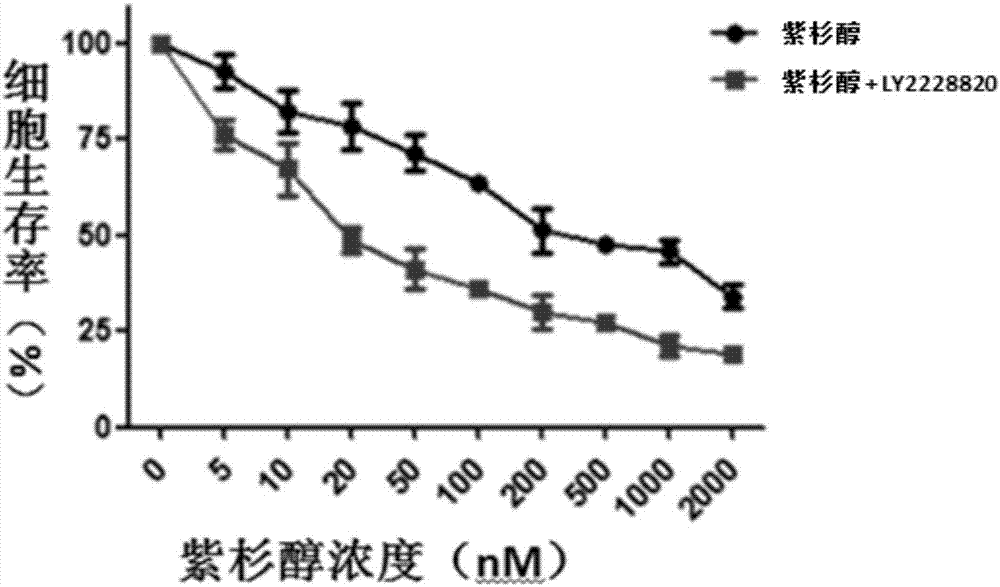
Application of compound LY2228820 to preparation of sensitizer of anti-tumor chemotherapeutic drug and anti-tumor pharmaceutical composition
InactiveCN107349427AAvoid drug resistanceGood curative effectOrganic active ingredientsAntineoplastic agentsChemotherapeutic drugsCurative effect
The invention discloses an application of compound LY2228820 to preparation of a sensitizer of an anti-tumor chemotherapeutic drug and anti-tumor pharmaceutical composition. The anti-tumor pharmaceutical composition comprises the chemotherapeutic drug and the sensitizer, wherein the sensitizer is the compound LY2228820. Research finds that the p38 kinase inhibitor ceritinib can serve as the sensitizer of the anti-tumor chemotherapeutic drug and has the advantages that the resistance of tumor to the chemotherapeutic drug can be overcome and the curative effect of the chemotherapeutic drug on drug-resistant tumor cells is enhanced remarkably when combined with the chemotherapeutic drug for use, and novel ways and means are provided for effective tumor treatment.
Owner:ZHEJIANG UNIV
Combination of certinib with an EGFR inhibitor
The present disclosure relates to a pharmaceutical composition comprising two Tyrosine Kinase Inhibitors (TKIs), namely Ceritinib and an EGFR Inhibitor. The present combination can be administered independently or separately, in a quantity which is jointly therapeutically effective for the treatment of a TKI mediated disease, such as cancer. The disclosure also provides the use of such a combination for the manufacture of a medicament; the use of such a combination as a medicine; a kit of part comprising such a combination; and a method of treatment of such a combination.
Owner:NOVARTIS AG
Ceritinib pharmaceutical composition
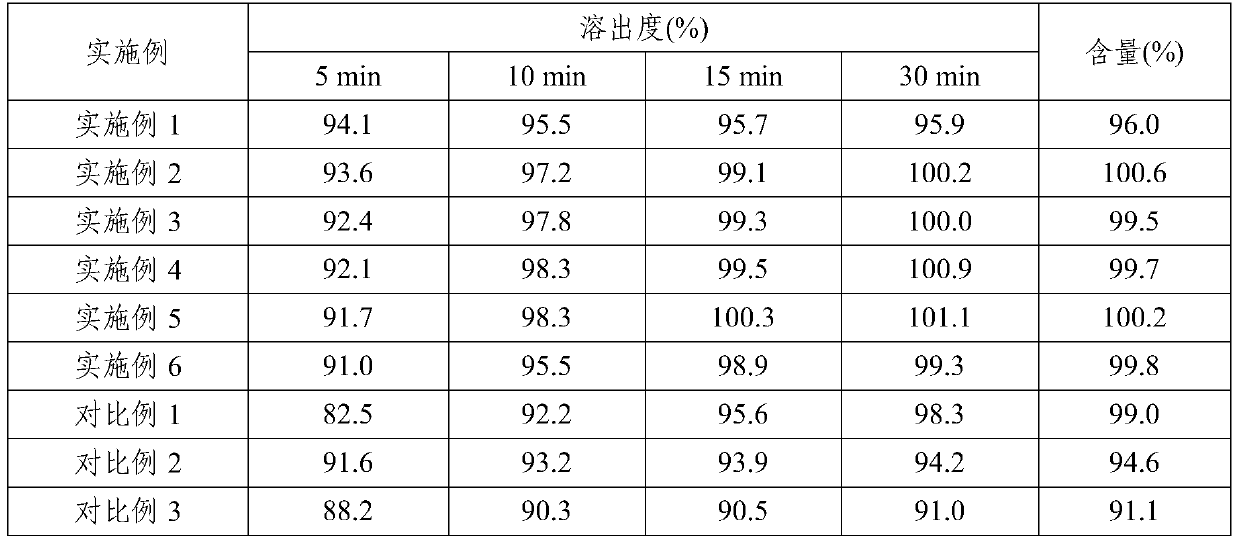
ActiveCN106176752BFast dissolution in vitroFully dissolvedOrganic active ingredientsAntineoplastic agentsCombinatorial chemistryPharmaceutical medicine
The invention relates to a pharmaceutical composition of ceritinib, which contains ceritinib and at least one pharmaceutically acceptable carrier, the particle size D90 of the raw material is in the range of 20 μm to 80 μm, and the pharmaceutical composition releases rapidly , stable quality; the present invention also relates to a preparation method of the pharmaceutical composition, the preparation method has a simple preparation process and is suitable for large-scale industrial production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Application of ceritinib in preparation of medicine for treating thyroid-associated ophthalmopathy
ActiveCN113244236AInhibit expressionValidate therapeutic effectOrganic active ingredientsSenses disorderInflammatory factorsCeritinib
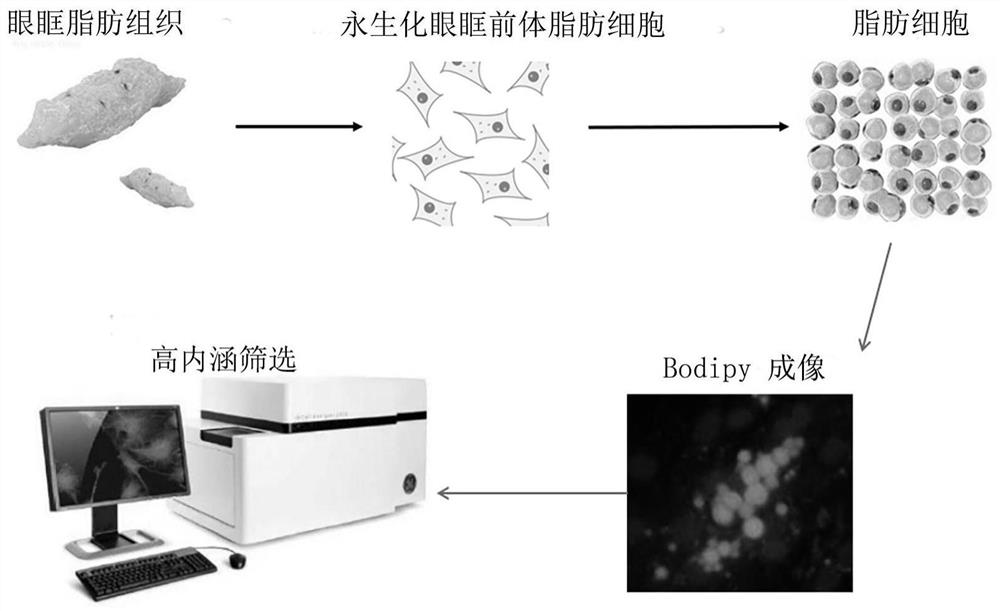
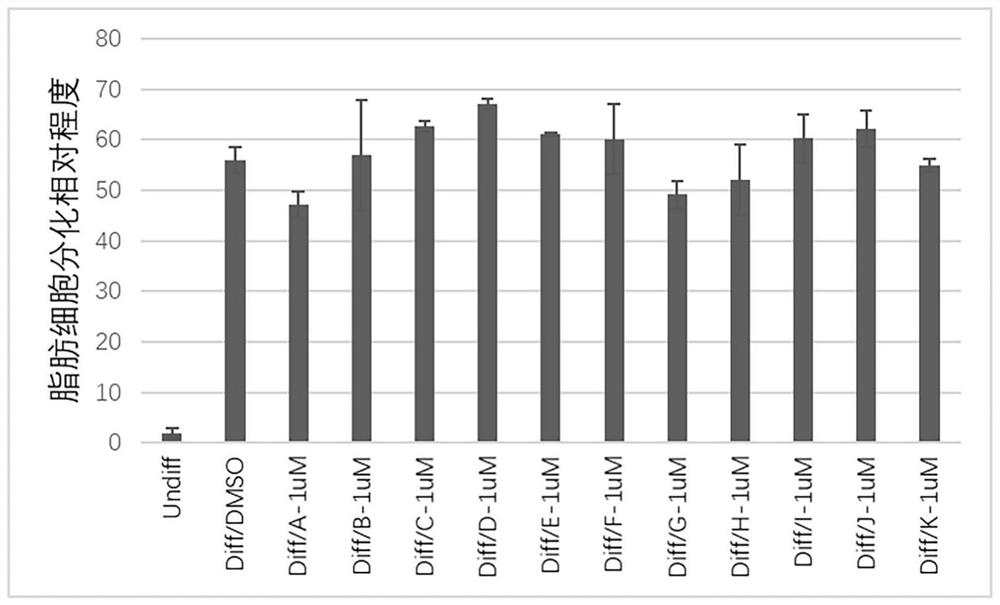
The invention discloses application of ceritinib in preparation of medicine for treating thyroid-associated ophthalmopathy, and relates to the field of medicines. The pathogenesis of thyroid-related ophthalmopathy is complex, and clinical phenotypes such as eyeball protrusion and intraocular pressure increase are finally caused by adipose precursor cell differentiation enhancement in eye sockets, large-amount secretion of inflammatory factors and the like. According to the invention, lipid accumulation condition of cells subjected to complete fat induced differentiation is evaluated by virtue of Bodipy dyeing and high-content instrument analysis so as to screen drugs capable of effectively inhibiting fat differentiation and lipid accumulation. The result shows that ceritinib has an obvious inhibiting effect on fat differentiation of human orbital fibroblasts, and can inhibit expression of inflammatory factors induced by IL-17A. Thus, the treatment effect of ceritinib on thyroid-related eye diseases is verified.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Preparation method for ceritinib analog
The invention discloses a preparation method for a ceritinib analog, i.e., 5-chloro-N2-(2-isobutyl-5-methyl-4-(1,2,3,6-tetrahydropyridin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine dihydrochloride. The method comprises the following steps: with 1-benzyl-4-(5-isopropyloxy)-2-methyl-4-nitrophenyl)-1,2,3,6-tetrahydropyridine as a starting material, subjecting the starting material and trichloroethyl chloroformate to a nucleophilic substitution reaction so as to form a compound I; then reducing the nitro group of the compound into an amino group under the action of a reducing agent so as to form a compound II; then carrying out deprotection under the action of a reducing agent to obtain a compound III; and finally, subjecting the hydrochloride of the compound III and acompound IV to a nucleophilic substitution reaction to form a compound V, i.e., 5-chloro-N2-(2-isobutyl-5-methyl-4-(1,2,3,6-tetrahydropyridin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine dihydrochloride. The preparation method of the invention has the advantages of short synthetic route, simple operation, good safety, high product yield, high quality and low synthesis cost.
Owner:EAST CHINA NORMAL UNIV +1
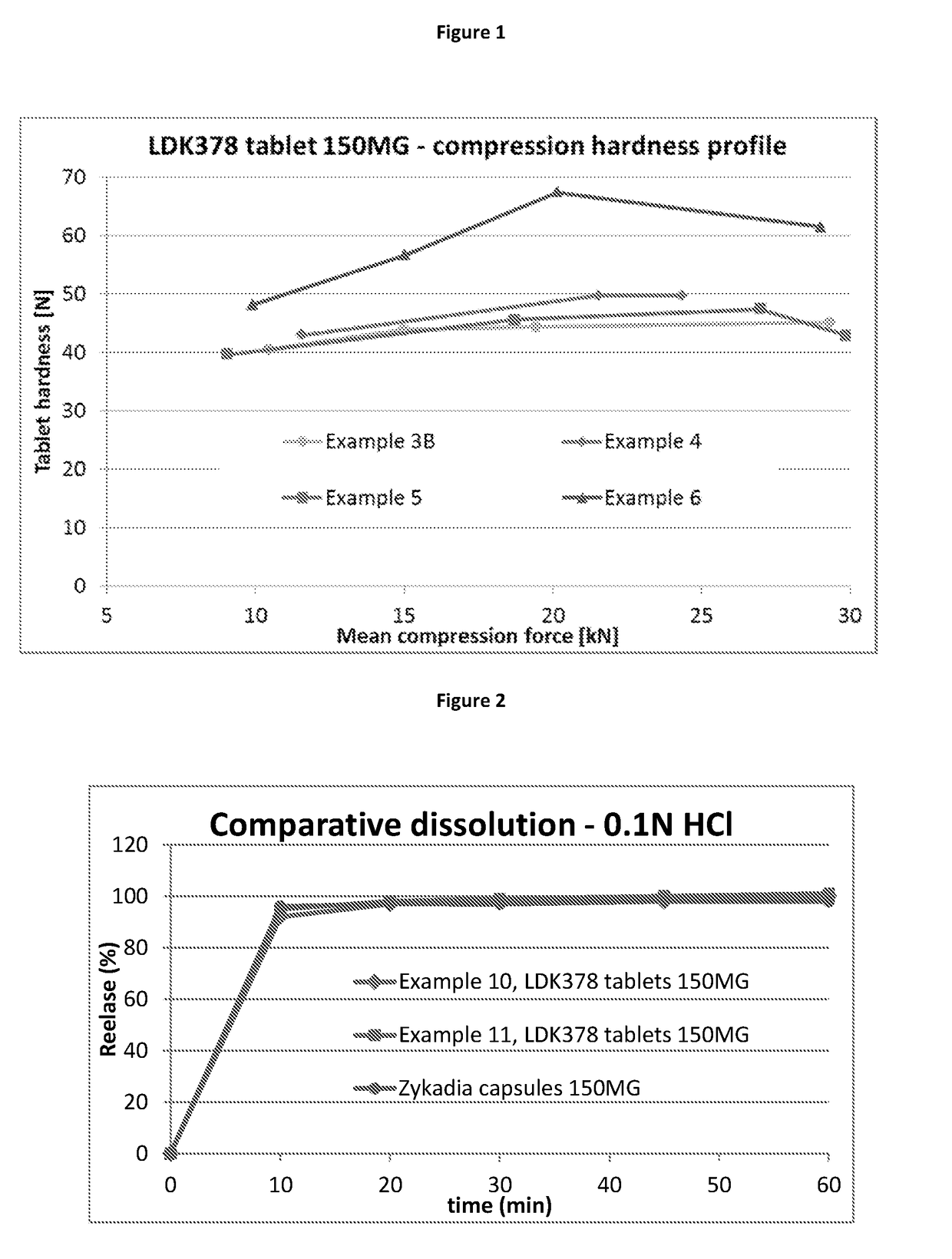
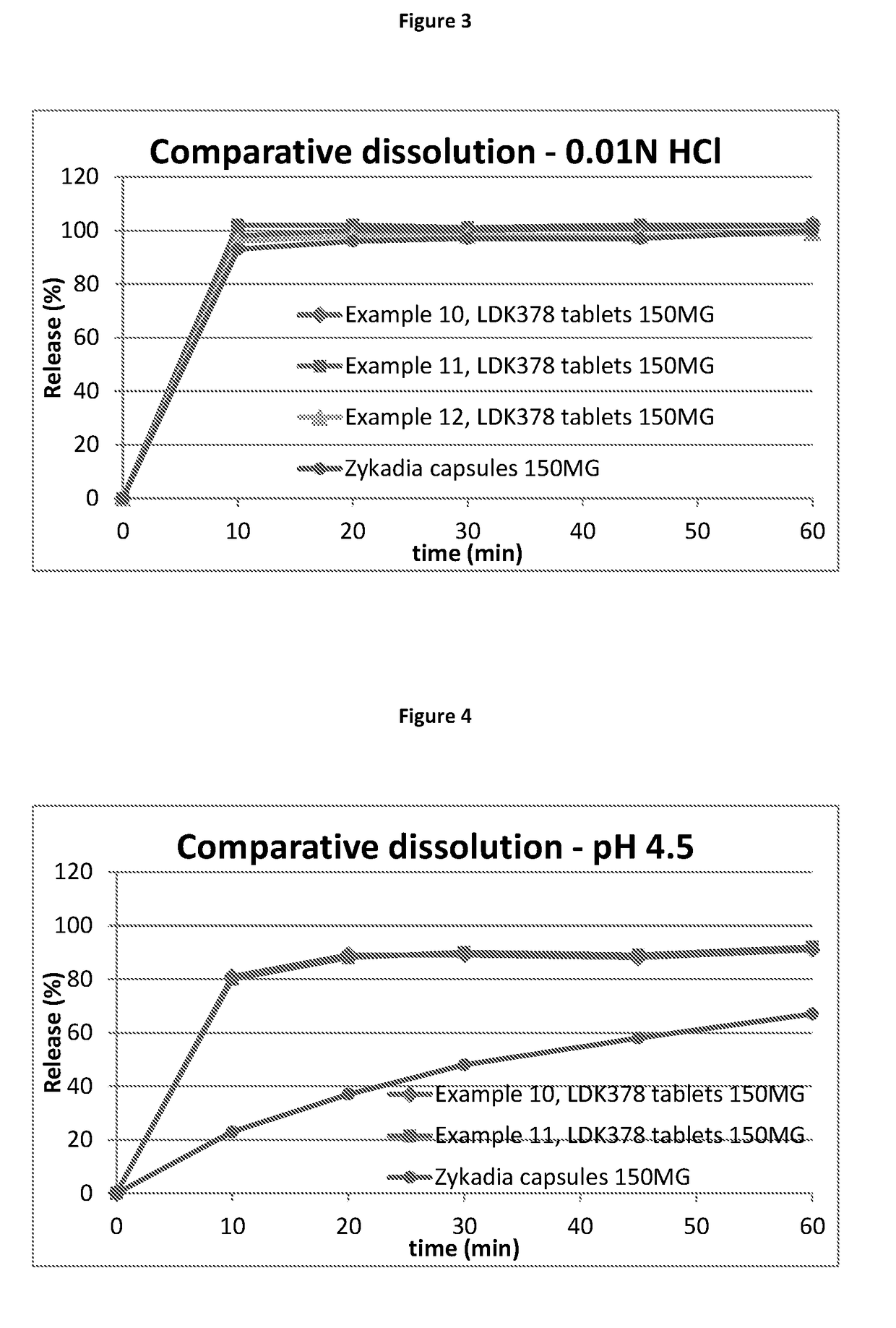
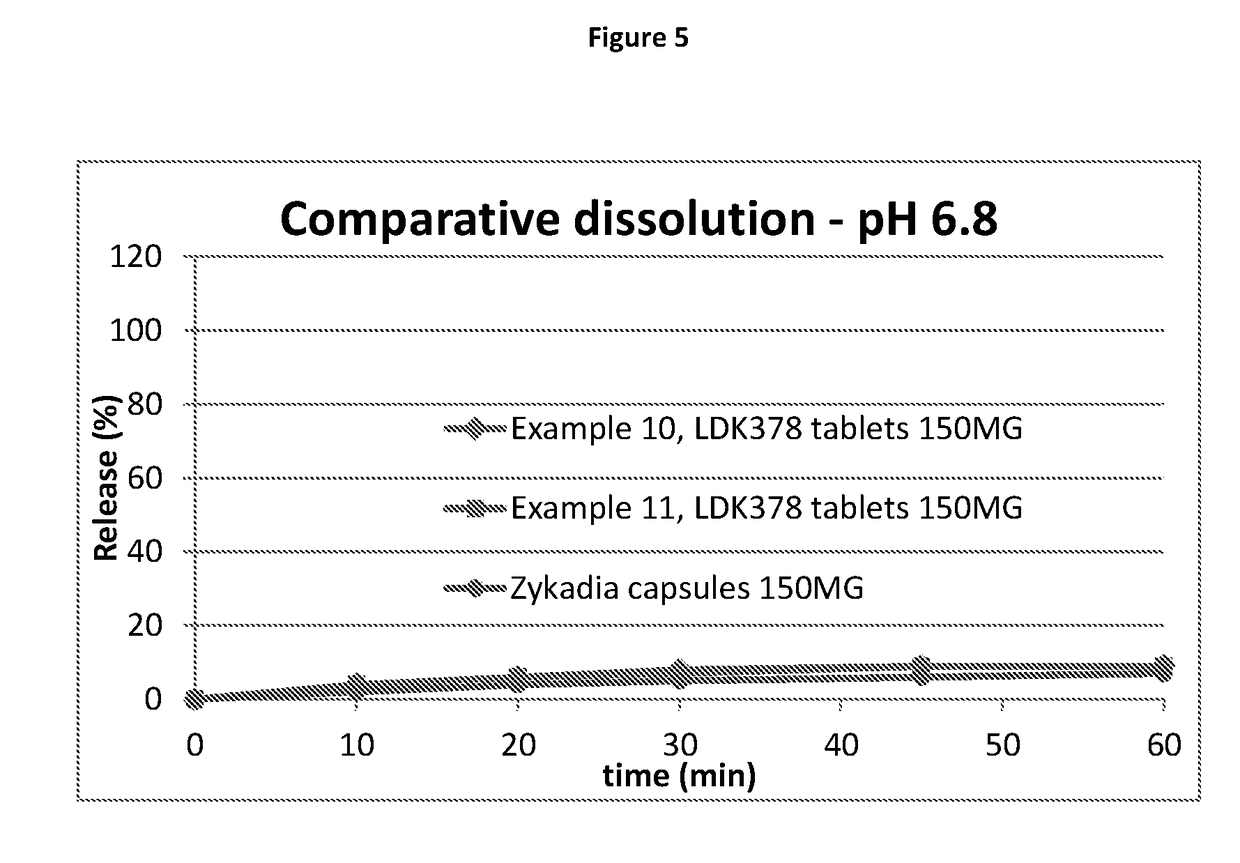
Ceritinib formulation
InactiveUS20170112834A1Prolong progression-free survivalExtended durationOrganic active ingredientsPill deliveryCeritinibExcipient
The present disclosure relates to a new pharmaceutical composition comprising Ceritinib. Particularly it is directed to the tablet that is prepared by wet granulation, wherein povidone is used as a binder. Further feature of the composition is that the drug and the binder form the inner phase, whereas all other excipients are added in a powder form as an outer phase. This way, the sticking of the composition is prevented and sufficient tablet hardness can be reached.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com