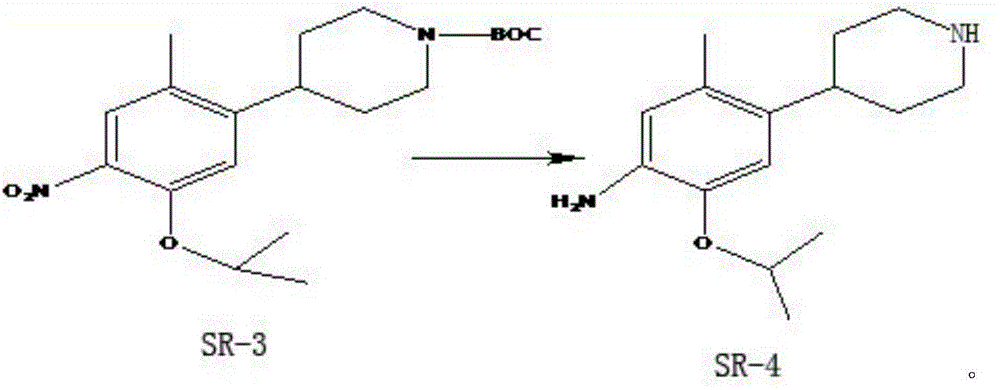
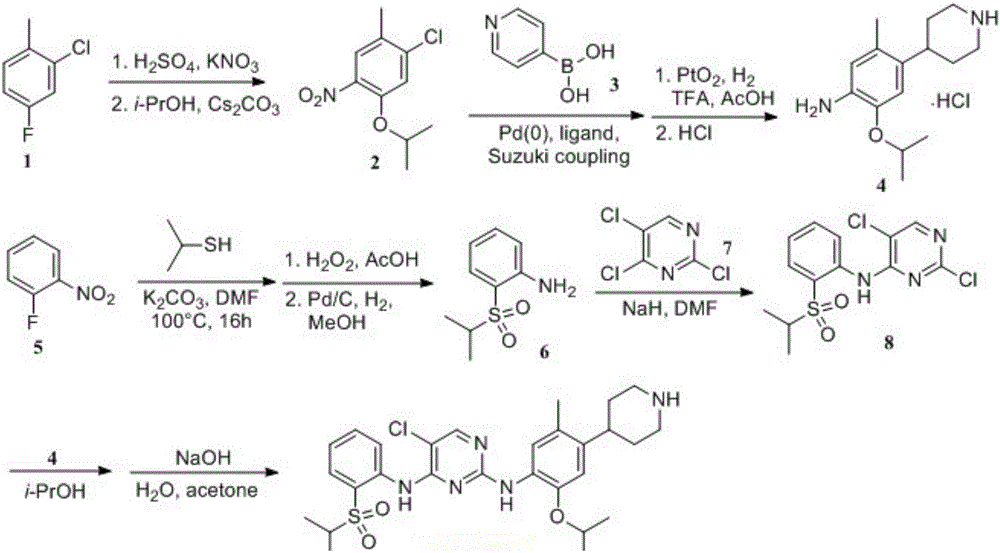
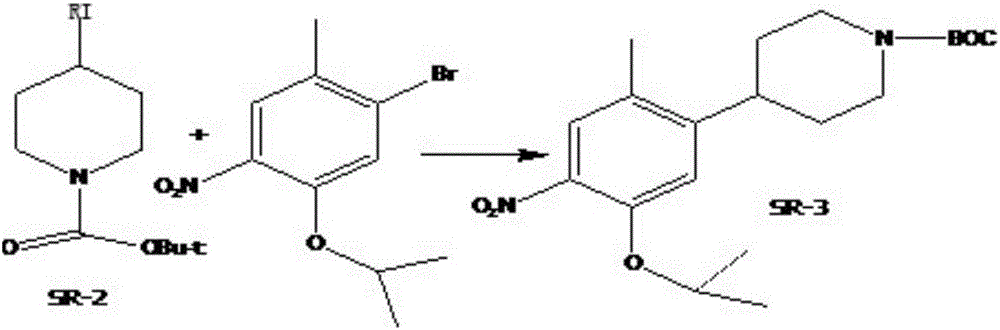
A kind of preparation method of ceritinib intermediate
A technology of ceritinib and intermediates, which is applied in the field of synthesis of organic compounds, can solve the problems of short route and low cost, and achieve the effects of low cost, mild reaction conditions and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Step 1: Add 422mg (6.45mmol) of zinc powder and 4ml of DMAC (dimethylacetamide) into a dry four-necked bottle under the protection of nitrogen gas. 90 microliters (1 mmol) of 1,2-dibromoethane, heated to an internal temperature of 56 degrees, a gray suspension in the reaction bottle, after heating for 5 minutes, cooling the reaction solution to about 33 degrees, TMSCl (trimethyl Chlorosilane (170 microliters, 1.34mmol) was added, exothermic, the temperature naturally rose to 57 degrees, the reaction solution was cooled to 40 degrees, and kept for 15min, SR-1 (1.02g, 3.26mmol), DMAC0.5ml mixed solution Add dropwise, exothermic reaction, the reaction solution is cooled to room temperature, stirred for 1H, after the reaction is complete, suction filtration is used under nitrogen protection and directly used in the next step reaction (to be used within 1HR, the concentration of the solution is titrated for iodine content).
[0017] The second step: In a 500ml four-neck flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com