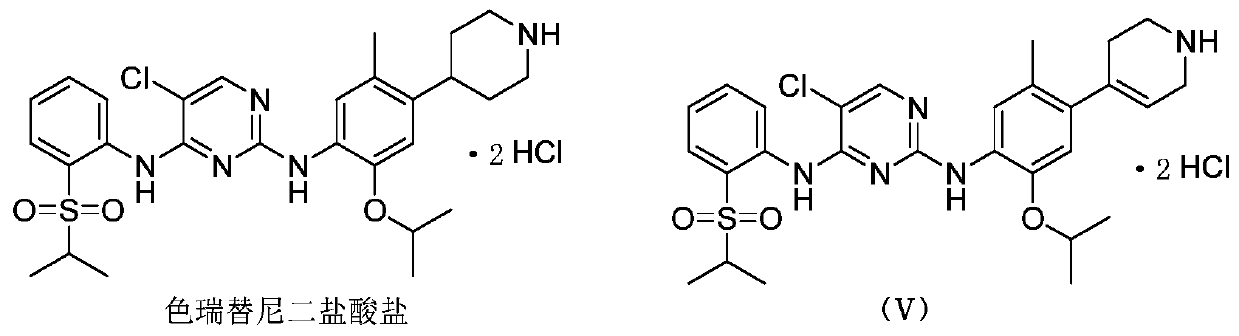
Preparation method for ceritinib analog
A technology of ceritinib and analogs, which is applied in the field of compound preparation and achieves the effects of easily controllable conditions, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
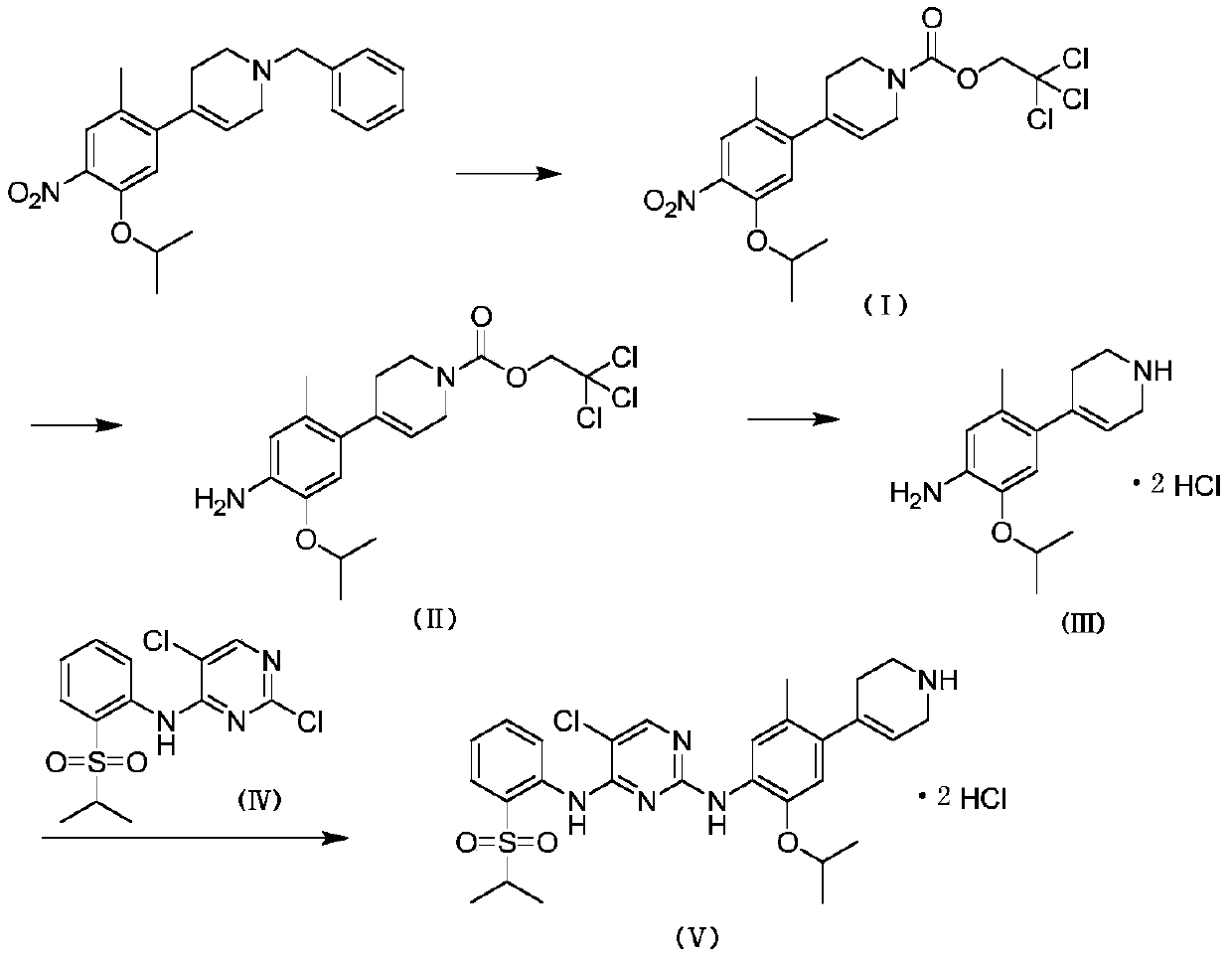
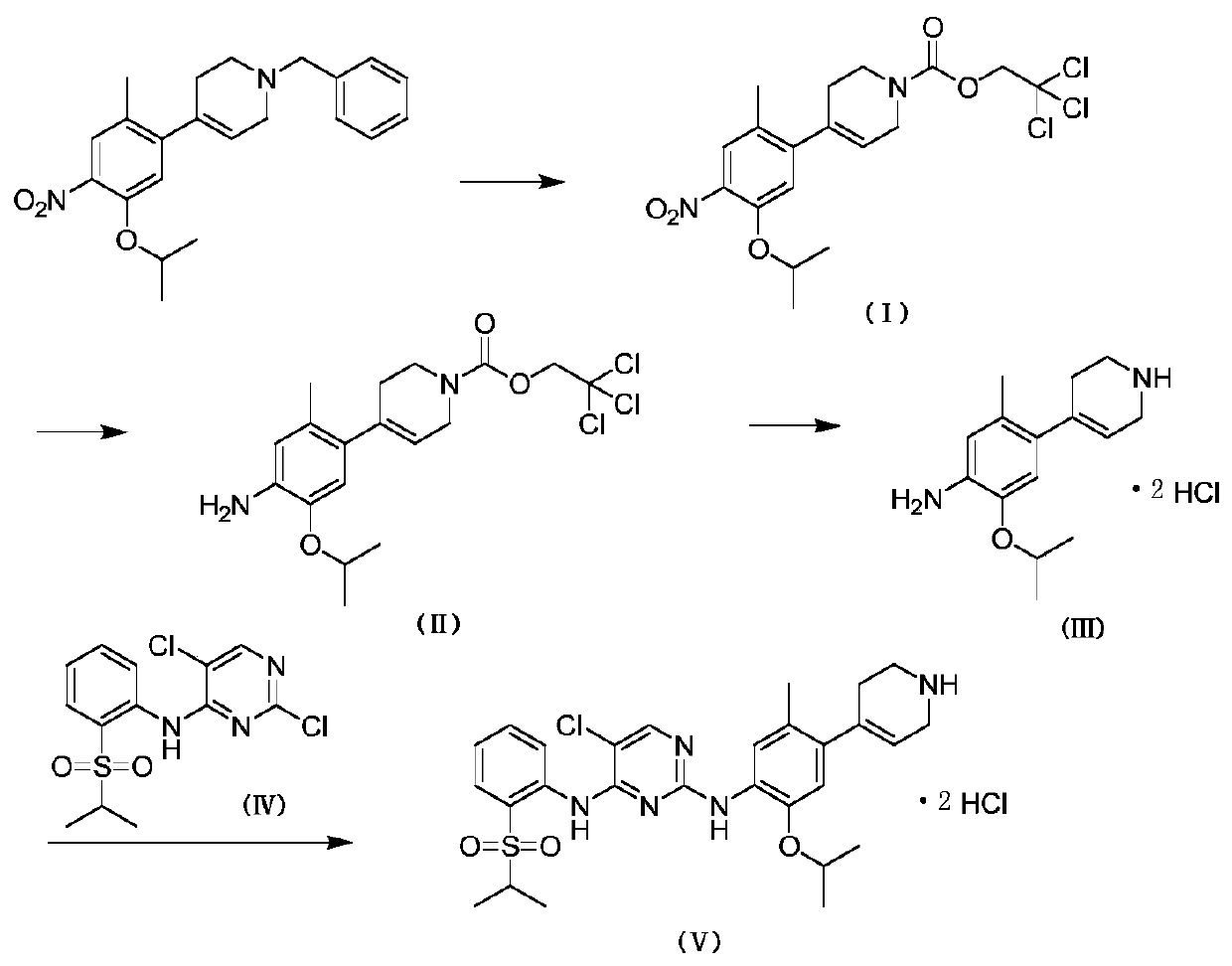
[0020] 1.1 Compound I is 2,2,2-trichloroethyl 4-(5-isopropoxy-2-methyl-4-nitrophenyl)-3,6-dihydropyridine-1(2H)- Synthesis of tert-Butyl Carboxylate
[0021] Dissolve 1 g of 1-benzyl-4-(5-isopropoxy-2-methyl-4-nitrophenyl)-1,2,3,6-tetrahydropyridine in 7 mL of acetonitrile in a one-necked bottle , Add 0.58g trichloroethyl chloroformate, react at room temperature. The reaction was carried out for 2 hours, and TLC detected that the reaction of the raw materials was complete. The acetonitrile was evaporated to dryness, and the volume ratio of ethyl acetate to petroleum ether was 1:8 for column chromatography purification. Obtain compound I namely 2,2,2-trichloroethyl 4-(5-isopropoxy-2-methyl-4-nitrophenyl)-3,6-dihydropyridine-1(2H)- 1.07 g of tert-butyl carboxylate, the yield was 87%.
[0022] 1 H NMR: (500MHz, CDCl 3 )δ7.64(s,1H),6.80(s,1H),5.67(d,J=17.9Hz,1H),4.83(s,2H),4.65-4.61(m,1H),4.23(d,J =23.2Hz,2H),3.80(d,J=25.1Hz,2H),2.40(br,2H),2.26(s,3H),1.40(d,J=6.1Hz,6H).
...
Embodiment 2
[0033] 2.1 Compound II is 2,2,2-trichloroethyl 4-(4-amino-5-isopropoxy-2-methylphenyl)-3,6-dihydropyridine-1(2H)-carboxyl Synthesis of tert-butyl ester
[0034] 4.0g 2,2,2-trichloroethyl 4-(5-isopropoxy-2-methyl-4-nitrophenyl)-3,6-dihydropyridine-1(2H )-tert-butyl carboxylate was dissolved in 40 mL of absolute ethanol, 0.29 g of ferric chloride was added, 0.58 g of activated carbon was added, and 10.64 g of 50% hydrazine hydrate was added, and the temperature was raised to 75°C. After 6 hours of reaction, TLC detected that the reaction of raw materials was complete. Suction filter with a pad of Celite, evaporate to dryness of absolute ethanol, add dichloromethane, wash with water twice, separate the organic phase, dry over anhydrous sodium sulfate, evaporate to dryness, and use ethyl acetate and petroleum ether at a volume ratio of 1:5 Purification by column chromatography was performed. Obtain compound II namely 2,2,2-trichloroethyl 4-(4-amino-5-isopropoxy-2-methylphenyl)-...
Embodiment 3
[0046] 3.1 Synthesis of Compound III, namely 2-isopropoxy-5-methyl-4-(1,2,3,6-tetrahydropyridin-4-yl)aniline dihydrochloride
[0047] 1.92g of 2,2,2-trichloroethyl 4-(4-amino-5-isopropoxy-2-methylphenyl)-3,6-dihydropyridine-1(2H )-tert-butyl carboxylate was dissolved in 15 mL of mixed solvent (V (acetonitrile): V (glacial acetic acid) = 4: 1), added 9.6 g of zinc powder, and reacted at room temperature. The reaction was carried out for 4 hours, and TLC detected that the reaction of the raw materials was complete. Suction filter with a pad of celite, evaporate the reaction solvent to dryness, add saturated sodium carbonate solution to the system to adjust the pH to 9-10, extract with dichloromethane, wash with water, dry over anhydrous sodium sulfate, and evaporate to dryness. The obtained compound was dissolved in 5 mL of anhydrous methanol, 0.57 mL of concentrated hydrochloric acid was added, stirred at room temperature for 20 min, and evaporated to dryness of anhydrous meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com