A ceritinib preparing method
A technology of ceritinib and isopropoxy, which is applied in the field of synthesis of organic compounds, achieves the effects of easy availability of raw materials, low cost, low synthetic route, mild reaction conditions and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
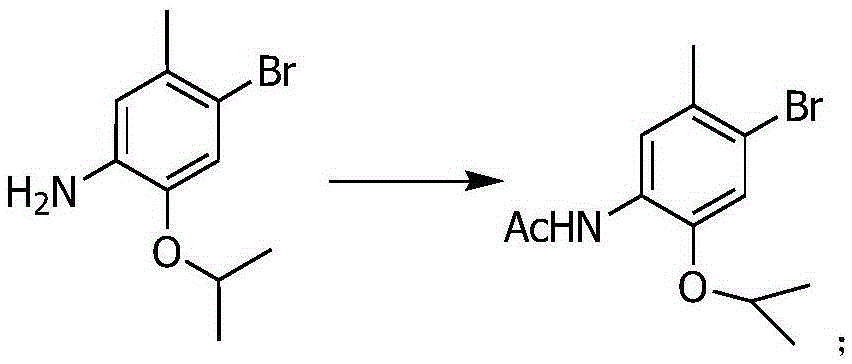
[0020] The first step: put 4-bromo-2-isopropoxy-5-methylaniline (56G, 231.1mmol) into the 1000ml reaction flask, add in 200ml glacial acetic acid at room temperature, start stirring, add dropwise acetic anhydride ( 26.2ml, 277mmol), then heated to reflux for 30min, cooled to room temperature, poured into 500ml of ice water, stirred for 10min, filtered with suction, washed with water, and dried to obtain N-(4-bromo-2-isopropoxy-5-toluene base) acetamide 62.7 g (95% yield).
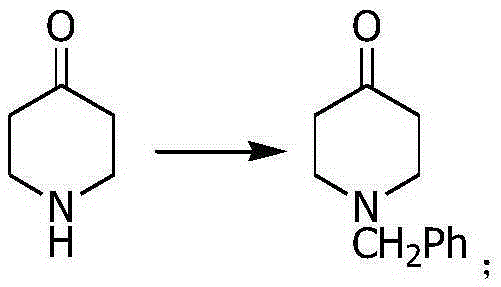
[0021] Step 2: Add 4-piperidone (99.13g, 1mol), potassium carbonate (138g, 1mol), DMF 500ml in turn into a 2000ml reaction flask, start stirring, stir at room temperature for 30min, dropwise add chlorobenzene (112g, 1mol ), after the dropwise addition, heat to 80° and keep warm for 5H, then cool down to room temperature, add 1000ml of water, stir for 30min, extract with 800ml*3 ethyl acetate, wash the ethyl acetate layer twice with 300ml brine, add anhydrous sulfuric acid It was dried over sodium and con...
Embodiment 2
[0027] The first step: put 4-bromo-2-isopropoxy-5-methylaniline (56G, 231.1mmol) into the 1000ml reaction flask, add in 200ml glacial acetic acid at room temperature, start stirring, add dropwise acetic anhydride ( 26.2ml, 277mmol), then heated to reflux for 45min, cooled to room temperature, poured into 500ml ice water, stirred for 10min, filtered with suction, washed with water, and dried to obtain N-(4-bromo-2-isopropoxy-5-toluene base) acetamide 62.7 g (95% yield).
[0028] Step 2: Add 4-piperidone (99.13g, 1mol), potassium carbonate (138g, 1mol), DMF 500ml in turn into a 2000ml reaction flask, start stirring, stir at room temperature for 30min, dropwise add chlorobenzene (112g, 1mol ), after the dropwise addition, heat to 80° and keep warm for 6H, then cool down to room temperature, add 1000ml of water, stir for 60min, extract with 800ml*3 ethyl acetate, wash the ethyl acetate layer twice with 300ml brine, add anhydrous sulfuric acid It was dried over sodium and concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com