Ceritinib intermediate and preparation method thereof
A technology of ceritinib and intermediates, applied in the field of drug synthesis, can solve problems such as operator injury and irritation, and achieve the effects of less side reactions, lower production costs, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
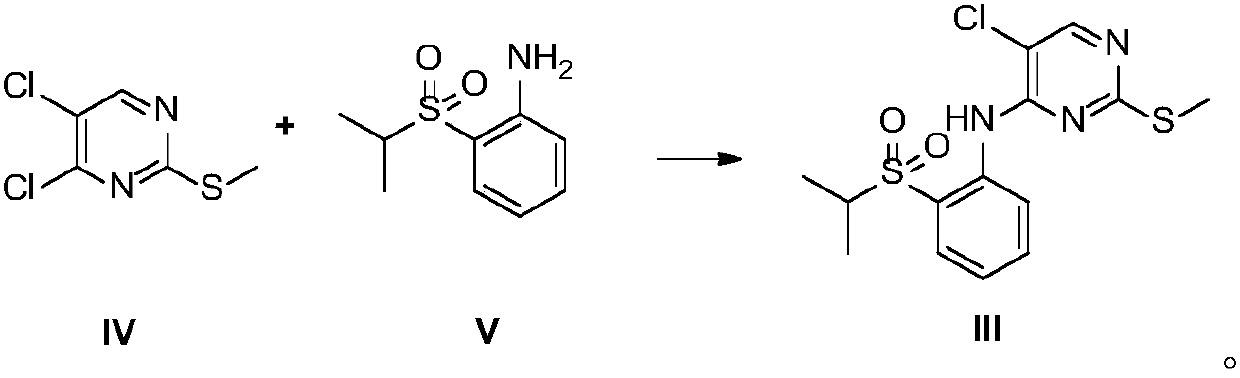
[0056] Preparation of 2-methylthio-5-chloro-N-[2-[(1-methylethyl)sulfonyl]phenyl]-4-pyrimidinamine (Intermediate III)
[0057] In a dry three-necked flask, filled with nitrogen, added sodium hydride (1.5g, 0.0375mol) and DMSO (25mL), cooled to 0°C, and added dropwise 2-(isopropylsulfonyl)aniline (5g, 0.025mol) DMSO (12.5mL) solution (dropping rate is 1 / d / s), after the addition is complete, stir at 25°C for 1h, then add dropwise 4,5-dichloro-2-methylthiopyrimidine (5.4g, 0.028mol) DMSO (12.5 mL) solution (dropping rate: 1 / d / s), stirred at 25°C for 2 h, quenched with ice water (150 mL), precipitated solid, filtered, washed with water to obtain a yellow solid. The resulting solid was added with n-heptane (40 mL), stirred for 2 h, and filtered to obtain 7 g of a yellow solid with a yield of 78% and a purity of 99.2% by HPLC. ESMS m / z 358.0[M+H] +.1 H NMR (DMSO-D 6 ):δ9.66(s,1H),8.51(d,1H),8.46(s,1H),7.81-7.88(m,2H),7.41(t,1H),3.46-3.53(m,1H), 2.47(s,3H),1.16(d,6H).
Embodiment 2
[0059] Preparation of 2-methylthio-5-chloro-N-[2-[(1-methylethyl)sulfonyl]phenyl]-4-pyrimidinamine (Intermediate III)
[0060] In a dry three-necked flask, filled with nitrogen, added sodium hydride (1.5g, 0.0375mol) and DMF (25mL), cooled to 0°C, and added dropwise 2-(isopropylsulfonyl)aniline (5g, 0.025mol) DMF (12.5mL) solution (dropping rate is 1 / d / s), after the addition, stirred at 25°C for 1h, then added dropwise 4,5-dichloro-2-methylthiopyrimidine (5.4g, 0.028mol) DMF (12.5 mL) solution (dropping rate: 1 / d / s), stirred at 25°C for 2 h, quenched with ice water (150 mL), precipitated solid, filtered, and washed with water to obtain a yellow solid. The resulting solid was added with n-heptane (40 mL), stirred for 2 h, and filtered to obtain 7.4 g of a yellow solid with a yield of 82.4% and a HPLC purity of 99%.
Embodiment 3
[0062] Preparation of 2-methylthio-5-chloro-N-[2-[(1-methylethyl)sulfonyl]phenyl]-4-pyrimidinamine (Intermediate III)
[0063] In a dry three-necked flask, fill with nitrogen, add sodium hydride (1.3g, 0.0325mol) and acetonitrile (25mL), cool to 0°C, add dropwise 2-(isopropylsulfonyl)aniline (5g, 0.025mol) Acetonitrile (25mL) solution (dropping rate is 1 / d / s), after addition, stirred at 25°C for 1h, then added dropwise 4,5-dichloro-2-methylthiopyrimidine (9.6g, 0.05mol) Acetonitrile (25 mL) solution (dropping rate 1 / d / s), stirred at 25°C for 3 h, quenched with ice water (150 mL), precipitated solid, filtered, washed with water to obtain a yellow solid. The resulting solid was added with n-heptane (40 mL), stirred for 2 h, and filtered to obtain 6.8 g of a yellow solid, with a yield of 75.5% and a HPLC purity of 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com