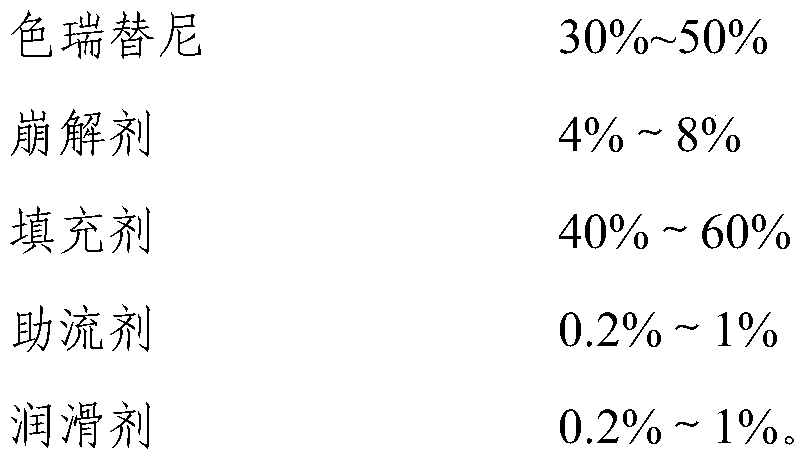
Ceritinib pharmaceutical composition
A technology of ceritinib and its composition, which is applied in the field of ceritinib pharmaceutical composition and its preparation, can solve problems such as difficult rapid dissolution, and achieve the effects of avoiding delayed absorption in vivo, rapid dissolution and sufficient dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6 and comparative example 1-2
[0041] Examples 1-6 and Comparative Examples 1-2: Preparation of pharmaceutical compositions with different particle sizes of ceritinib
[0042] Preparation method: mix ceritinib with microcrystalline cellulose, low-substituted hydroxypropyl cellulose and carboxymethyl starch sodium evenly, add water for wet granulation, after drying, add silicon dioxide and magnesium stearate Mix well, and fill the final mixture into gelatin capsules to prepare capsules.
[0043] Table 1 The prescription composition of embodiment 1-6 and comparative example 1-3 ceritinib pharmaceutical composition
[0044] prescription Weight (mg) weight percentage Ceritinib 150.0 40% microcrystalline cellulose 205.5 54.8% Low-substituted hydroxypropyl cellulose 7.5 2% Carboxymethyl Starch Sodium 7.5 2% silica 1.5 0.4% Magnesium stearate 3.0 0.8% gross weight 375.0 100%
[0045] Table 2 The particle size of raw materials in the pharmaceutical...
Embodiment 7
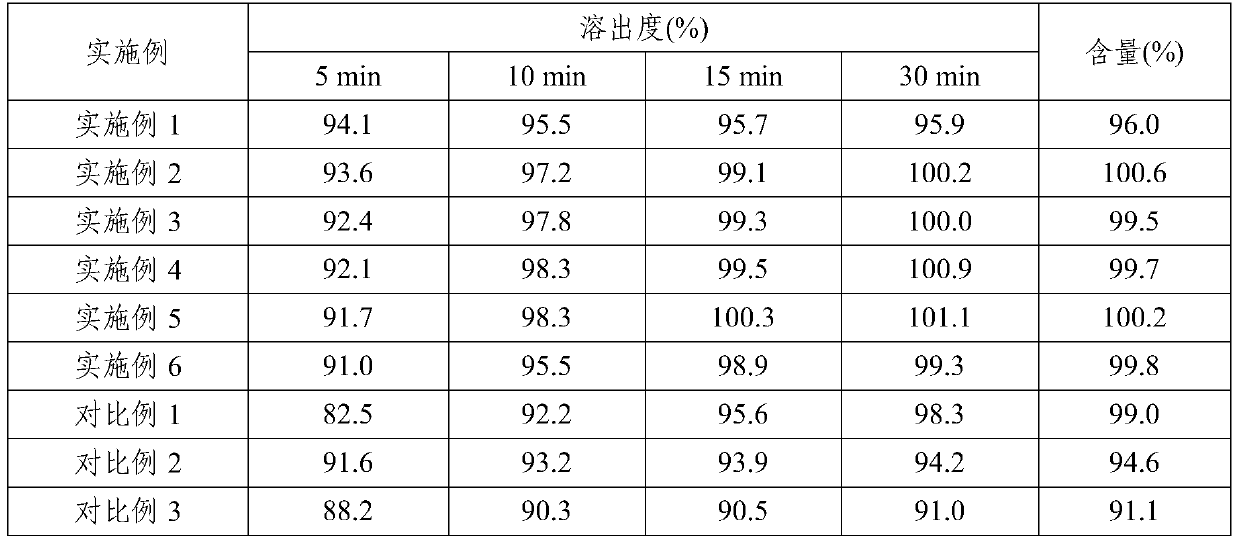
[0047] Embodiment 7: Dissolution and content investigation of the pharmaceutical composition prepared in Examples 1-6 and Comparative Examples 1-3
[0048] Dissolution: According to the dissolution determination method (the second method of appendix XC of the Chinese Pharmacopoeia in 2010), 900mL of 0.1M hydrochloric acid solution was used as the dissolution medium, and the rotating speed was 60 revolutions per minute. Operate according to the law, respectively at 5, 10, and 15 , Sampling for 30 minutes, filtered, and the filtrate was taken as the test solution. According to ultraviolet-visible spectrophotometry (Chinese Pharmacopoeia 2010 edition two appendix IVA), measure absorbance at 339nm wavelength place.
[0049] Content: Determined according to high performance liquid chromatography (Appendix VD of Part Two of the Chinese Pharmacopoeia 2010 Edition). Precisely measure 20 μL each of the test solution and the reference solution and inject them into the liquid chromatogr...
Embodiment 8-10 and comparative example 4-5
[0053] Examples 8-10 and Comparative Examples 4-5: Preparation of ceritinib pharmaceutical composition with different additions of disintegrants
[0054] The disintegrants are low-substituted hydroxypropyl cellulose and sodium starch glycolate, and the particle size D90 of ceritinib is 50.6 μm.
[0055] Refer to Example 1 for the preparation method.
[0056] Table 4 The prescription composition of the pharmaceutical composition prepared with different additions of disintegrants
[0057]
[0058] Remarks: the weight unit in the above table is mg; the percentage is the weight percentage of the pharmaceutical composition, and the unit is %; the total weight of the composition is 375.0mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com