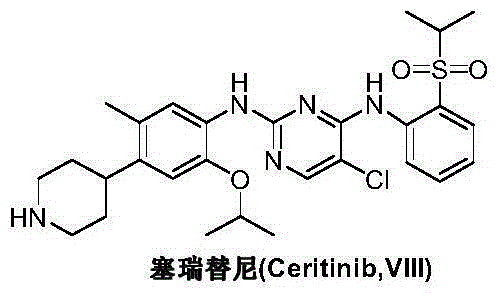
Preparation method of ceritinib and its intermediate
A technology of ceritinib and intermediates, which is applied in the field of preparation of ceritinib and its intermediates, can solve the problems of increased side reactions, cumbersome operations, reduced total yield, etc. Reaction reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine (II) (2.72g, 10mmol) and 10% palladium carbon (0.27g, 10%w / w), 25mL saturated hydrogen chloride isopropanol solution and 100mL isopropanol, according to the hydrogenation reaction operation procedure, pass in hydrogen, keep 50-60°C, 8-10Kg / cm 2 The reaction was stirred for 16 hours until hydrogen was no longer consumed. Cool, filter and recover palladium carbon, concentrate under reduced pressure to recover the solvent, dissolve the residue with dichloromethane and wash with saturated sodium bicarbonate, separate the organic phase, concentrate under reduced pressure, and the resulting crude product is washed with ethyl acetate and n-hexane (1:1) Recrystallization gave 2.27 g of off-white solid 2-isopropoxy-5-methyl-4-(piperidin-4-yl)aniline (III), with a yield of 91.5%.
Embodiment 2
[0032] Add 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine (II) (2.72g, 10mmol), Raney nickel (0.54g), 0.1mL concentrated Sulfuric acid and 120mL isopropanol, according to the hydrogenation reaction operating procedure, pass in hydrogen, keep at 40-50°C, 5-8Kg / cm 2 The reaction was stirred for 22 hours until hydrogen was no longer consumed. Cool, filter to recover the catalyst, concentrate under reduced pressure to recover the solvent, dissolve the residue with dichloromethane and wash with saturated sodium bicarbonate, separate the organic phase, concentrate under reduced pressure, and weigh the obtained crude product with ethyl acetate and n-hexane (1:1). Crystallized to obtain 2.21 g of off-white solid 2-isopropoxy-5-methyl-4-(piperidin-4-yl)aniline (III), with a yield of 89.1%.
Embodiment 3
[0034]Add 2-isopropoxy-5-methyl-4-(piperidin-4-yl)aniline (III) (2.48g, 10mmol), 5mL of 40% hydrobromic acid solution and 20mL of water into the three-necked flask, keep At 0-5°C, a 20 mL aqueous solution of sodium nitrite (1.38 g, 20 mmol) was added dropwise, and the reaction was continued for 2 hours after the drop was completed. Add cuprous bromide (1.57g, 11mmol), heat up to 50-60°C, react for 2 hours, cool, filter to remove solids, extract the mother liquor 3 times with dichloromethane, combine the organic phases, and successively wash with saturated sodium bicarbonate solution Wash with saturated brine, dry over anhydrous sodium sulfate, recover the solvent under reduced pressure, and the resulting oil is 2-isopropoxy-5-methyl-4-(piperidin-4-yl)bromobenzene (I) 2.58g , yield 83.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com