Preparation method for ceritinib
A technology of ceritinib and compound, applied in the field of ceritinib synthesis, can solve the problems of high time cost and raw material cost, difficult to show economic benefits, long operation cycle, etc., and achieves good application prospects, environmental friendliness, and operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
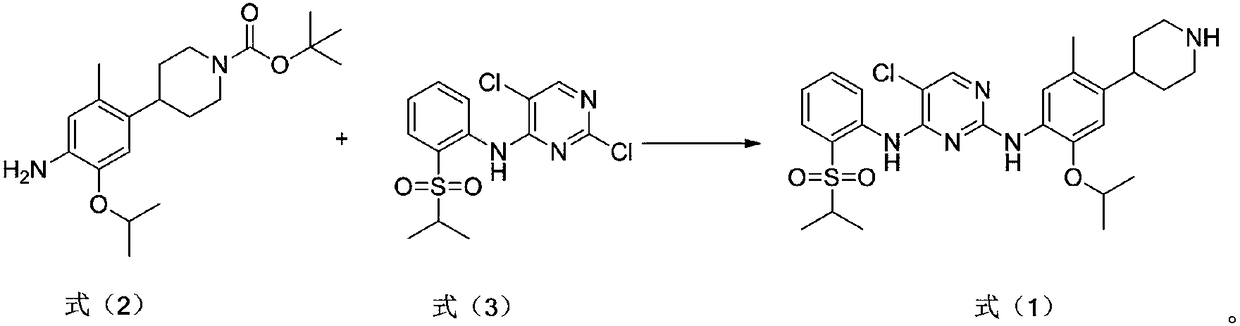
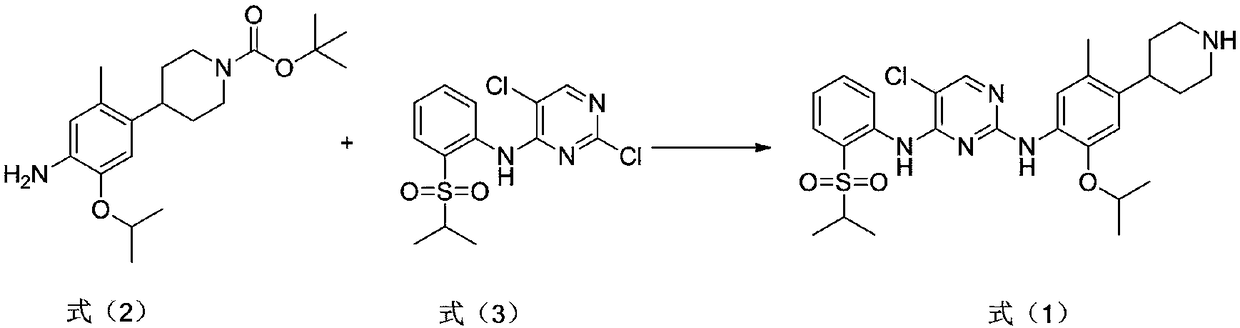
[0022] Under nitrogen protection, the compound (3.5g, 1eq) of formula (3), the compound (2.8g, 0.8eq) of formula (2) were added in diisopropylethylamine (16.8g), and the temperature was raised to 80 ℃ reaction, after the completion of the reaction, evaporate the organic base, add ethyl acetate to extract, wash with water, add hydrogen chloride in ethyl acetate solution, stir for 2 hours, filter to obtain a solid, adjust the base of the solid, and suction filter to obtain the compound of formula (1). Compound ceritinib (2.5 g).
Embodiment 2
[0024] Under the protection of nitrogen, the compound of formula (3) (3.5g, 1eq) and the compound of formula (2) (4.2g, 1.2eq) were added to 1,8-diazabicyclo[5.4.0]undeca- In 7-ene (50.4g), the temperature was raised to 120°C for reaction. After the reaction was completed, the organic base was evaporated, extracted with ethyl acetate, washed with water, added with ethyl acetate solution of hydrogen chloride, stirred for 2 hours, and filtered to obtain a solid. The solid was adjusted to base and suction filtered to obtain the compound ceritinib (3.2 g) of formula (1).
Embodiment 3
[0026] Under the protection of nitrogen, the compound of formula (3) (3.5g, 1eq) and the compound of formula (2) (3.5g, 1eq) were added to triethylenediamine (28g), and the temperature was raised to 90°C for reaction. After completion, evaporate the organic base, add ethyl acetate to extract, wash with water, add hydrogen chloride in ethyl acetate solution, stir for 2 hours, filter to obtain a solid, adjust the base of the solid, and suction filter to obtain the compound of formula (1) ceretyl Nil (1.8g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com