Ceritinib compound and pharmaceutical composition thereof
A compound and composition technology, applied in the field of medicinal chemistry, can solve the problems of not providing Ceritinib crystal information, not obtaining the free base solid form, and not seeing Ceritinib public reports, etc., and achieve the effect of simple preparation process and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Embodiment 1 Ceritinib Compound Amorphous Crystalline Form
[0046] At room temperature, dissolve Ceritinib dihydrochloride (5g) in 25g of purified water, add dichloromethane (75ml), slowly add 50% sodium hydroxide solution dropwise under stirring, adjust the pH of the aqueous solution to 7-10, and after standing The organic layer was separated, and the organic layer was concentrated to dryness under reduced pressure at 45°C to obtain a foamy solid. X-ray powder diffraction pattern shows an amorphous crystalline form.
Embodiment 2
[0047] Preparation of Embodiment 2 Ceritinib Formula I Compound Crystal Form
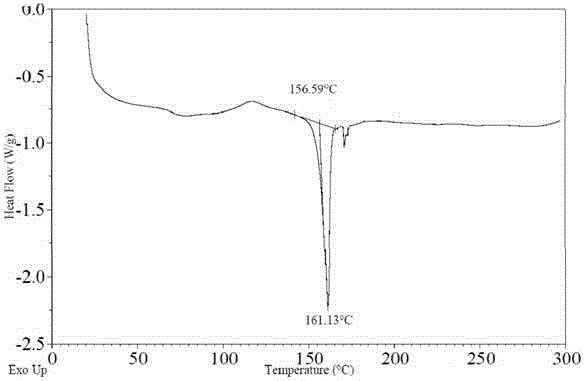
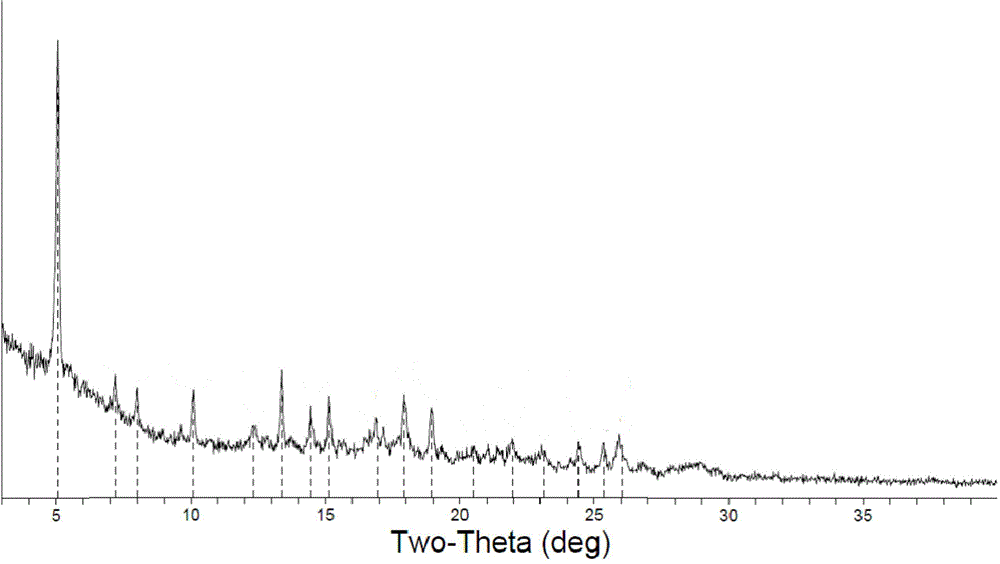
[0048] The Ceritinib amorphous crystalline form (3g) obtained in Example 1 was added to purified water (15g), stirred and heated to 60°C for beating for 2-5h, filtered, and dried under reduced pressure at 40°C for 3h to obtain 2.5g of solid. X-ray powder diffraction diagram, DSC diagram, TGA diagram are respectively attached figure 1 , figure 2 , image 3 shown.
Embodiment 3
[0049] Preparation of Embodiment 3 Ceritinib Formula I Compound Crystal Form
[0050] Take the Ceritinib amorphous crystal form (3g) obtained in Example 1, add it to purified water (30g), stir and heat to 80°C for beating for 2-5h, filter, and dry under reduced pressure at 40°C for 3h to obtain a solid, X-ray powder diffraction pattern same figure 1 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com