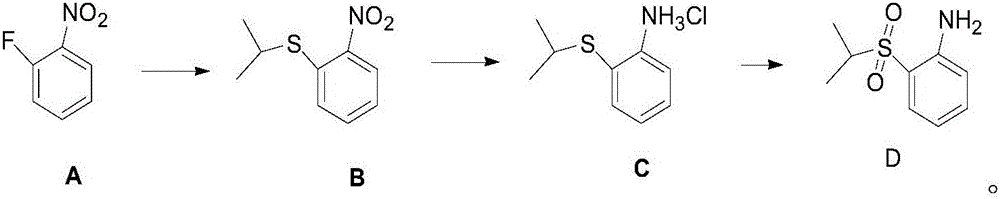
Anti-cancer drug ceritinib intermediate 1-amino-2-(isopropyl sulfonyl)benzene synthetic method
A technology of isopropylsulfonyl and ceritinib, applied in chemical instruments and methods, preparation of organic compounds, preparation of sulfides, etc., can solve the problems of cumbersome operation and high energy consumption, and achieve the improvement of reaction rate and energy consumption. Low, simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In the reaction bottle that is equipped with liquid caustic soda tail gas absorption device, add DMF (423.3g), 60% sodium hydride (44g, 1.1moL), o-fluoronitrobenzene (141.1g, 1.0moL), ice-salt bath drops to 0 Below ℃, add isopropyl mercaptan (91.4g, 1.2moL) dropwise, after the dropwise addition is completed, return to 25℃ and react for 2.0-3.0h, the central control o-fluoronitrobenzene disappears, and the reaction is completed, and the reaction solution is slowly poured into ice Water (1.41kg), extracted with toluene (280ml*3), organic solvent, washed with water and layered, dried and concentrated to obtain 209g of crude compound B, yield 105.9%, HPLC area normalization method 98.3%.
Embodiment 2
[0021] Add DMF (564.4g), 60% sodium hydride (48g, 1.2moL) and o-fluoronitrobenzene (141.1g, 1.0moL) to the reaction flask equipped with liquid caustic soda tail gas absorption device, and the ice-salt bath is reduced to 0 Below ℃, add isopropyl mercaptan (99g, 1.3moL) dropwise, return to 25℃ and react for 2.0~3.0h, the central control o-fluoronitrobenzene disappears, and the reaction is completed, slowly pour the reaction solution into ice water (1.41kg), extracted with ethyl acetate (280ml*3), organic solvent, washed with water and layered, dried and concentrated to obtain 212g of crude compound B, yield 107.4%, HPLC area normalization method 98.5%.
Embodiment 3
[0023] In the reaction bottle that is equipped with liquid caustic soda tail gas absorption device, add DMF (564.4g), 60% sodium hydride (60g, 1.5moL), o-fluoronitrobenzene (141.1g, 1.0moL), ice-salt bath drops to 0 Below ℃, add isopropyl mercaptan (114.2g, 1.5moL) dropwise. After the dropwise addition is completed, return to 25℃ and react for 2.0~3.0h. Water (1.41kg), extracted with dichloromethane (280ml*3), organic solvent, washed with water to separate layers, dried and concentrated to obtain 210g of crude compound B, yield 106.4%, HPLC area normalization method 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com