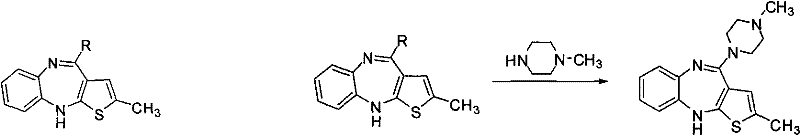
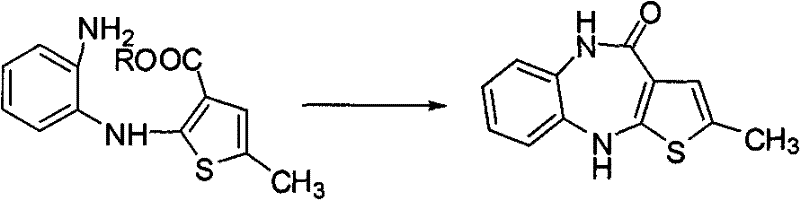
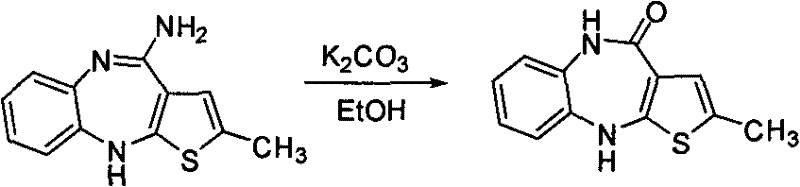Preparation method of antipsychotic drug olanzapine
An antipsychotic, olanzapine technology, applied in organic chemistry and other directions, can solve the problems of high production cycle and cost, complex process and post-processing, and high requirements for reaction conditions, and achieve cost reduction, high product yield, and post-processing. Handling convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Malononitrile (66.1g, 1.0mol), sulfur (32.0g, 1.0mol), triethylamine (10.1g, 0.1mol) and DMF (200mL) were placed in a 1000mL reaction flask, and propionaldehyde ( 61.0g, 1.05mol) and DMF (100mL) solution, dripping over 1h. After dropping, keep warm at 40-50°C for 3 hours. The reaction solution was poured into ice water (1000 mL), stirred for 30 min, filtered and washed with water. The obtained crude product was recrystallized by ethanol-water system to obtain pure compound 9 (60.7 g, 0.439 mol), with a yield of 43.9%.
Embodiment 2
[0068] Malononitrile (66.1g, 1.0mol), sulfur (32.0g, 1.0mol), triethylamine (10.1g, 0.1mol) and THF (200mL) were placed in a 1000mL reaction flask, and propionaldehyde ( 61.0g, 1.05mol) and THF (100mL) solution, dripping over 1h. After dropping, keep warm at 40-50°C for 3 hours. The reaction solution was poured into ice water (1000 mL), stirred for 30 min, filtered and washed with water. The obtained crude product was recrystallized from ethanol-water system to obtain pure compound 9 (55.4 g, 0.401 mol), with a yield of 40.1%.
Embodiment 3
[0070] Malononitrile (66.1g, 1.0mol), sulfur (32.0g, 1.0mol), triethylamine (10.1g, 0.1mol) and DMSO (200mL) were placed in a 1000mL reaction flask, and propionaldehyde ( 61.0g, 1.05mol) and DMSO (100mL) solution, dripping over 1h. After dropping, keep warm at 40-50°C for 3 hours. The reaction solution was poured into ice water (1000 mL), stirred for 30 min, filtered and washed with water. The obtained crude product was recrystallized from ethanol-water system to obtain pure compound 9 (52.0 g, 0.376 mol), with a yield of 37.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com