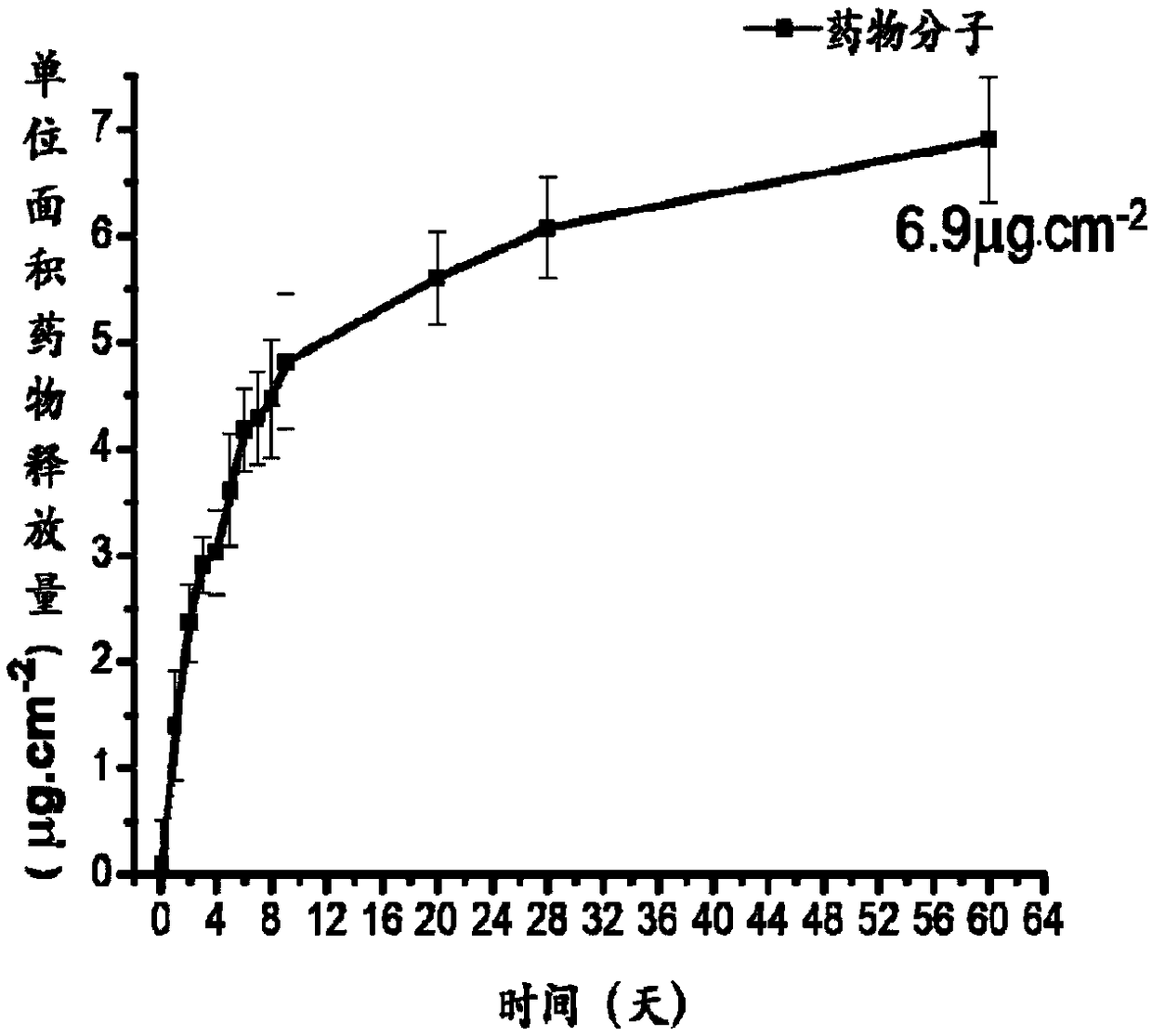
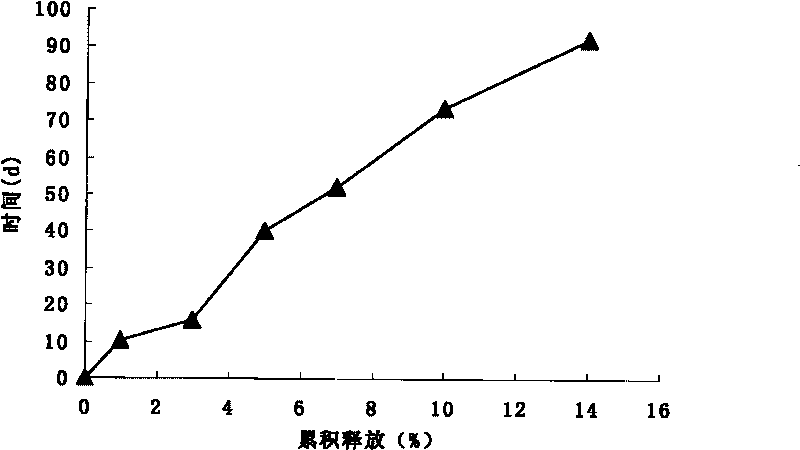
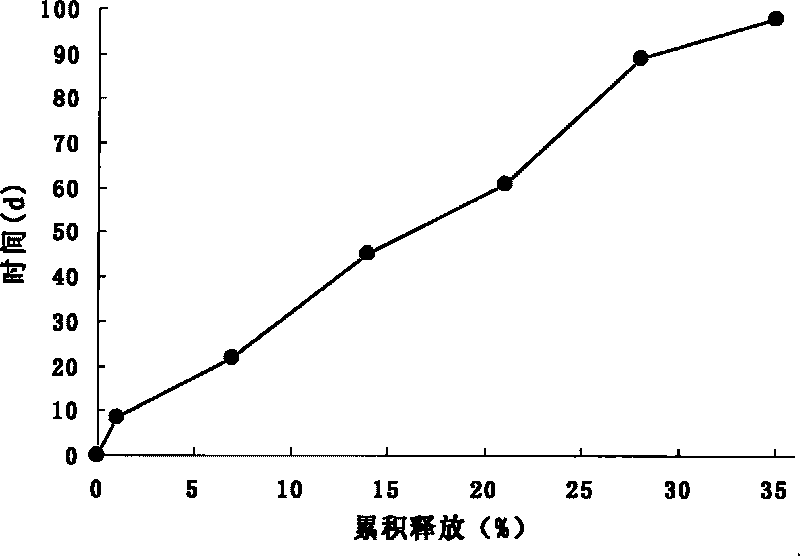
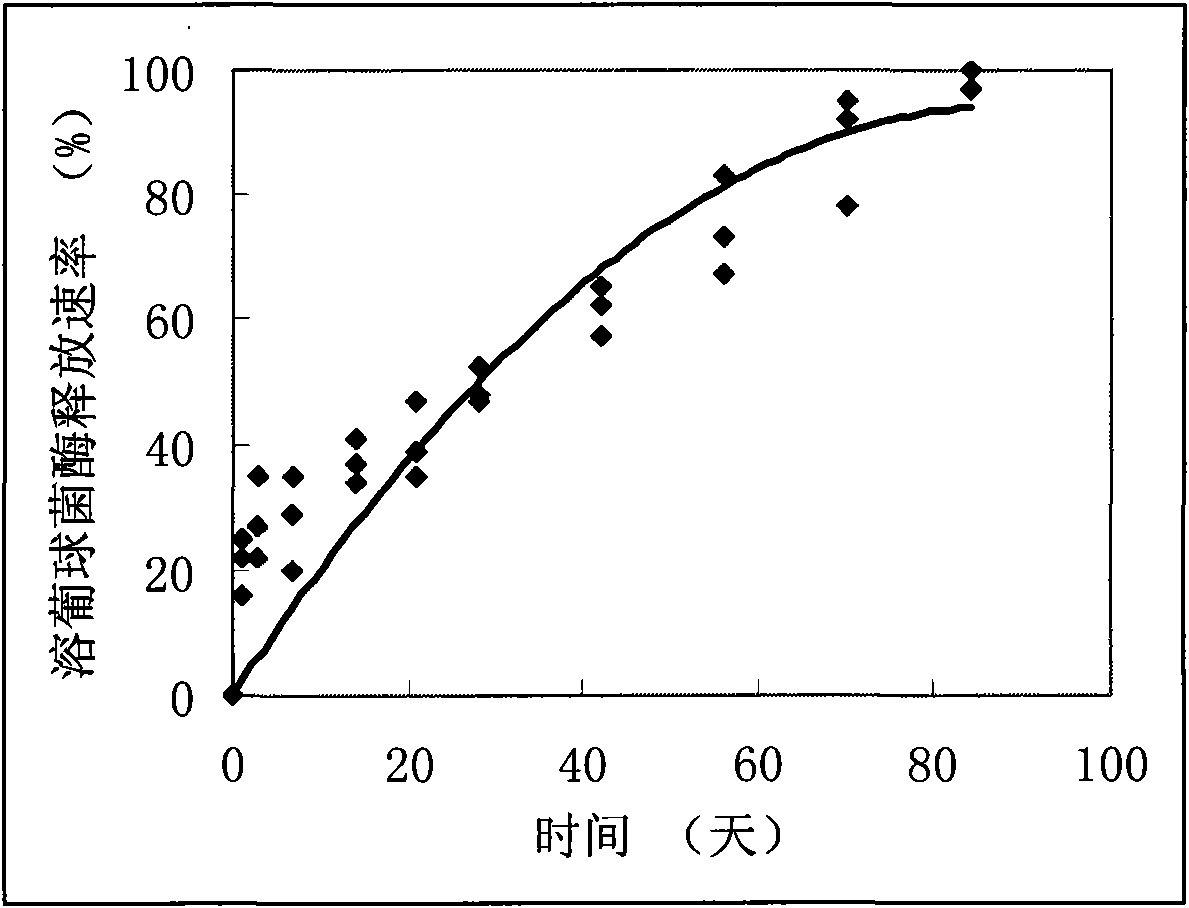
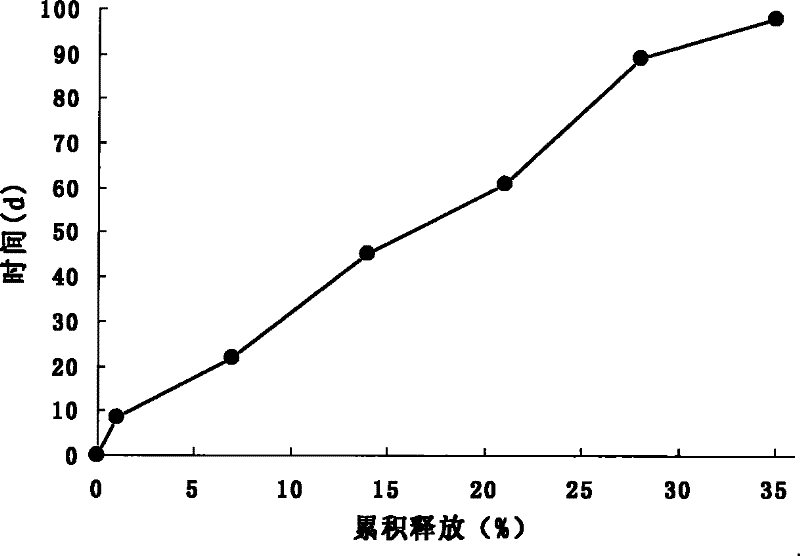
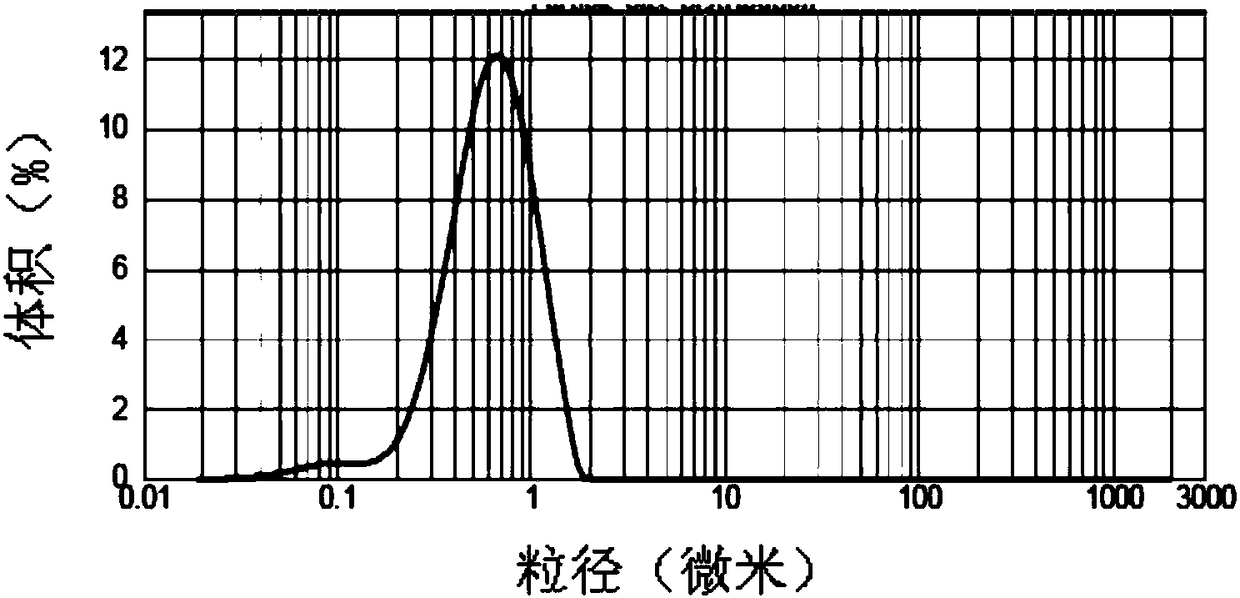
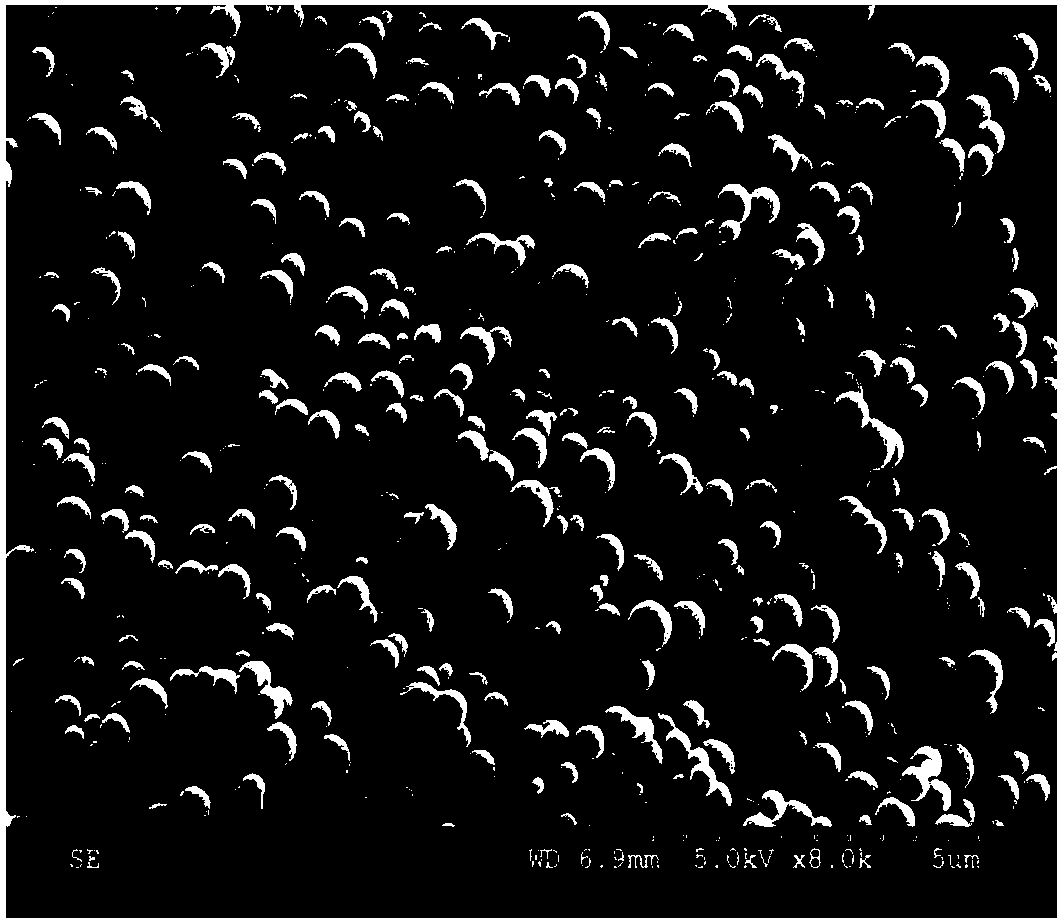
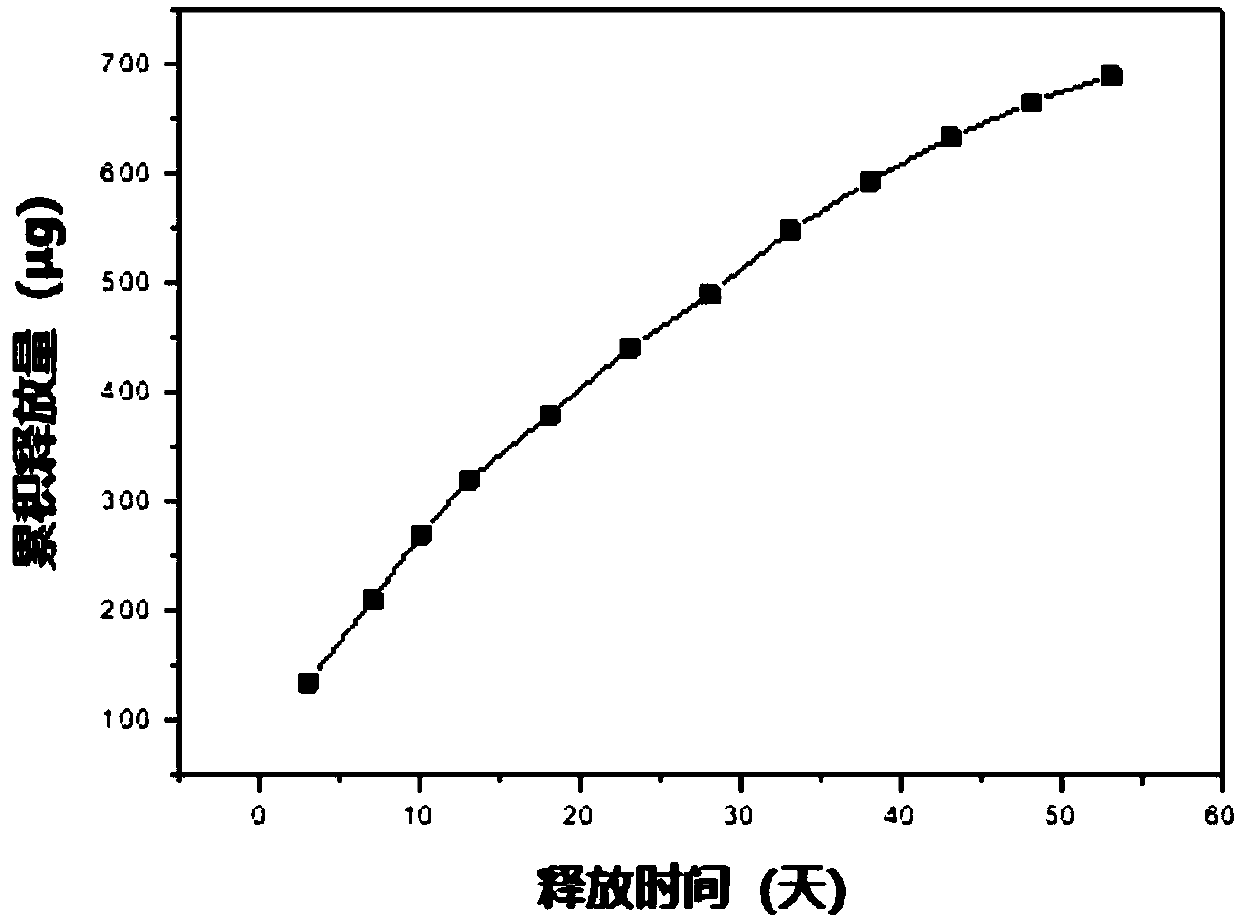
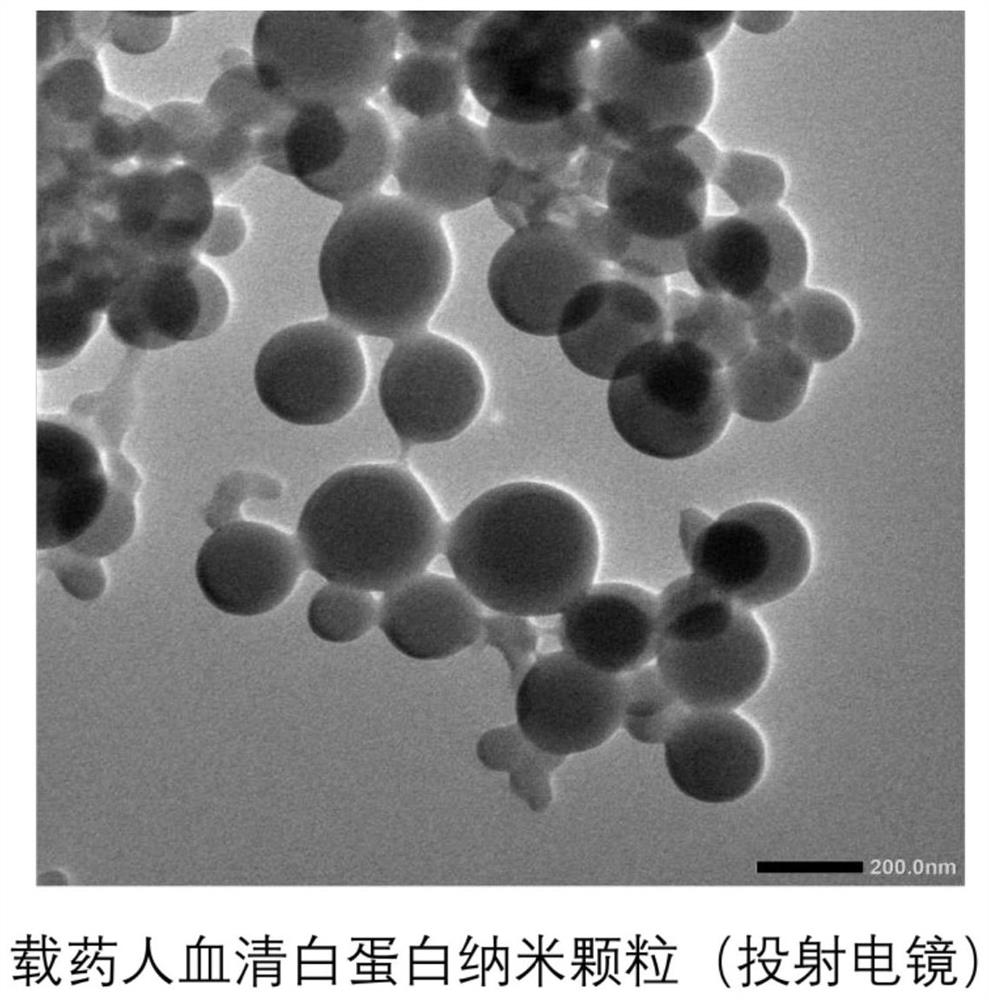
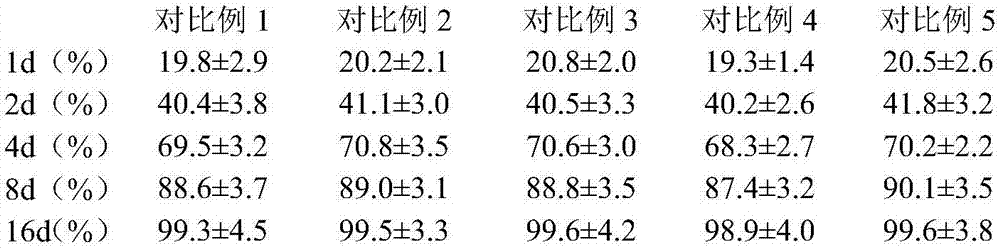
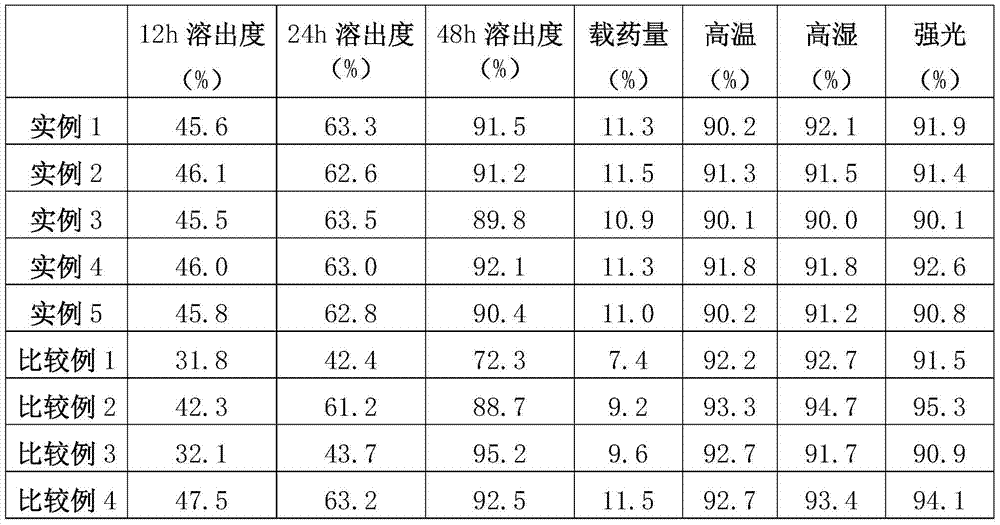
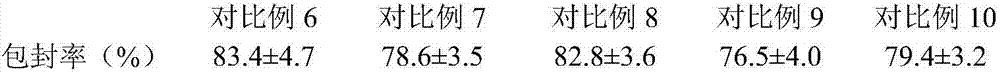Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Long acting release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multi-drug controllable-loading and long-acting slow-released biomedical coating material and preparation method thereof
ActiveCN108815552AControl loading typeControl releaseSurgical adhesivesAbsorbent padsLayer by layer self assemblyDopamine
The invention provides a multi-drug controllable-loading and long-acting slow-released biomedical coating material and a preparation method thereof. A preparation process comprises the following steps: polishing, washing and drying a substrate material, soaking in dopamine solution (a negative charge layer), and using a catechol-modified multi-amino biomacromolecule (with a positive charge), electronegative macromolecular solution (with a negative charge), and micelle (with a negative charge) loading multiple drugs as three components of layer-by-layer self-assembly, and preparing a coating modified material on a dopamine-processed substrate by using the above three components through a layer-by-layer self-assembly method. Multiple assembling layers can be repeatedly coated, and multiple drug molecules are largely and orderly fixed on a self-assembling coating, so that controllable long-acting release of a drug is realized.
Owner:SICHUAN UNIV
Galanthamine long-acting release injectable microsphere composite and preparation method thereof
The invention discloses galanthamine microspheres which contain 5-40wt% of basic group of galanthamine and 60-95wt % of polylactic acid / hydroxyacetic acid copolymer. The invention also provides a galanthamine long-acting release injectable microsphere composite which comprises the following components by weight percent: 60-90% of galanthamine microspheres, 5-25% of frozen dry proppant, 0.5-5% of suspending agent, 0.5-5% of wetting agent and 1-25% of osmotic pressure regulator. The invention combines the vibration nozzle method with the emulsion process (O / W)-solvent evaporation method so that the method has the advantage that the droplet-generating speed is fast, the efficiency is high, the containers used in preparation are easy to use in the aseptic operation, the production process can be carried out continuously, the technology is applicable to scale-up application, etc.
Owner:SHANDONG NEWTIME PHARMA
Controlled Long Acting Release Pharmaceutical Preparation For Use In The Oral Cavity
InactiveUS20080085248A1Convenient treatmentSimple treatmentCosmetic preparationsOrganic active ingredientsDentistryPharmaceutical formulation
The present invention relates to a controlled long acting release pharmaceutical preparation for use in the oral cavity either for the treatment of diseases of the oral cavity or; for releasing said pharmaceutical preparation into said oral cavity. Said pharmaceutical preparation comprises at least a therapeutic agent, a binder and a lubricant (hereinafter “the preparation” or “the pharmaceutical preparation”).
Owner:CALCIDENT ACTIVE
Local sustained release preparation for preventing and treating osteomyelitis, preparation method and application thereof
InactiveCN101618209AImprove the bactericidal effectImprove release abilityAntibacterial agentsPeptide/protein ingredientsMicrospherePhosphoric acid
The invention discloses a local sustained release preparation for preventing and treating osteomyelitis, a preparation method and application thereof. The preparation comprises the following components in percentage by weight: 0.01 to 1 percent of staphylococcus lysozyme, 1 to 20 percent of polylactic acid (PLA), 0.5 to 10 percent of polylactide-co-glycolide (PLGA), and 70 to 90 percent of calcium phosphate cement (CPC). The preparation takes the staphylococcus lysozyme as a bactericidal active component to obtain PLA-PLGA polymer microspheres containing the staphylococcus lysozyme through supercritical fluid microparticle preparation technology, and the PLA-PLGA polymer microspheres are compounded with the calcium phosphate cement (CPC) to obtain a novel sustained release degradable antibacterial composite artificial bone carrying staphylococcus lysozyme microspheres. The preparation has good physical and chemical properties and strong sterilization effect, ensures that the in vitro medicament release process is long up to three months, has steady release rate, and can maintain steady and long-acting release at administration positions.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
Resveratrol composite membrane agent and preparation method thereof
The invention relates to a resveratrol composite film agent. The film agent takes L-polylactic acid and lignin-grafted D-polylactic acid copolymer as a main film forming material; by utilizing a solution blending method and simultaneously introducing resveratrol, the composite film agent with good mechanical property, thermal stability, UV resistance, biocompatibility and drug slow release is prepared in a compounding manner. On one hand, a stereocomplex formed by L-polylactic acid and D-polylactic acid molecular chains can significantly improve the compatibility of the polylactic acid with the lignin and improve the mechanical properties of the lactic acids; on the other hand, the lignin and resveratrol have a similar polyphenol structure and a certain ultraviolet resistance, so that thestability of the resveratrol can be improved, and the long-acting release is realized.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
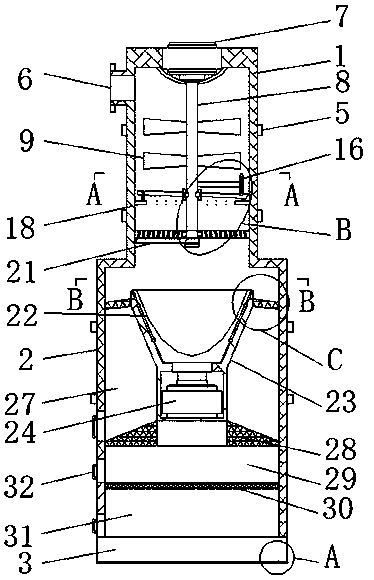
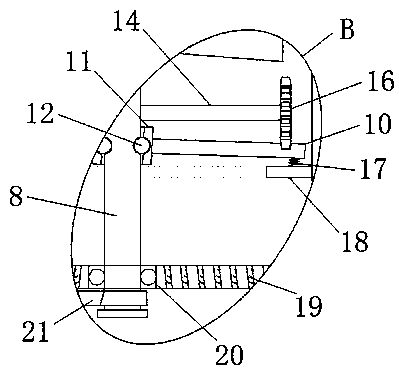
Long-acting release control fertilizer granulation device with screening function for cultivation of onobrychis viciaefolia
ActiveCN109395659AConducive to long-term controlled release useStable deliverySievingScreeningControlled releaseFixed frame
The invention discloses a long-acting release control fertilizer granulation device with a screening function for cultivation of onobrychis viciaefolia. The long-acting release control fertilizer granulation device comprises a cutting separating box, a first motor, a material distributing plate, a granulation hopper and a leaking plate; and a granulation storage box is mounted on the lower part ofthe cutting separating box, partition plates and fixing rods are mounted on the lower parts of stirring rods correspondingly, the material distributing plate is mounted on the lower part of a bearingplate, the granulation hopper is located in the middle of the top of the granulation storage box, a second motor is mounted on the bottom side in a fixing frame, the leaking plate is fixed to the outer side of the top of the fixing frame, and a filter plate is fixed to the bottom of a second accommodating cavity in a welded mode. According to the long-acting release control fertilizer granulationdevice with the screening function for cultivation of the onobrychis viciaefolia, the discharging speed can be adjusted, synchronous adjusting is conducted conveniently, complete conveying of materials can be ensured, fertilizer grains can be sieved, fine and small particles in fertilizer are screened out advantageously, and long-term controlled release using of the release control fertilizer isfacilitated.
Owner:GUANGDONG OCEAN UNIVERSITY
Galanthamine long-acting release injectable microsphere composite and preparation method thereof
The invention discloses galanthamine microspheres which contain 5-40wt% of basic group of galanthamine and 60-95wt % of polylactic acid / hydroxyacetic acid copolymer. The invention also provides a galanthamine long-acting release injectable microsphere composite which comprises the following components by weight percent: 60-90% of galanthamine microspheres, 5-25% of frozen dry proppant, 0.5-5% of suspending agent, 0.5-5% of wetting agent and 1-25% of osmotic pressure regulator. The invention combines the vibration nozzle method with the emulsion process (O / W)-solvent evaporation method so thatthe method has the advantage that the droplet-generating speed is fast, the efficiency is high, the containers used in preparation are easy to use in the aseptic operation, the production process canbe carried out continuously, the technology is applicable to scale-up application, etc.
Owner:SHANDONG NEWTIME PHARMA
Microencapsulated multivesicular liposome drug carrier and preparation method thereof
ActiveCN104622847AReduced release rateAchieving co-releasePharmaceutical non-active ingredientsMicrocapsulesSide effectCationic polymerization
The invention provides a microencapsulated multivesicular liposome drug carrier and a preparation method thereof. The method comprises the following steps: with a sucrose solution as an inner water phase, glucose and an L-lysine solution as outer water phases, and a dichloromethane solution dissolved with lipid as an oil phase, carrying out high-speed homogenate emulsification on the inner water phase and the oil phase to form colostrums; adding the outer water phase, carrying out vortex emulsification to form a compound emulsion, carrying out rotary evaporation to remove dichloromethane, so as to obtain a multivesicular liposome suspension; dispersing the multivesicular liposome into a sodium alginate solution, dispersing uniformly, and then dropping into a calcium chloride solution through a high-pressure microcapsule molding device and an injection pump, so as to prepare calcium alginate gel beads embedded with the multivesicular liposome; and finally forming a film from the gel beads and a cationic polymer solution, so as to form a sample, wherein the drug is carried on at least one part in the multivesicular liposome or out of the multivesicular liposome in the microcapsule. According to the microencapsulated multivesicular liposome drug carrier, space allocation of different drugs is ensured; the long-acting release and absorption of the drug are improved; and toxic and side effects caused by simple use of the drug are reduced.
Owner:HUAQIAO UNIVERSITY
Tower-type melt granulation bi-directional regulation compound fertilizer and preparation method thereof
InactiveCN107311778AReliefGood dispersionAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersDispersitySlurry
The invention discloses tower-type melt granulation bi-directional regulation compound fertilizer and a preparation method thereof and belongs to the technical field of fertilizer. The fertilizer is prepared from a fertilizer core, a first-layer encapsulating agent and a second-layer encapsulating agent, wherein the mass ratio of the fertilizer core to the first-layer encapsulating agent to the second-layer encapsulating agent is 100 to 3 to 5. The preparation method comprises the following steps: mixing urea formaldehyde with other organic and inorganic fertilizer components, and granulating; then carrying out encapsulating treatment on the surface of a mixture for two times to obtain a finished product. The tower-type melt granulation bi-directional control compound fertilizer has rapidly-released nutrients and long-acting release nutrients, and the nutrients are slow and assisted in release; meanwhile, modified nanometer titania is added in the tower-type melt granulation bi-directional control compound fertilizer to serve as a raw material, so that the dispersity of slurry in the granulation process can be improved, the granulation uniformity is enhanced, and the nutrients are released more uniformly; besides, the components also can be used for effectively absorbing heavy metal ions in soil, so that the planting safety of crops is improved.
Owner:史丹利化肥扶余有限公司
NSAID sustained-release nanoparticles and preparation method thereof
InactiveCN108451933AGood slow releaseQuick effectOrganic active ingredientsAntipyreticActive componentBiocompatibility Testing
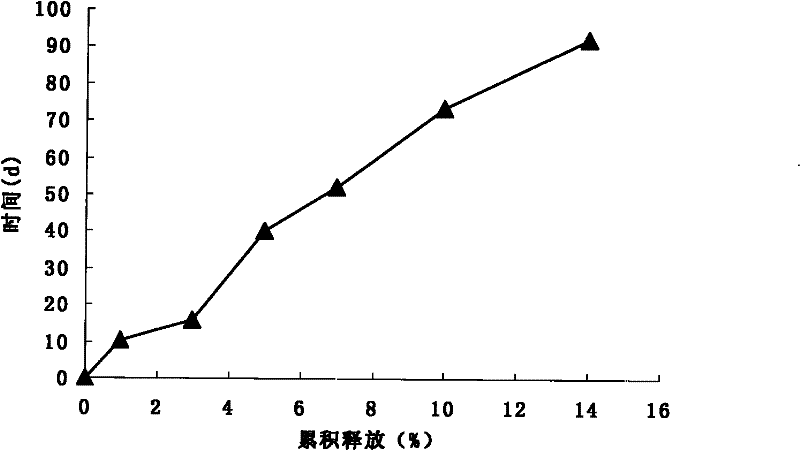
The invention discloses nonsteroidal anti-inflammatory drug (NSAID) sustained-release nanoparticles and a preparation method thereof, wherein the NSAID sustained-release nanoparticles contain an active component and a carrier, wherein the active component is NSAID, the carrier is polylactic acid polyglycolic acid copolymer (PLGA) nanoparticles, and the active component is loaded in the carrier. According to the present invention, the NSAID sustained-release nanoparticles have good biocompatibility and good slow-release effect, wherein the long-acting release time can reach 15-50 d.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Middle ear anti-adhesion medicine slow-release system and preparation method and application thereof
ActiveCN109568298AFacilitated releaseLong releaseOrganic active ingredientsSenses disorderMass ratioMiddle ear
The invention relates to a middle ear anti-adhesion medicine slow-release system and a preparation method and application thereof. The middle ear anti-adhesion medicine slow-release system comprises adiaphragm, a coating covering the diaphragm and medicines, wherein the medicines are loaded onto the diaphragm and the coating. The middle ear anti-adhesion medicine slow-release system has a structure of the internal diaphragm and the external coating, and both the diaphragm and the coating are loaded with the medicines so that the medicines can be released fast, and long-acting release can be achieved; by controlling intrinsic viscosity of diaphragm preparation raw materials and coating preparation raw materials and controlling a mass ratio of the medicines contained in the diaphragm and the coating, a medicine release curve can be made to be more smooth, and a medicine release process can be made to be more stable.
Owner:PUYI (SHANGHAI) BIOTECHNOLOGY CO LTD
Paracetamol and caffeine sustained release preparation and preparation method thereof
ActiveCN103110638ASmooth releaseSmall fluctuations in blood concentrationAntipyreticAnalgesicsBULK ACTIVE INGREDIENTActive ingredient
The invention discloses a paracetamol and caffeine sustained release preparation and a preparation method thereof. The paracetamol and caffeine sustained release preparation comprises active ingredients, a slow-release substrate matrix, a filler, a surfactant, a lubricant and an additive, wherein the active ingredients consist of paracetamol and caffeine in ratio by weight of 1:0.13. The paracetamol and caffeine sustained release preparation disclosed by the invention is capable of realizing the long-acting release and stably and regularly releasing the active ingredients within 24 hours.
Owner:CP PHARMA QINGDAO CO LTD
Attractant for stored product pest stegobium paniceum and preparation method thereof
The invention relates to a pest control technology, in particular to an attractant for a stored product pest stegobium paniceum and a preparation method thereof. The attractant is prepared by utilizing a radix notoginseng extract, an orange blossom extract, a lemon peel extract, a mint leaf extract, betaine, sodium alginate, guar gum, modified silicone and water. The attractant disclosed by the invention has the characteristics of small toxic and side effects, small residual quantity, efficient trapping and killing, long-acting release and high broad-spectrum property. The attractant disclosedby the invention is selected from natural components, is small in toxic and side effects and has strong attracting action on stored product pests such as the stegobium paniceum, tribolium castaneum,oryzaephilus surinamensis linne and sitophilus oryzae.
Owner:GUIYANG UNIV
Sustained release trepostinil-compound microparticle compositions
PendingUS20210022994A1Convenient treatmentPowder deliveryOrganic active ingredientsTreprostinilPulmonary artery
Provided herein are new compositions comprising novel microparticles that are configured to provide a long acting release of one or more treprostinil compounds when administered to mammalian subjects. The microparticles of the invention are biocompatible and typically injectable through a needle or other injection system. The invention also provides methods of using such compositions, such as in the treatment of pulmonary arterial hypertension.
Owner:LUPIN HLDG BV
Novel agricultural fertilizer
The invention relates to a novel agricultural fertilizer, and relates to an agricultural fertilizer field. The novel agricultural fertilizer is composed of the following components: bentonite, humic acid, microbial agents, urea, calcium superphosphate, potassium nitrate and trace elements. The novel agricultural fertilizer has an effect of slow and long-acting release of nutrients, the loss rate of nutrient elements in soil is lowered, the absorption utilization rate of crops is raised, multi-time fertilizer addition is not needed, and the economic benefit of crops is raised. The ratio of nutrient elements of nitrogen, phosphor and potassium and trace elements is reasonable, nutrition balance is achieved, and nutrients required by crop growth are met. The microbial agents improve the soil structure, the conversion speed of organic and inorganic fertilizers in soil is accelerated, the capability of absorbing nutrition components of crops is raised effectively, fertilizer waste is reduced, and soil hardening and desertification and environment pollution caused by application of excess inorganic fertilizer are avoided.
Owner:杨文昊
Ketoconazole gel and preparation method thereof
ActiveCN102525885AImprove stabilitySensitive to temperatureAntibacterial agentsOrganic active ingredientsPatient complianceKetoconazole
The invention provides a ketoconazole gel and a preparation method thereof. The ketoconazole gel is used for treating vulval and vaginal infection and vaginal mixed infection which are caused by bacteria, trichomonad and candida albicans. The ketoconazole gel mainly consists of ketoconazole, hydroxypropyl-beta-cyclodextrin, carbomer, chitosan and poloxamer, improves stability of the ketoconazole and quanlity, does not cause drug resistance phenomena, and is high in safety and suitable for treatment of vaginal infection of women in the gestation period. The ketoconazole gel is characterized by having temperature susceptibility and biological adhesion and slowly performing long-acting release so that the ketoconazole gel has the advantages of facilitating use of patients and being high in patient compliance, convenient to take, safe, hygienic and the like. The quality standard is improved comprehensively, pharmacy variety is enriched after dosage form is changed, and the ketoconazole gel and the preparation method have great significant meaning for further meeting and guaranteeing pharmacy requirements of users.
Owner:BEIJING ACTING PHARMA BIOTECH
Slow-release nitrogen fertilizer granule and preparation method thereof
InactiveCN109438070ASlow release rateLong release periodCalcareous fertilisersAmmonium salt fertilisersCross-linkFilm-forming agent
The invention provides a slow-release nitrogen fertilizer granule and a preparation method thereof. The slow-release nitrogen fertilizer granule comprises a nitrogen fertilizer granule layer, a slow-release layer and a coating layer which are arranged from inside to outside, wherein the weight ratio of the components in the slow-release layer, namely the ratio of a nitrogen fertilizer to a cross-linking agent to a film-forming agent to a proportion regulator is 1:0.5-5:0.2-2:20-100, and the coating layer is a polyurethane coating. According to the slow-release nitrogen fertilizer granule and the preparation method thereof, two protective layers can be formed on the outer layer of a nitrogen fertilizer core, one is the slow-release layer, the other is the coating layer, the slow-release layer can help the nitrogen fertilizer to form the granule with the smooth surface and has the slow release effect, the proportion of the nitrogen fertilizer granule can further be changed, the coating layer can help to realize the long-acting release of the nitrogen fertilizer, and the utilization rate of the fertilizer is improved.
Owner:广西金盛农联农业科技有限公司
Preparation method of targeted controlled release arsenical medicine
InactiveCN104173378ASolve complexityFix stability issuesInorganic active ingredientsPharmaceutical non-active ingredientsSide effectBiocompatibility Testing
The invention discloses a preparation method of a targeted controlled release arsenical medicine. The preparation process comprises three steps: preparation of an arsenical medicine-loaded magnetic Fe3O4 core, coating of the magnetic arsenical medicine by nanosilicon dioxide and biocompatibility of the arsenical medicine. According to the preparation method disclosed by the invention, the arsenical medicine and porous magnetic Fe3O4 are coprecipitated and the arsenical medicine is adsorbed on the surface of the magnetic Fe3O4, so that the release speed is delayed. The arsenical medicine is coated by using porous nanosilicon dioxide so as to realize targeted long-acting release and controlled release of the arsenical medicine. Surface treatment is carried out on the arsenical medicine by using a polyvinylpyrrolidone macromolecular sol, so that the biocompatibility is improved. The preparation method disclosed by the invention can be used for solving the problem that an existing controlled release arsenical medicine preparation process is complex and unstable in release speed, and can be used for realizing targeted delivery, reducing the side effects of the medicine and improving the curative effect.
Owner:TIANJIN VOCATIONAL INST
Microcapsule aqueous suspension of herbicide sethoxydim and preparation method thereof
InactiveCN101427672BLong durationReduce usageBiocideAnimal repellantsBULK ACTIVE INGREDIENTActive ingredient
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Nitrogenous fertilizer
The invention relates to a nitrogenous fertilizer, and relates to the agricultural fertilizer field. The nitrogenous fertilizer is composed of the following ingredients: a fertilizer matrix, urea, ammonium chloride, bentonite, humic acid, microorganism bacteria, white stone powder and a nitrogen-fixing bacterium agent. The nitrogenous fertilizer has an effect of slow and long-acting release of nutrients, the loss rate of the nitrogen nutrient element in soil is lowered, the absorption utilization rate of crops is raised, the nitrogen amount required by the plant growth stage is met through combined action of ammonium chloride and urea, multi-time additional fertilization is not needed, resources are saved and investment is saved. The microorganism bacteria improve the soil structure and promote activity of soil microorganisms, thus soil is loose, the conversion rate of organic and inorganic fertilizers in soil is raised plant chlorophylls are increased, the plant photosynthesis is raised, and soil hardening and desertification and environment pollution caused by application of excess inorganic fertilizers are prevented.
Owner:杨文昊
A biomedical coating material with multi-drug controllable loading and long-acting sustained release and preparation method thereof
ActiveCN108815552BControl loading typeControl releaseSurgical adhesivesAbsorbent padsLayer by layer self assemblyDopamine
The invention provides a biomedical coating material with multi-drug controllable loading and long-acting sustained release and a preparation method thereof. The preparation process is as follows: the base material is polished, cleaned, dried, and then soaked in a dopamine solution (negatively charged layer), and then use catechol-modified polyamino biomacromolecules (positively charged), negatively charged macromolecular solutions (negatively charged), and micelles loaded with various drugs (negatively charged) as layer-by-layer self-assembly The above three components are used to prepare coating modified materials by layer-by-layer self-assembly method on the dopamine-treated substrate, which can be repeatedly coated with multiple assembly layers to achieve a large number of drug molecules and effective The sequence is immobilized on the self-assembled coating, thereby realizing the controlled long-term release of the drug.
Owner:SICHUAN UNIV
Preparation method of elemental sulfur based ammonium phosphate slow release fertilizer
InactiveCN108752097AControl release speedLong-term releaseAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersNitrogenWell control
The invention relates to the technical field of fertilizer processing, in particular to a preparation method of an elemental sulfur based ammonium phosphate slow release fertilizer. The preparation method comprises the following steps of preparation of sulfur sol, mixing, pelleting, coating and cleaning. The preparation method provided by the invention is simple in technology and safe to operate;a prepared coated elemental sulfur based ammonium phosphate product well controls the release rate of nitrogen, phosphorus and sulfur, realizes long-acting release of nutrient elements; and in addition, the contained sulfur and silicon components can promote absorption of other nutrient elements and improve the stress resistance growth of crops.
Owner:GUIYANG KAILIN FERTILIZER CO LTD +2
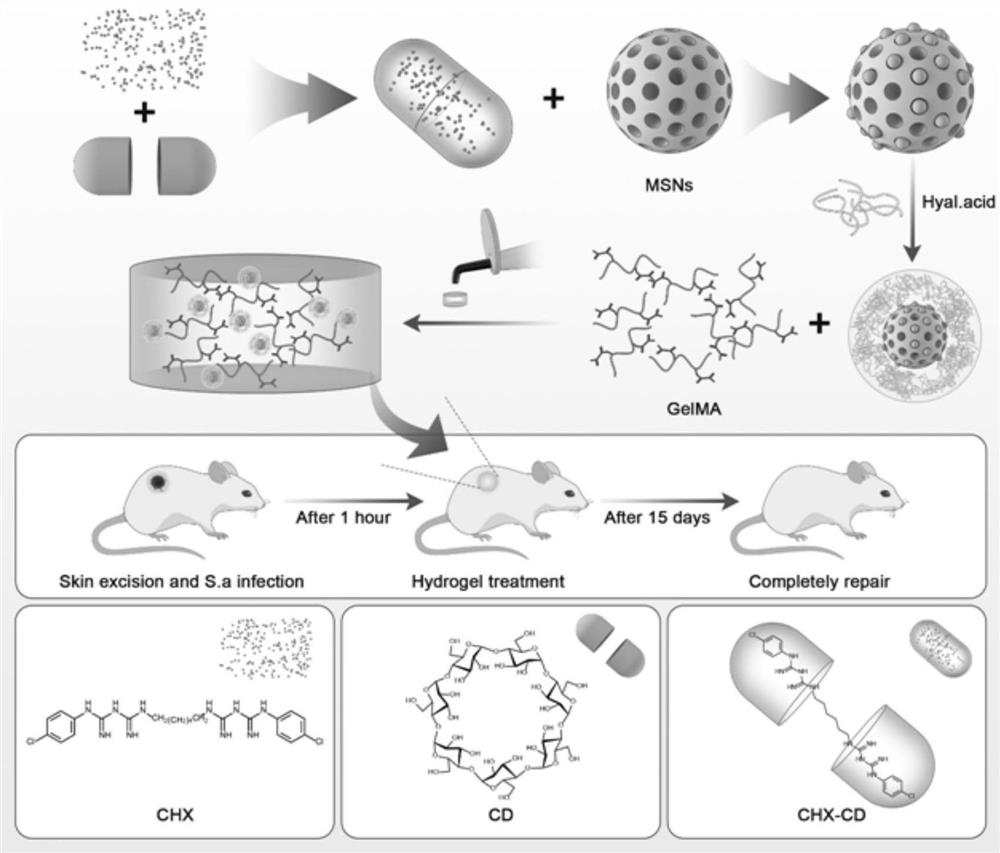
Ultra-long-acting controllable slow-release mesoporous-hyaluronic acid hybrid targeted antibacterial nano-material as well as preparation method and application thereof
ActiveCN113440503ARealization of ultra-long-acting controlled and sustained releaseAchieve pH-responsive releaseAntibacterial agentsOrganic active ingredientsNanoparticleDrug carrier
The invention provides an ultra-long-acting controllable slow-release mesoporous-hyaluronic acid hybrid targeted antibacterial nano-material. The nano-material comprises a drug carrier, an active drug and hyaluronic acid, wherein the drug carrier is mesoporous silica nanoparticles; the active drug is included in cyclodextrin to form an inclusion compound; the inclusion compound is loaded on the mesoporous silica nanoparticles; and the hyaluronic acid wraps the surfaces of the mesoporous silica nanoparticles loaded with the inclusion compound, so that the mesoporous-hyaluronic acid hybrid targeted antibacterial nanomaterial is formed. The invention also provides a preparation method and application of the ultra-long-acting controllable slow-release mesoporous-hyaluronic acid hybrid targeted antibacterial nano-material. According to the nano-material disclosed by the invention, the active drug can be subjected to dual slow release, the ultra-long-acting slow release of the active drug is realized, the pH response release of the drug can be realized, and the long-acting release of the active drug is controllable.
Owner:温州医科大学附属口腔医院
Long-acting and environmental-protection release agent and preparation method thereof
InactiveCN105175274AReduce production processLow cost of industrializationOrganic compound preparationAmino-carboxyl compound preparationAir atmosphereDecomposition
The invention discloses a long-acting and environmental-protection release agent and a preparation method thereof. The preparation method of the release agent comprises the following steps: carrying out shearing emulsification on Carbomer resin, polyethylene glycol and an aqueous solution of chloroacetic acid under the action of an anionic surfactant to form a stable chloroacetic acid emulsion system, and slowly adding hexylenediamine at normal temperature through adopting an emulsion synthesis process to prepare the release agent. The emulsion reaction obstructs molecular bumping rate of chloroacetic acid and hexylenediamine, so the reaction rate is alleviated, thereby the reaction is carried out in normal temperature air atmosphere; the release agent is a non-migratory long-acting release agent, can coordinate with iron and copper ions on the surface of a die through an association effect to form a hydrophilic and oil repellent firm micro-film on the surface of the die, so contact between an oil-soluble material and the die is reduced, the thickness of the film is not reduced, and the demolding frequency under high temperature conditions reaches 40 or above; and the release agent also has the advantages of high decomposition temperature, non-volatility and no influences on the quality of performances of a finished product.
Owner:SHAANXI NORMAL UNIV
Dual-protein delivery carrier programmed drug delivery system and preparation method thereof
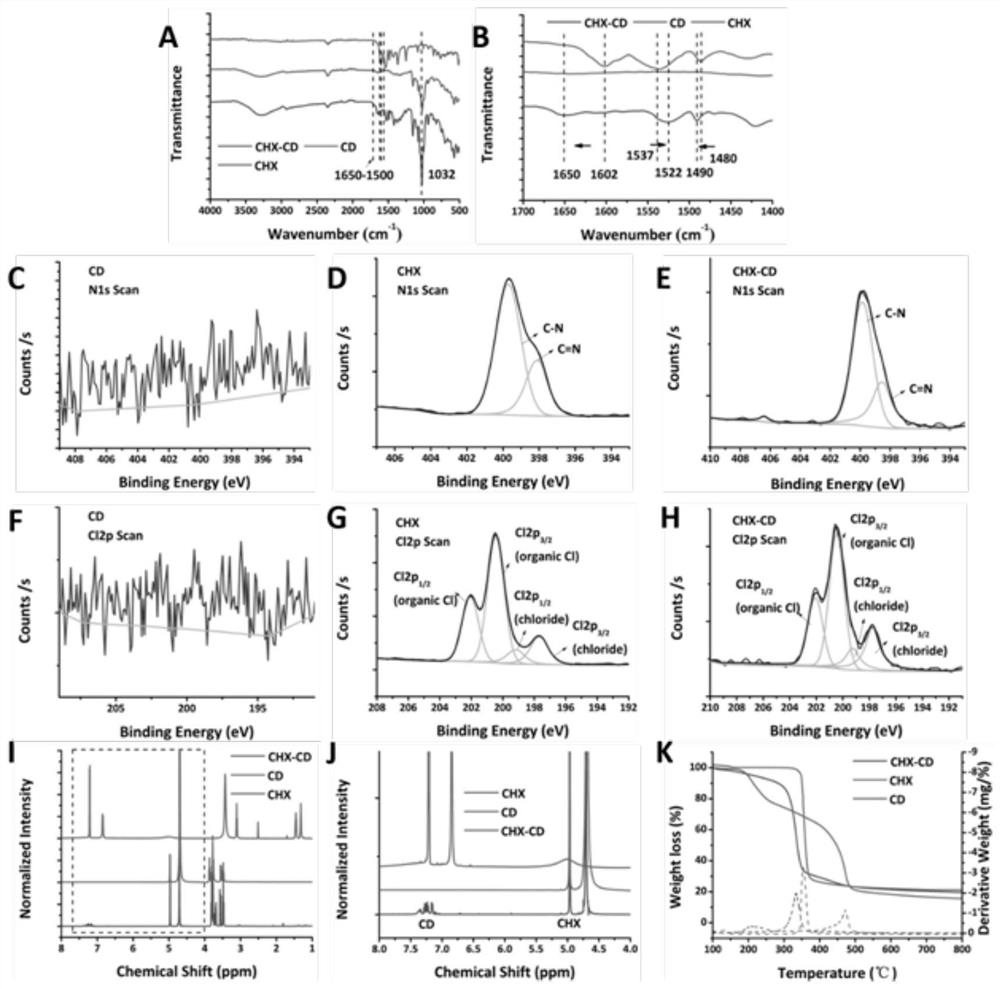
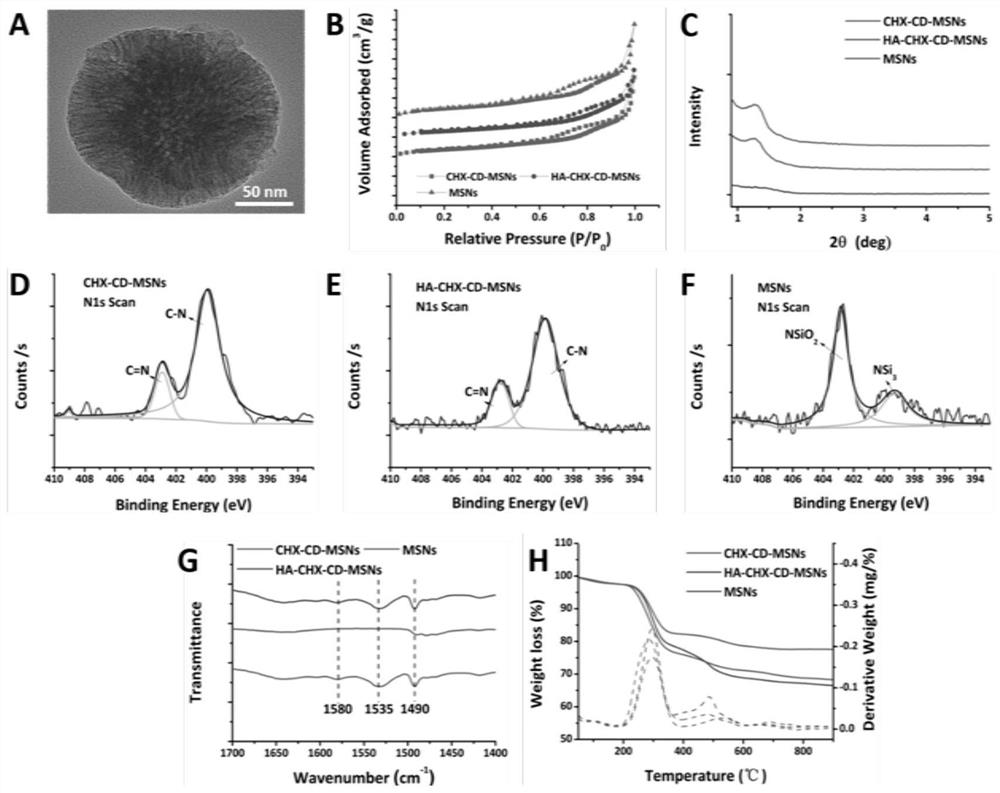
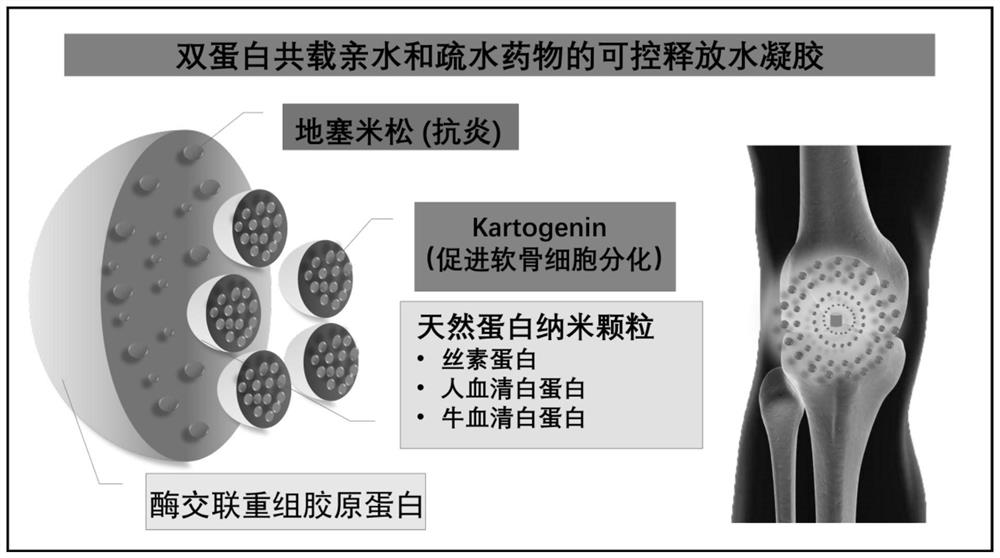
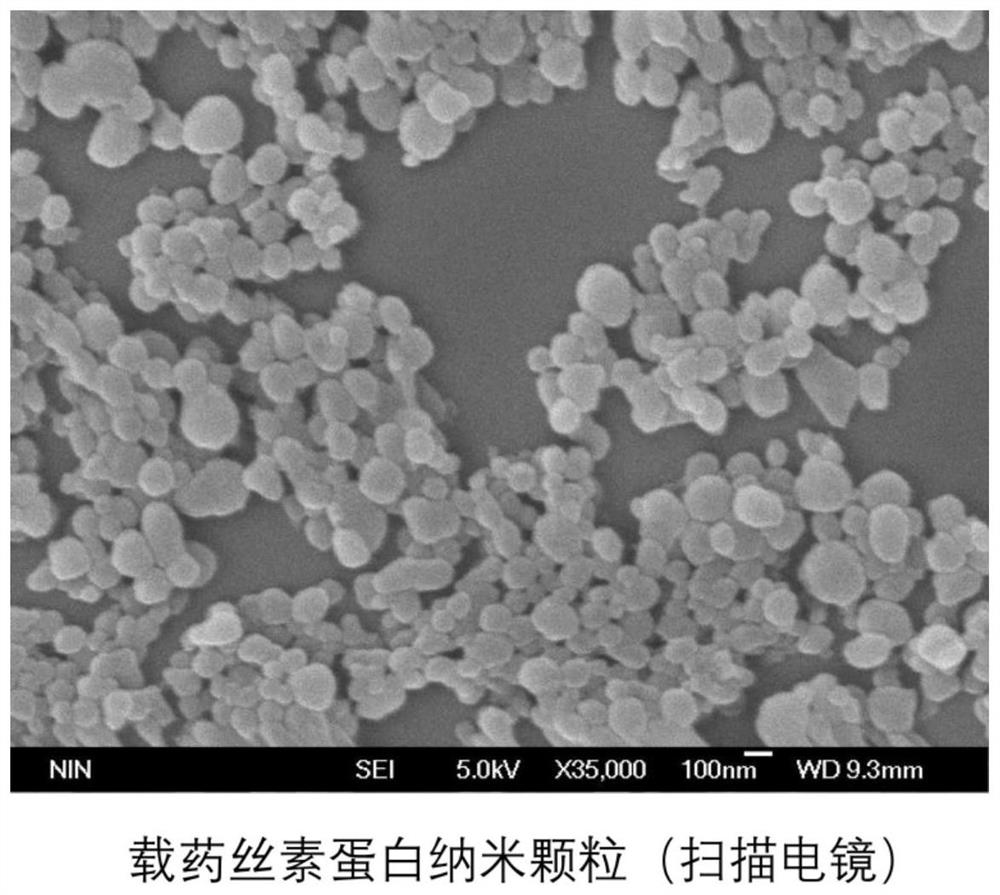
PendingCN114652856AAchieve ultra-long-lasting releaseRealize the procedural release of the systemPowder deliveryOrganic active ingredientsNanoparticlePharmaceutical Substances
The invention discloses a dual-protein delivery carrier programmed drug delivery system and a preparation method thereof. The dual-protein delivery carrier programmed drug delivery system comprises nanoparticles of a first protein, a first drug and hydrogel of a second protein, wherein the nanoparticles of the first protein are dispersed in the hydrogel of the second protein and are covalently linked with the second protein, and the first drug is loaded on the nanoparticles of the first protein. The dual-protein delivery carrier programmed drug delivery system provided by the invention is a multi-drug co-delivery system, and can realize co-system programmed release of hydrophilic drugs and hydrophobic drugs and ultra-long-acting release of the hydrophobic drugs; the invention is especially suitable for the situation that the first drug is a hydrophobic drug and is administered in a sustained-release manner, and the second drug is a hydrophilic drug and is administered in a quick-release manner.
Owner:NORTHWEST UNIV(CN)
Topiroxostat microspherical preparation and preparation method thereof
ActiveCN107441090AHigh encapsulation efficiencyUniform particle sizeOrganic active ingredientsSkeletal disorderLong actingChemistry
The invention relates to the technical field of medicines and in particular relates to a topiroxostat microspherical preparation and a preparation method thereof. According to the technical scheme, the topiroxostat microspherical preparation is characterized by comprising the following components in parts by weight: 10-20 parts of topiroxostat, 30-100 parts of a macromolecular carrier, 0-5 parts of an emulsifier and 0-10 parts of a topiroxostat supporting agent. The topiroxostat microspheres are high in encapsulation efficiency, uniform in grain size, stable in quality and simple in preparation process, and the administrating path of topiroxostat is increased, and the patient compliance is enhanced; and moreover, the topiroxostat microspherical preparation also has a long-acting releasing effect of topiroxostat and achieves an unexpected technical effect.
Owner:CP PHARMA QINGDAO CO LTD
Sustained-release coating of degradable medicaments coating bracket
InactiveCN101259295ASmooth releaseDuring release, the drug releases at a uniform rateStentsSurgerySide effectDrug-Coated Stents
The invention belongs to a drug release coating of a degradable drug-coated stent of coronary stents in the medicinal material field. The drug release coating is composed of drug for inhibiting thrombosis and tissue hyperplasia and a polymer carrier. The drug can be rapamycin, tacrolimus or paclitaxel, wherein, the polymer carrier in the drug release coating is poly ortho ester (POE). The invention is the drug release coating of the degradable drug-coated stent which is especially applied to coronary interventional operations. With the poly ortho ester used as the carrier, the stent can lead to the long-acting release of the drug coated on the surface of the stent, improve the stability of the drug, achieve more ideal effects of the drug release, prolong the physical activity of the drug and reduce the toxic and side effects of the drug, thereby preventing the occurrence of postoperative inflammation effectively.
Owner:天津市凯迪亚医疗器械有限公司
Propylene glycol marinate sulfate-containing sustained-release preparation and preparation method thereof
ActiveCN103099796BSmooth releaseSmall fluctuations in blood concentrationOrganic active ingredientsMetabolism disorderMedicineSulfate
The invention discloses a propylene glycol marinate sulfate sustained-release preparation and a preparation method thereof. The propylene glycol marinate sulfate sustained-release preparation comprises: propylene glycol marinate sulfate, a sustained-release skeleton matrix, a filler, a surfactant, a lubricant and a binder. The propylene glycol marinate sulfate sustained-release preparation disclosed in the invention can achieve long-acting release, and can release drug ingredients stably and evenly within 24h.
Owner:CP PHARMA QINGDAO CO LTD
A kind of nifedipine long-acting sustained-release pellets and preparation method thereof
InactiveCN105055325BReduce aggregationReduce cakingOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsPolyethylene glycol
The invention provides a nifedipine long-acting sustained-release pellet, which comprises the following materials in parts by weight: 8-12 parts of nifedipine, 30-49 parts of welan gum, 5-10 parts of stearic acid, polyethylene glycol Diol 4000 15-20 parts, sorbitol 10-15 parts, lactose 5-10 parts, lubricant 2-5 parts. The preparation method is as follows: firstly, the nifedipine dispersion is prepared with polyethylene glycol and sorbitol as the carrier, then mixed evenly with other raw materials in the sustained-release pellets, added with ethanol aqueous solution to moisten to obtain a soft material, and then the The soft material is respectively prepared into a spherical product through an extrusion machine and a spheronizer, and dried at 45-50° C. for 20-30 hours to obtain sustained-release pellets. The preparation method of the invention is simple, easy to be prepared in large quantities, and the process reproducibility is good. The obtained long-acting sustained-release pills are uniform and stable, can effectively control the release speed of the medicine, and basically have no stimulating effect on the gastrointestinal mucosa.
Owner:BAOTOU MEDICAL COLLEGE OF INNER MONGOLIA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com