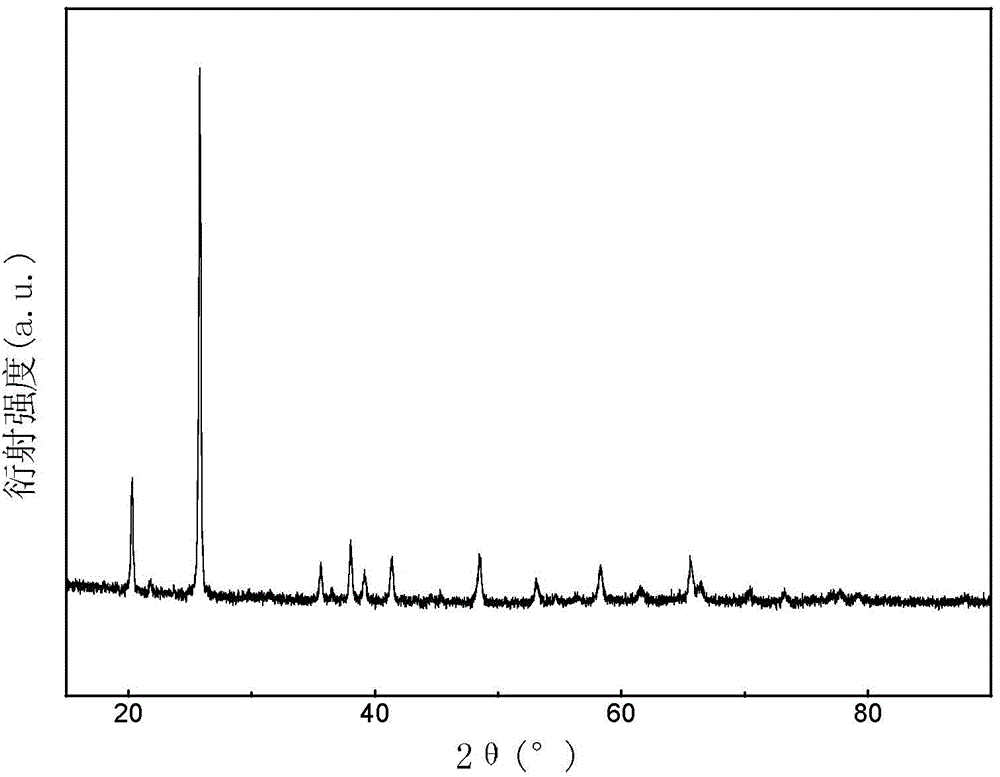
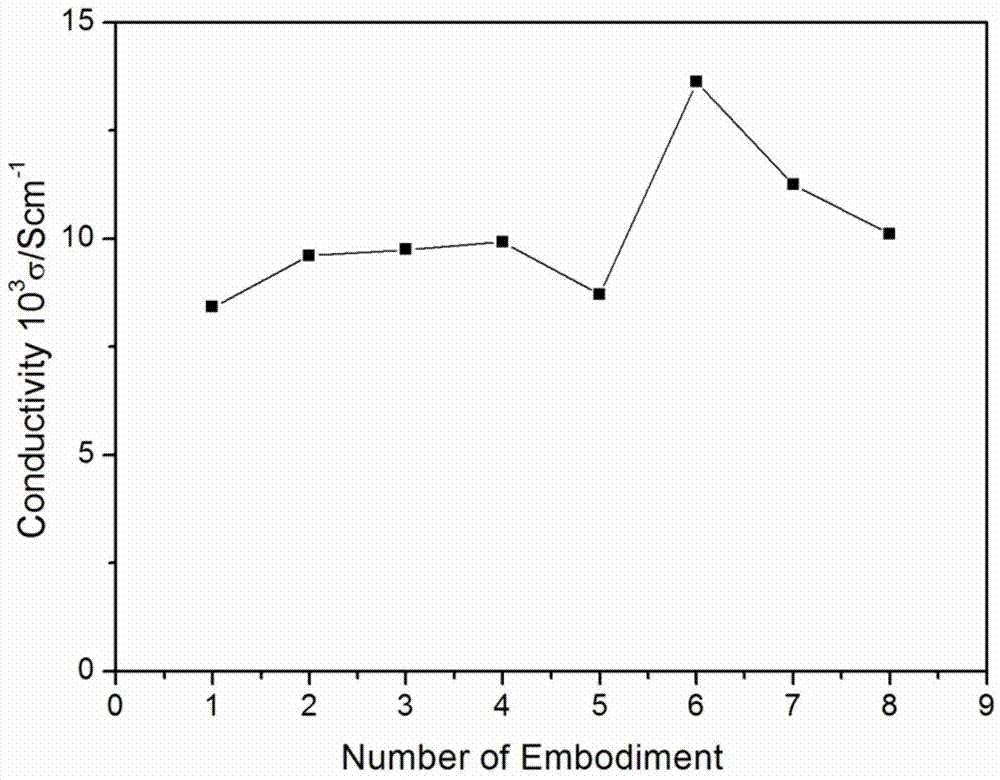
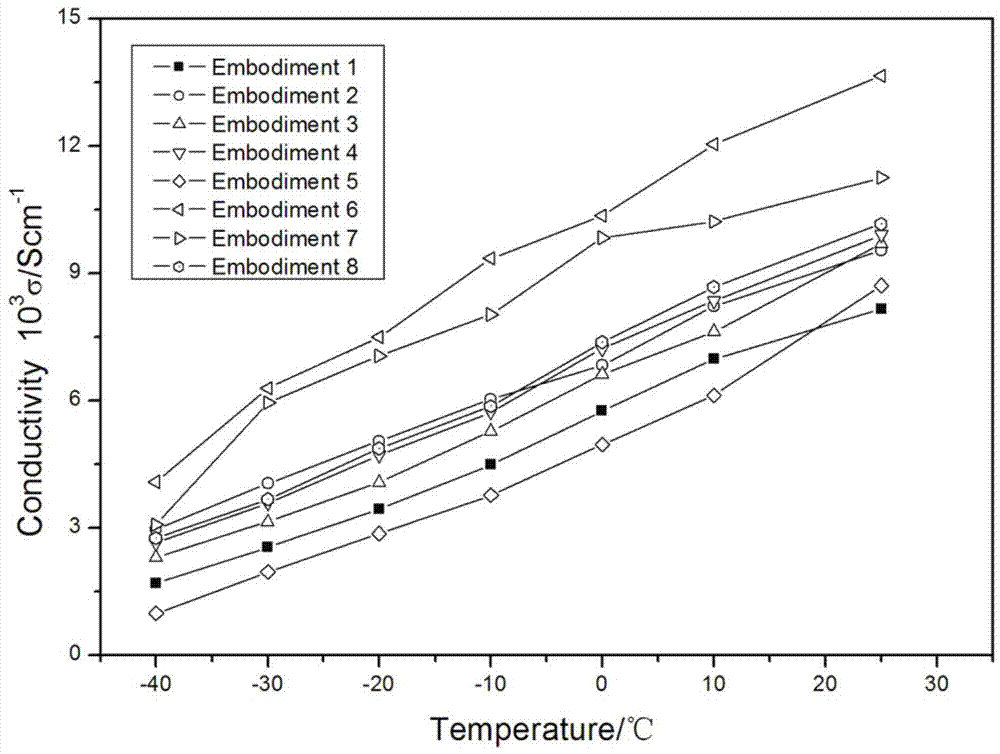
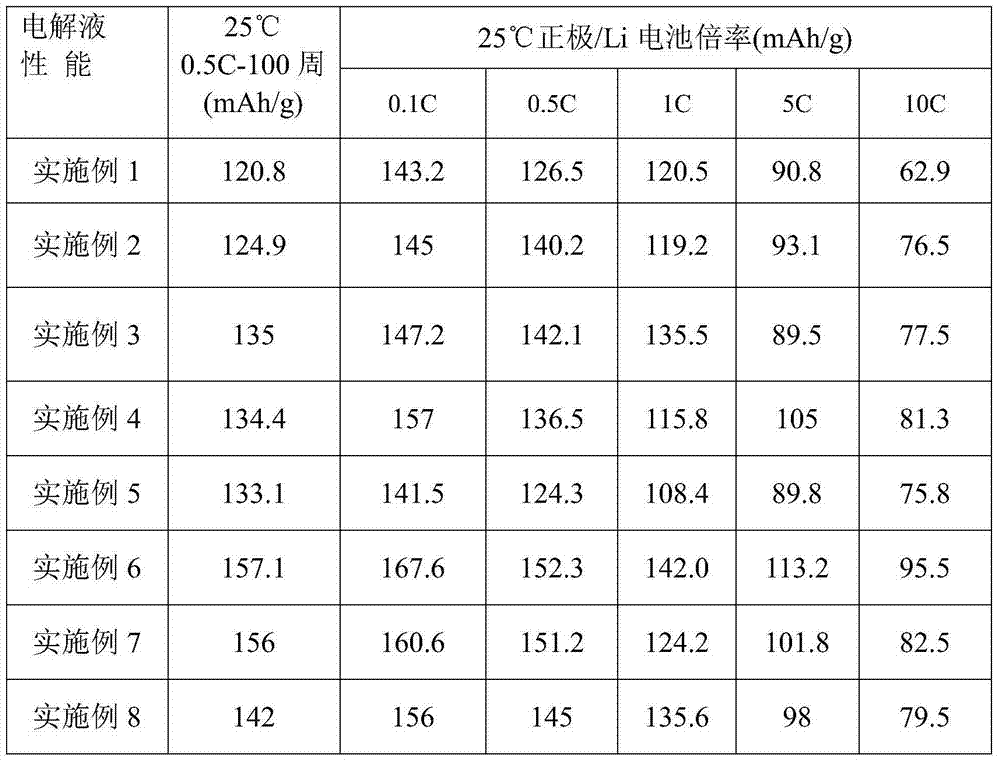
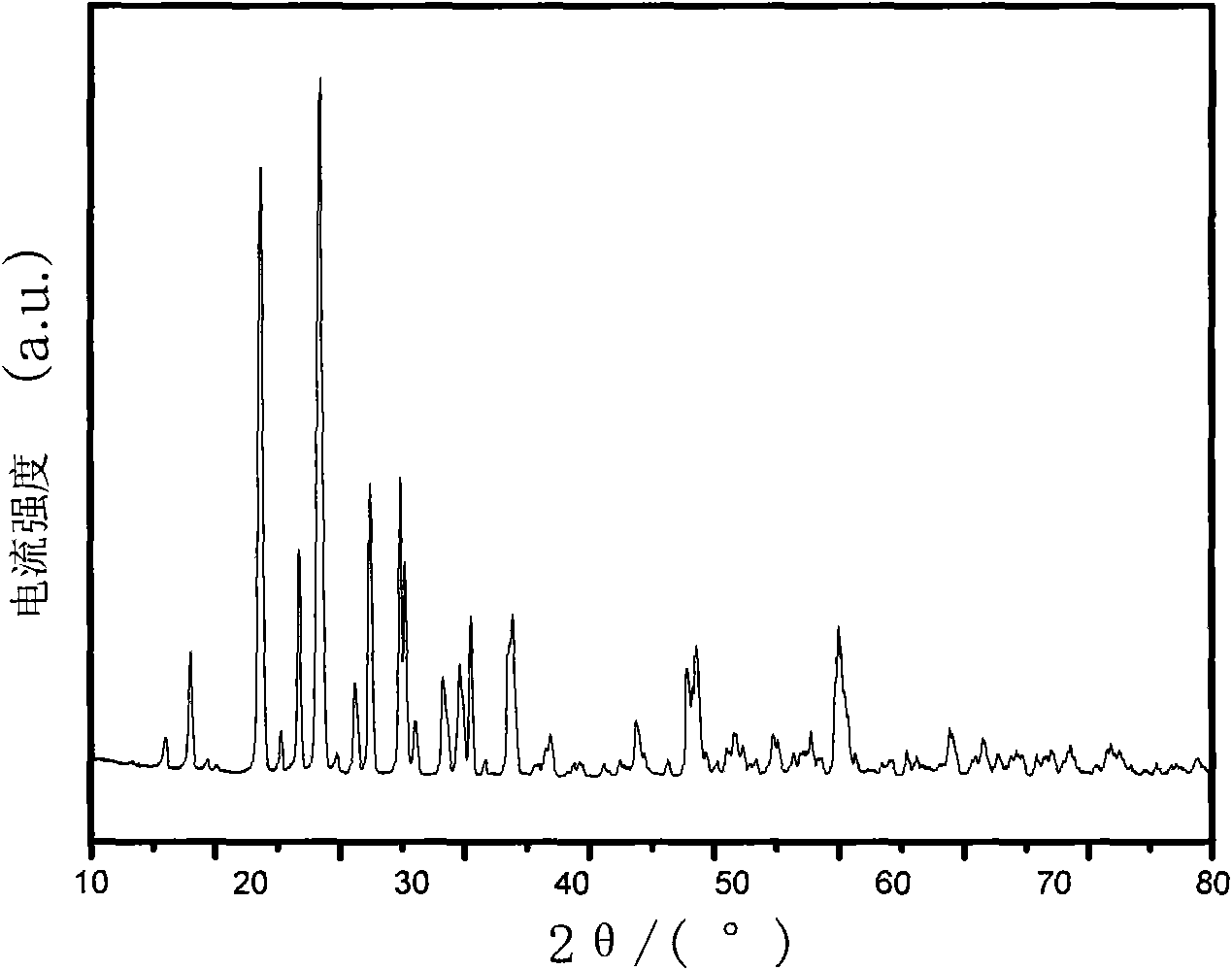
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
267 results about "Lithium vanadium phosphate battery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A lithium vanadium phosphate (LVP) battery is a proposed type of lithium ion battery that uses a vanadium phosphate in the cathode. As of 2016 they have not been commercialized.
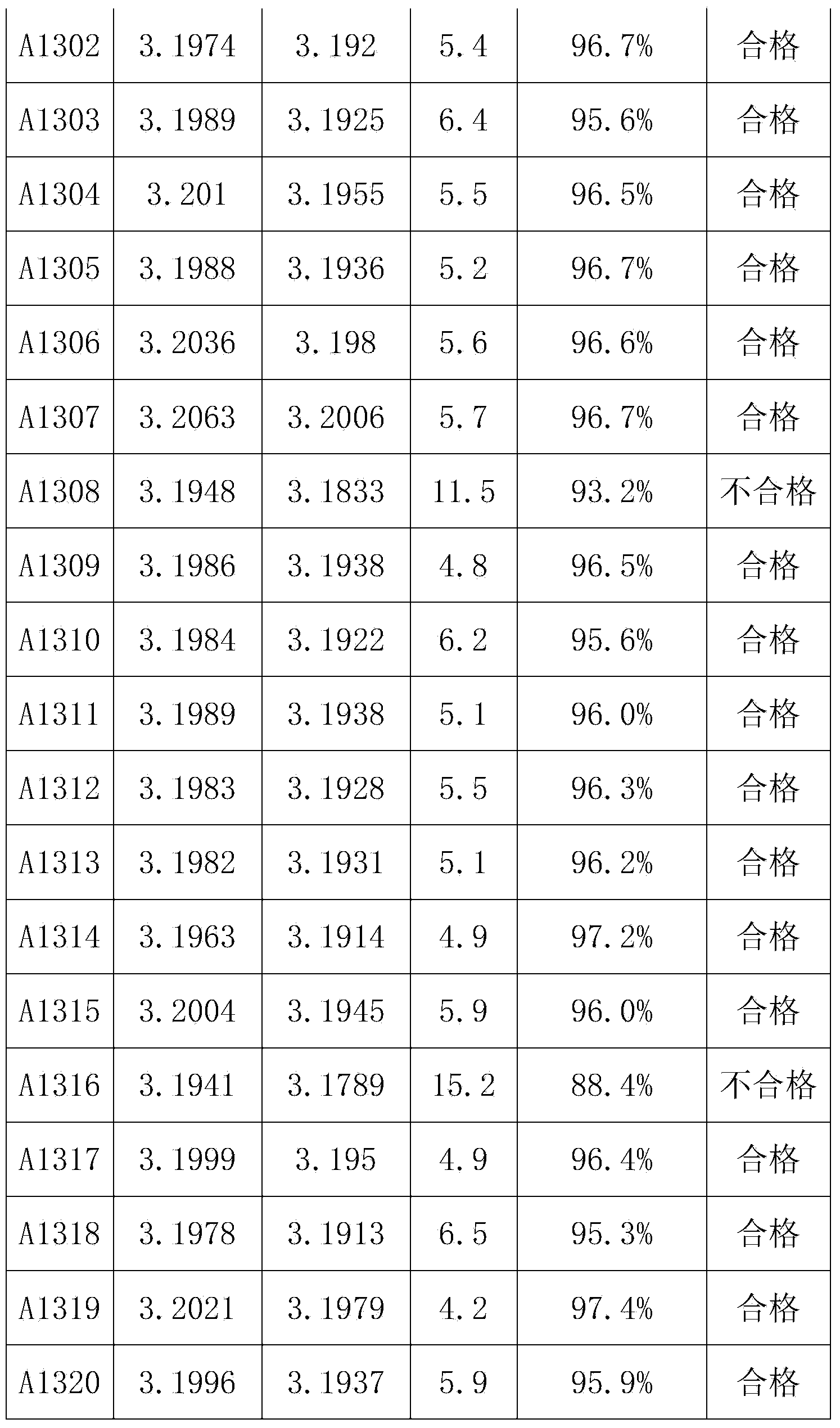
Self-discharge screening method for lithium ion phosphate battery
ActiveCN102117937ASolving the Difficult Problem of Self-Discharge ScreeningImprove consistencyFinal product manufactureElectrolyte accumulators manufacturePhosphateScreening method
The invention discloses a self-discharge screening method for a lithium ion phosphate battery, belongs to the technical field of lithium ion batteries, and aims to provide a method for effectively screening the lithium ion phosphate battery with high self-discharge rate by using shelving in a charging state. According to the technical key points, the method comprises the following steps of: adding laminar lithium nickel cobalt manganese oxide or spinel lithium nickel manganese oxide which comprises 0.5 to 5 weight percent of lithium ion phosphate and has high voltage platform into a lithium ion phosphate-containing compound positive electrode; assembling the lithium ion phosphate battery by taking graphite as a negative electrode; fully charging the battery and then shelving the battery at an ambient temperature of between 20 and 45 DEG C; recording voltages before and after the shelf and shelf time; calculating the voltage difference before and after the shelf or a voltage variation value in unit time; and determining a critical value of the voltage difference of the batteries with the high self-discharge rate which are shelved in the same period or voltage variation in the unit time, and determining that the self-discharge rate of the battery of which the voltage difference or the voltage variation value in the unit time is greater than the critical value is high.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
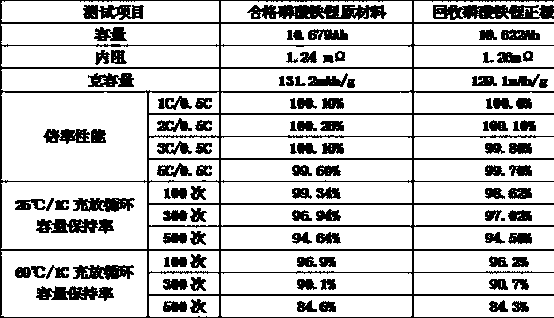
Method for recycling battery-grade iron phosphate in lithium iron phosphate battery and preparing lithium iron phosphate positive material by utilizing waste lithium ion phosphate battery
ActiveCN104953200AAchieve synthesisRealize the recovery of added valueWaste accumulators reclaimingBattery recyclingPhosphatePhosphoric acid
The invention relates to a method for recycling battery-grade iron phosphate in a lithium iron phosphate battery and preparing a lithium iron phosphate positive material by utilizing a waste lithium ion phosphate battery, and relates to a method for recycling a battery and preparing the battery positive material by utilizing the waste battery recycled material, solving the problems of the traditional method for recycling the LiFePO4 lithium ion battery positive electrode that the purity of the obtained element or substances is low and the obtained element or substances cannot be used for preparing the LiFePO4 lithium ion battery positive electrode. The method comprises the steps: I, crushing a positive pole piece, and carrying out heat treatment; II, dissolving the crushed positive pole piece in an acid solution; III, charging a surface active agent; IV, charging an alkaline solution, thereby obtaining a battery-grade iron phosphate; V, charging sodium carbonate to obtain a lithium carbonate; VI, mixing iron phosphate, lithium carbonate and a carbon source reduction agent; and VII, calcining. In the process for recycling the battery-grade iron phosphate in the lithium iron phosphate battery and preparing the lithium iron phosphate positive material by utilizing the waste lithium iron phosphate battery, no secondary pollution is produced, and the comprehensive and high-added-value recycling of the waste lithium iron phosphate battery can be realized.
Owner:HARBIN INST OF TECH
Lithium ion battery phosphatic composite cathode material and preparation method thereof
ActiveCN102244263AEasy to processImprove electrochemical performanceCell electrodesMicro structurePhosphoric acid
The invention discloses a lithium ion battery phosphatic composite cathode material and a preparation method thereof. The composite material is a multinuclear core shell structure composed of a plurality of cores and a housing layer, the cores are lithium iron phosphate particles wrapped by lithium vanadium phosphate and the housing layer is amorphous carbon. Preparation of the lithium iron phosphate particles wrapped by lithium vanadium phosphate comprises the following steps: preparing precursor sol with a sol gel method, adding lithium iron phosphate powder to disperse uniformly, carrying out spray drying on the above mixture, calcining the above resultant in inert gas, and followed by cooling and grinding to obtain the lithium iron phosphate particles wrapped by lithium vanadium phosphate. Preparation of the composite cathode material comprises the following steps: dissolving a carbon source compound into deionized water, adding core materials, dispersing the above resultant uniformly, carrying out second spray drying, calcining the above resultant in inert gas, and followed by cooling to obtain the composite cathode material. The composite material prepared in the invention has good electronic conduction performance, good ionic conduction performance and excellent electrochemistry performance. Because of existence of lithium vanadium phosphate, energetic density of a material is raised. Because of the multinuclear core shell structure like nano / micro structures, the composite material has good processing performance, and tap density of the material is greatly raised.
Owner:CENT SOUTH UNIV
Comprehensive recycling method of lithium ion battery anode material
ActiveCN107267759AReduce sorting costsImprove recycling economicsWaste accumulators reclaimingProcess efficiency improvementSlagElectrical battery
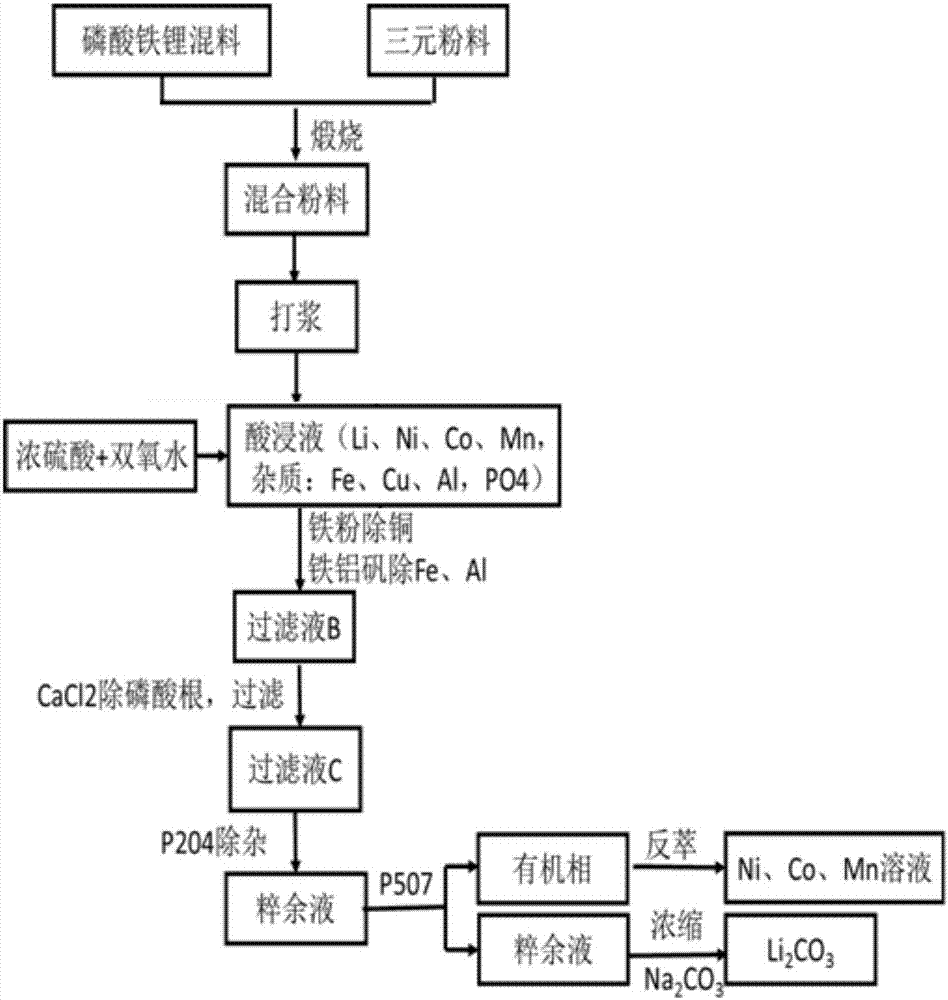
The invention provides a comprehensive recycling method of a lithium ion battery anode material. The method comprises the steps that the anode material of lithium iron phosphate and the anode material of a ternary battery are subjected to high-temperature pretreatment; a product is added into water for pulping processing; concentrated sulfuric acid and hydrogen peroxide are added, and filtering is performed to remove undissolved substances; iron powder is added, filtering is performed to remove copper elements, and heating is performed to generate iron aluminum vanadium slag; a calcium chloride solution is added, and filtering is performed to remove phosphate radicals; series-connection countercurrent extraction is performed through an extraction agent P204 to remove Fe and Ca impurities, and series-connection countercurrent extraction is performed through an extraction agent P507 to separate Ni, Co, Mn elements from Li elements; the organic phase is subjected to reverse extraction with sulfuric acid, a Ni, Ca and Mn solution is obtained, and recycling of nickel, cobalt and manganese is achieved; and the water phase is concentrated, and then a saturated sodium carbonate solution is added to generate lithium carbonate precipitation. By means of the comprehensive recycling method, the anode material of a lithium iron phosphate battery and the anode material of the ternary battery are recycled simultaneously, the battery separation cost is lowered, and the economic benefits of lithium battery recycling are increased.
Owner:南京国轩新能源有限公司
Self-discharge detection method of lithium iron phosphate battery
ActiveCN104316877AAffect consistencyExtended service lifeElectrical testingLithium vanadium phosphate batteryLithium iron phosphate
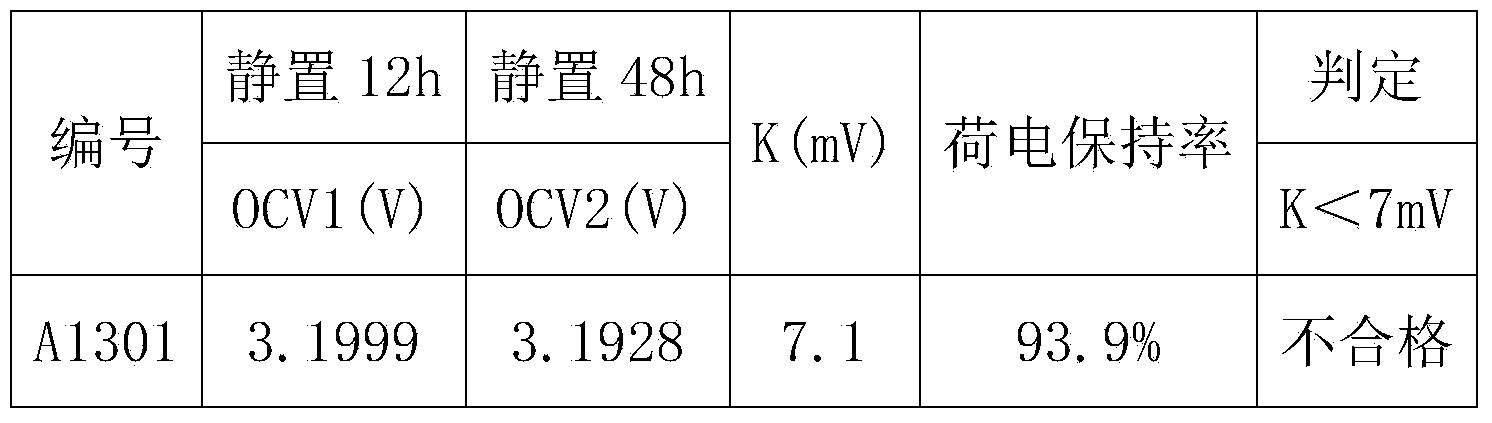
The invention discloses a self-discharge detection method of a lithium iron phosphate battery. Charging and discharging pretreatment is carried out on lithium iron phosphate batteries after capacity sorting processing; the batteries are placed for standing for some time respectively in different temperature environments and open-circuit voltages OCV1 and OCV2 are tested; a difference value K between the OCV1 and the OCV2 is calculated; and according to the K value, a battery with a high self-discharge value is determined and selected. According to the invention, the electrode polarization is eliminated by pretreatment, so that the influence on the voltage by polarization and unstable system factors can be minimized; and the battery with the high self-discharge value can be screened out rapidly in short time before grouping. The test is accurate and effective; the product quality is substantially improved; and the consistency of the whole group of batteries can be prevented from being influenced by the battery with the high self-discharge value. Therefore, the service life of the battery group can be prolonged.
Owner:中创新航科技(江苏)有限公司
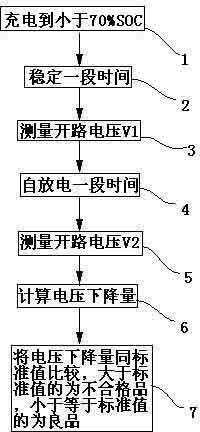
Method for detecting and sorting self-discharge performance of lithium iron phosphate battery
ActiveCN102303023AHigh sorting accuracyReduce sorting timeSortingLithium vanadium phosphate batteryElectricity
The invention relates to a method for detecting and sorting self-discharge performance of a lithium iron phosphate battery. The method comprises the following steps of: charging to less than 70 percent of a SOC ( State Of Charge); 2, storing for a period of time, wherein the period of time is called as a stabilization time; 3, testing an open-circuit voltage of the battery, wherein the tested voltage in the step is called as V1; 4, storing for one period of time, wherein the period of time is called as a self-discharge time; 5, testing an open-circuit voltage of the battery, wherein the tested voltage in the step is called as V2; 6, calculating a voltage drop amount of the battery, the voltage drop amount is called as a delta V, wherein the delta V is equal to the V1 minus the V2; and 7, determining that a battery of which the delta V is greater than a standard value is an unqualified product, and determining that a battery of which the delta V is smaller than the standard value is a good product. According to the method provided by the invention, the self-discharge performance of the lithium iron phosphate battery can be sorted in a short time without needing a high temperature aging, the problem of high detection cost caused by shortening the detection time by using the traditional method needing using the high temperature aging to accelerate the electricity discharge speed is solved.
Owner:WANXIANG 123 CO LTD
Non-aqueous electrolyte for lithium iron phosphate battery
The invention discloses a non-aqueous electrolyte for a lithium iron phosphate battery. The non-aqueous electrolyte comprises 70 to 85 weight percent of carbonic ester compound, 3 to 20 weight percent of various function additives and 11 to 17 weight percent of lithium hexafluorophosphate, wherein the carbonic ester compound is one of ethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate and diethyl carbonate or a mixture of more of the ethylene carbonate, the propylene carbonate, the butylene carbonate, the dimethyl carbonate and the diethyl carbonate; and the additives comprise one of 0.5 to 10 percent of film-forming additive, 0.5 to 10 percent of high-temperature additive, 0.5 to 10 percent of low-temperature additive, 0.5 to 10 percent of overcharge-preventing additive and 0.001 to 2 percent of stability additive, and a mixture of more of the additives. The non-aqueous electrolyte for the lithium iron phosphate battery has the advantages that the solubility and dissociation of the lithium hexafluorophosphate are improved, and electric conductivity is improved; the low temperature resistance of a solid electrolyte interphase (SEI) is reduced; the overall stability of the battery is improved, the overall service life of the battery is prolonged, the compatibility of an electrolyte and a cathode is improved, circulation of the battery is improved, and the service life is prolonged; and the non-aqueous electrolyte can have high performance at high temperature.
Owner:广东金光高科股份有限公司
Composite positive electrode material of graphene and lithium iron phosphate battery and preparation method thereof
InactiveCN103872287ASimple filling processLow priceSecondary cellsPositive electrodesLithium vanadium phosphate batteryLithium iron phosphate
The invention discloses a composite positive electrode material of a graphene and lithium iron phosphate battery and a preparation method thereof. The composite positive electrode material of the graphene and lithium iron phosphate battery is composed of graphene and a LiFePO4 lithium ion battery positive electrode material in the mass percentage range of 0.1%-8%. The composite material sufficiently utilizes the good conductive performance of the graphene so that the electronic conductivity and the discharging rate capability of the positive electrode material of the lithium iron phosphate ion battery are improved; a graphene filling process is simple and the price is low.
Owner:CHONGQING TECH & BUSINESS UNIV
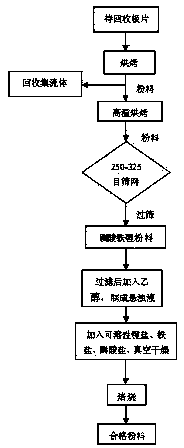
Method for recycling lithium iron phosphate wastes in manufacturing link of lithium iron phosphate batteries
ActiveCN104362408AImprove electrochemical performanceHigh reuse rateWaste accumulators reclaimingBattery recyclingDecompositionLithium-ion battery
The invention discloses a method for recycling lithium iron phosphate wastes in the manufacturing link of lithium iron phosphate batteries and relates to lithium ion batteries. The recycling method is characterized by comprising the following steps: after a pole piece to be recycled is put into a muffle furnace, baking is performed for 2-3h at the temperature of 400-600 DEG C, the decomposition of a binder fails, and an active material lithium iron phosphate and conductive agent powder completely fall off from a current collector aluminum foil; after the powder is put in the muffle furnace, baking is performed for 4-6h at the temperature of 650-800 DEG C, sieving is performed to obtain lithium iron phosphate power; filtering is performed, the lithium iron phosphate power is washed with deionized water, and an ethanol wetting agent after washing is added to obtain turbid liquid; soluble lithium salt, molysite and phosphate are proportionally mixed into an ethanol solution, the mixed solution is added into the turbid liquid for mixing, and vacuum drying is performed at the temperature of 120-140 DEG C; roasting is performed for 3-6h at the temperature of 650-850 DEG C in an inert gas atmosphere to obtain acceptable materials. The method has the advantages of being good in electrochemical performance of recycled materials, high in reuse rate and low in cost.
Owner:SHANDONG SACRED SUN POWER SOURCES
Preparation method of lithium vanadium phosphate (Li3V2(PO4)3)/graphene composite material for positive electrode of lithium ion battery
InactiveCN102623708AHigh purityHigh crystallinityCell electrodesAluminium-ion batterySodium-ion battery
The invention relates to a preparation method of a lithium vanadium phosphate (Li3V2(PO4)3) / graphene composite material for a positive electrode of a lithium ion battery. The method comprises the steps that firstly, a mixed precursor solution is prepared; pretreatment of spray drying is conducted and a precursor is obtained; and then the lithium vanadium phosphate positive electrode material of the lithium ion battery is prepared through a calcination reaction under an inert atmosphere condition. Compared with the prior art, the preparation method has the advantages that the combination mode of the Li3V2(PO4)3 and the graphene, the contents of carbon and the graphene, and the particle size of the material are effectively controlled, thereby improving the stability and the electrical conductive performance of the material.
Owner:SHANGHAI ZHIRONG TECH
Lithium ion cell anode material lithium vanadium phosphate and preparation method thereof
ActiveCN101145611ASmall and evenly dispersedAvoid reunionElectrode manufacturing processesPhosphorus compoundsHigh rateConductive materials
The invention discloses a lithium vanadium phosphate anode material used in a lithium battery and a preparation method thereof, and solves the technical problem that can improve the electrical conductivity and high-rate discharge property of the anode material. The inventive anode material comprises a matrix coated outside with a conductive nano-composite material with a particle size of 5-50 Mu m and a specific surface area of 5-25m<2> / g. The inventive anode material is prepared by the following steps: wet-method superfine ball mill, liquid-phase mixture reaction, spray drying, pretreatment, calcinations treatment, coating with conductive composite material, and fusion. Compared with the prior art, the invention can synthesize lithium vanadium phosphate anode material by the secondary molding liquid phase method of nanoparticles, and the product purity is high, the particle agglomeration is efficiently prevented. The synthetic lithium vanadium phosphate anode material has a discharge voltage about 4V, three discharge voltage platform zones, higher charge / discharge capacity, excellent rate discharge capability and loop stability, and low cost; and is suitable for the industrial production.
Owner:SHENZHEN CITY BATTERY NANOMETER TECH
Lithium iron phosphate battery positive electrode pulp, lithium iron phosphate battery using positive electrode pulp and preparation method thereof
ActiveCN101872856AQuality improvementGood flexibilityCell electrodesFinal product manufactureLithium vanadium phosphate batteryLithium iron phosphate
The invention provides lithium iron phosphate battery positive electrode pulp, a lithium iron phosphate battery using the positive electrode pulp and a preparation method thereof. The positive electrode pulp consists of the following ingredients in percentage by weight: 40 to 55 percent of lithium iron phosphate, 2 to 5 percent of conductive agents, 3 to 5 percent of aqueous bonding agents and 40 to 55 percent of solvents. The battery comprises an electrode sheet coated with the positive electrode pulp. The aqueous positive electrode pulp of the invention greatly improves the positive electrode lithium iron phosphate active material amount, the electrode sheet of the positive electrode has friendly effect on the moisture, and adverse phenomena such as water absorption powder dropping, material dropping and the like can not occur, and the electrode sheet of the positive electrode has good softness and adhesive performance after tabletting, and has good effect in the cutting and conversion operation processes. The solvents in the positive electrode pulp relatively have environment protection effect, and the safety performance of the aqueous bonding agents is stable. The battery of the invention has good integrated performance, high discharging power, capability of fast charging and long circulation life. The preparation method of the battery has good pulp dispersion effect and coating effect.
Owner:SHENZHEN MOTTCELL NEW ENERGY TECH CO LTD
Self-discharge sorting process for lithium iron phosphate batteries
InactiveCN104084383AReduce sorting costsHigh sorting accuracySortingLithium vanadium phosphate batteryElectricity
The invention discloses a self-discharge sorting process for lithium iron phosphate batteries. The process comprises the following steps: discharging a graded battery to cut-off voltage; putting the battery aside for a period of time T1, and recording the open-circuit voltage V1 of the battery; putting the battery aside for a period of time T2 again, and recording the open-circuit voltage V2 of the battery; calculating the voltage drop DeltaV which is equal toV1-V2 of the battery within the time T2 and voltage drop K which is equal to (V1-V2) / T2 of the battery per unit of time within the time T2; setting the top and bottom limitation of DeltaV and K, and removing rejects beyond the range. The process has the advantages that the open-circuit voltage of the battery in a power empty state after being statically put for a period of time is retested; the self-discharge sorting precision of the batteries is high, and meanwhile, the sorting cost of the batteries is low; the process is suitable for being promoted and applied in the industries.
Owner:ZHEJIANG XINGHAI ENERGY TECH
Positive pole material of lithium ion battery with nanometer structure and preparation method thereof
The invention discloses a positive pole material of a lithium ion battery with a nanometer structure and a preparation method thereof. The positive pole material is in a particle type core-shell structure, a core material consists of nanometer lithium iron phosphate, lithium vanadium phosphate or cobalt lithium oxide and graphene, and a shell material is porous carbon. The preparation method comprises the following steps of: taking lithium acetate, lithium oxalate, ammonium dihydrogen phosphate, ammonia metavanadate, phosphoric acid, lithium nitrate, cobalt nitrate and graphite oxide as raw materials, adopting a sol-gel method or a ball milling method to prepare a mixture, presintering the mixture in vacuum to obtain the core material, and mixing, grinding and calcining the core material with an organic carbon source to obtain the positive pole material with the particle type core-shell structure. The positive pole material has the advantages of good conductivity, good circulating performance, high capacity, small and uniform particle size, and simple preparation process and is easy for industrialized production.
Owner:深圳清研紫光科技有限公司
Lithium battery electrode material
ActiveCN101859891AImprove electrochemical performanceDiffuse fullyCell electrodesLithium vanadium phosphate batteryLithium iron phosphate
The invention discloses a lithium battery electrode material, comprising a plurality of uniformly distributed lithium iron phosphate / lithium vanadium phosphate composite particles. The material is characterized in that each lithium iron phosphate / lithium vanadium phosphate composite particle comprises a lithium vanadium phosphate particle and a lithium iron phosphate particle layer uniformly coated on the surface of the lithium vanadium phosphate particle, wherein, the lithium iron phosphate particle layer comprises a plurality of lithium iron phosphate particles.
Owner:TSINGHUA UNIV +1
Low-temperature electrolyte of lithium iron phosphate battery
InactiveCN103500850AEasy to buyFair priceSecondary cellsOrganic electrolytesLithium vanadium phosphate batteryLithium iron phosphate
The invention relates to a low-temperature electrolyte of a lithium iron phosphate battery. The low-temperature electrolyte includes the following solvents of, by volume, 30%-45% of carbonic ester solvent, 50%-65% of carboxylic ester solvent and 4%-10% of additive. The solvents contain solute lithium, the lithium is LiPF6 or a combination of the LiPF6 and LiBF4, and the concentration of the lithium is 0.8-1.4mol / L. The low-temperature electrolyte is a nonaqueous electrolyte, through optimization of kinds and proportioning combination of the solvents of the electrolyte, low-viscosity carbonic ester and low-melting-point carboxylic ester are selected and used, the freezing point at low temperature is lowered, and low-temperature conductivity is increased. According to the low-temperature electrolyte, the lithium of the electrolyte is optimized, the low-temperature additive is selected preferably, normal-temperature circulation ratio performance of the electrolyte is maintained, and meanwhile, the low-temperature capacity retention ratio of the lithium iron phosphate battery and the ratio performance of the lithium iron phosphate battery are improved. The commercial application requirements of the electrolyte can be met, the low-temperature performance of the electrolyte is improved particularly, and therefore the electrolyte is suitable for aerospace and plateau alpine environment.
Owner:SHANDONG UNIV
Method of recycling waste lithium iron phosphate battery and lithium manganate battery
ActiveCN106997975AReduce manufacturing costWaste accumulators reclaimingProcess efficiency improvementPower batteryLithium vanadium phosphate battery
The invention discloses a method of recycling waste lithium iron phosphate battery and lithium manganate battery. The method comprises the following steps of carrying out discharging, disassembling, soaking with an organic solvent, calcining, acid hydrolysis and filtering and the like on the lithium iron phosphate battery and the lithium manganate battery respectively, then mixing filtrates of anode materials of the two kinds of batteries according to a certain ratio, regulating a pH value of a solution to obtain a lithium iron manganese phosphate precursor, adding the lithium iron manganese phosphate precursor in a carbon source, and carrying out high-temperature calcining synthesis reaction to obtain the carbon-cladding lithium iron manganese phosphate anode material. By adopting the method provided by the invention, an anode material of the waste lithium iron phosphate battery and an anode material of the waste lithium manganate are treated by proper chemical means to be used as a manganese source, an iron source, a phosphorus source and a lithium source for synthesizing high-energy density anode material lithium iron manganese phosphate, so that the preparation cost of the lithium iron manganese phosphate is lowered, the recycling efficiency is high, the treatment speed is fast, and a brand new reference mode can be provided for a power battery enterprise to treat waste power batteries.
Owner:ANHUI ANKAI AUTOMOBILE
Method for preparing lithium vanadium phosphate as lithium ion battery anode material
InactiveCN101651205AUniform particle size distributionHigh specific capacityElectrode manufacturing processesMuffle furnacesLithium vanadium phosphate batteryMicrowave sintering
The invention discloses a method for preparing lithium vanadium phosphate as a lithium ion battery anode material, which comprises the following steps: (1) preparing reaction precursor gel of an anodematerial by a sol-gel method; (2) presintering the reaction precursor gel prepared in the step (1); and (3) placing a material obtained by presintering into a microwave sintering furnace to sinter. The invention combines the sol-gel method with a microwave sintering method and can prepare a product with favorable electrode plate processing ability, electrical conductivity and electrochemical performance by sintering in tens of minutes. The method has high production efficiency, low energy consumption, easy control of technical parameter, favorable batch stability and low production cost and is suitable for mass industrial production.
Owner:CHANGSHU INSTITUTE OF TECHNOLOGY
Method for preparing lithium carbonate by employing discarded lithium iron phosphate battery
InactiveCN106129519AHigh purityImprove extraction efficiencyWaste accumulators reclaimingLithium carbonates/bicarbonatesLithium vanadium phosphate batteryLithium iron phosphate
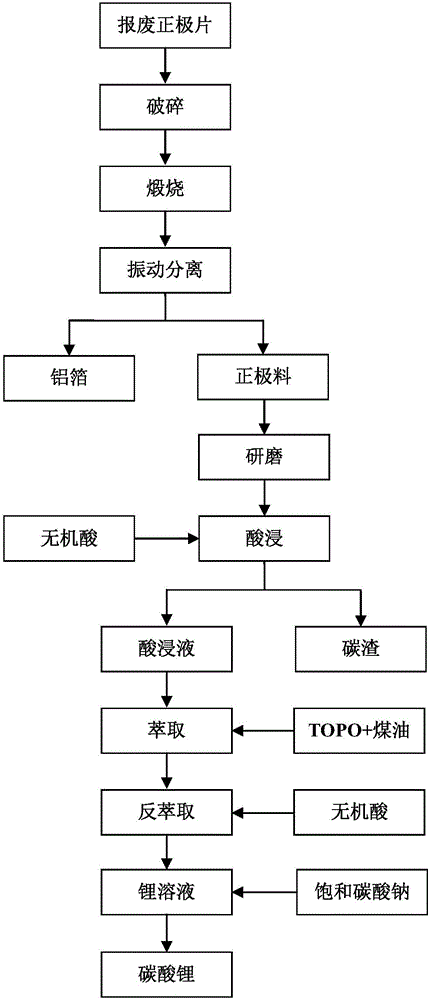
The invention discloses a method for preparing lithium carbonate by employing a discarded lithium iron phosphate battery. The method comprises the steps of crushing a discarded positive pole piece into blocks; calcining the massive positive pole piece in a high temperature furnace into which nitrogen is introduced; carrying out vibrating separation on the calcined positive pole piece through a vibrating screen; grinding a positive pole material which is obtained through vibrating separation; carrying out mixing and stirring reaction on the ground positive pole material and an acid liquid at a certain ratio; adding a TOPO-kerosene extracting agent to an acid leaching liquid to extract lithium and then carrying out reverse extraction through a stripping agent to obtain a lithium solution; and adding a sodium carbonate solution to the lithium solution and carrying out precipitation to prepare lithium carbonate. By the method for preparing the lithium carbonate by employing the discarded lithium iron phosphate battery, the recovery technology is simple; the equipment investment is low; the environmental pollution is small; the lithium loss is small; the extraction efficiency on lithium is high; the prepared lithium carbonate is high in purity; and the method is suitable for large-scale industrial production.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Self-discharge detection method for lithium iron phosphate batteries
InactiveCN102508173AEasy to filterElectrical testingLithium vanadium phosphate batteryLithium iron phosphate
The invention discloses a self-discharge detection method for lithium iron phosphate batteries, which relates to the technical field of screening of lithium batteries. The self-discharge detection method is characterized in that the batteries are fully charged, and placed in a uniform magnetic field with magnetic intensity ranging from 0.01T to 1.3T, self-discharge of the batteries is accelerated by the aid of an effect of the high-intensity magnetic field to the lithium iron phosphate batteries, the lithium iron phosphate batteries are taken out from the high-intensity magnetic field after standing in the high-intensity magnetic field for 1 day to 15 days, self-discharge rates of the shelved batteries are tested after the batteries are degaussed, and batteries with abnormal discharge properties can be selected according to actually measured data of self-discharge rates. The method is used for screening the self-discharge properties of the lithium batteries, disqualified batteries can be selected within a short period of time, and the method has an important popularization value.
Owner:JIANGSU FRONT NEW ENERGY
Conductive agent used for lithium iron phosphate battery and preparation method thereof
ActiveCN102136576ASlow down the sinking speedGood dispersionCell electrodesLithium vanadium phosphate batteryLithium iron phosphate
The invention discloses a conductive agent used for a lithium iron phosphate battery, and also discloses a preparation method for the conductive agent. The conductive agent consists of a carbon nanotube / carbon black composite material, graphene and a bonding agent in the weight ratio of 1:(0.001-0.1):(0.01-1). The preparation method comprises the following steps of: first, preprocessing the carbon nanotube / carbon black composite material; then, performing ultrasonic dispersion on the carbon nanotube / carbon black composite material and the bonding agent in secondary distilled water; next, adding the graphene into the secondary distilled water under the condition of ultrasonic waves; and finally, performing the ultrasonic dispersion to prepare the conductive agent. The preparation method for the conductive agent is simple; and the prepared conductive agent has uniform dispersion and high stability. The conductive agent has high electronic conductivity and uniform heat conduction; and the lithium iron phosphate battery prepared by adopting the conductive agent has remarkably improved electrochemical performance and obviously reduced alternating-current internal resistance.
Owner:中创新航科技(江苏)有限公司
Method for rapidly screening self discharge of lithium iron phosphate battery
InactiveCN102903957AIncrease occupancyIncrease wasteFinal product manufactureElectrolyte accumulators manufactureLithium vanadium phosphate batteryLithium iron phosphate
The invention discloses a method for rapidly screening self discharge of a lithium iron phosphate battery. The method comprises the following steps of: prolonging the laying time when the capacity grading working procedure charging of the lithium iron phosphate battery is finished, and recording the voltage of the battery at each time point in laying; prolonging the laying time when discharging is finished, and recording the voltage of the battery at each time point in laying; and selecting the voltage value corresponding to the specific time point as the basis for screening self discharge. The method has the beneficial effects that the self discharge screening is carried out through prolonging the technology time during the production process, and the data test record is directly completed on a test cabinet, thus the technology time is short, and the data accuracy and comparability are better.
Owner:合普新能源科技徐州有限公司
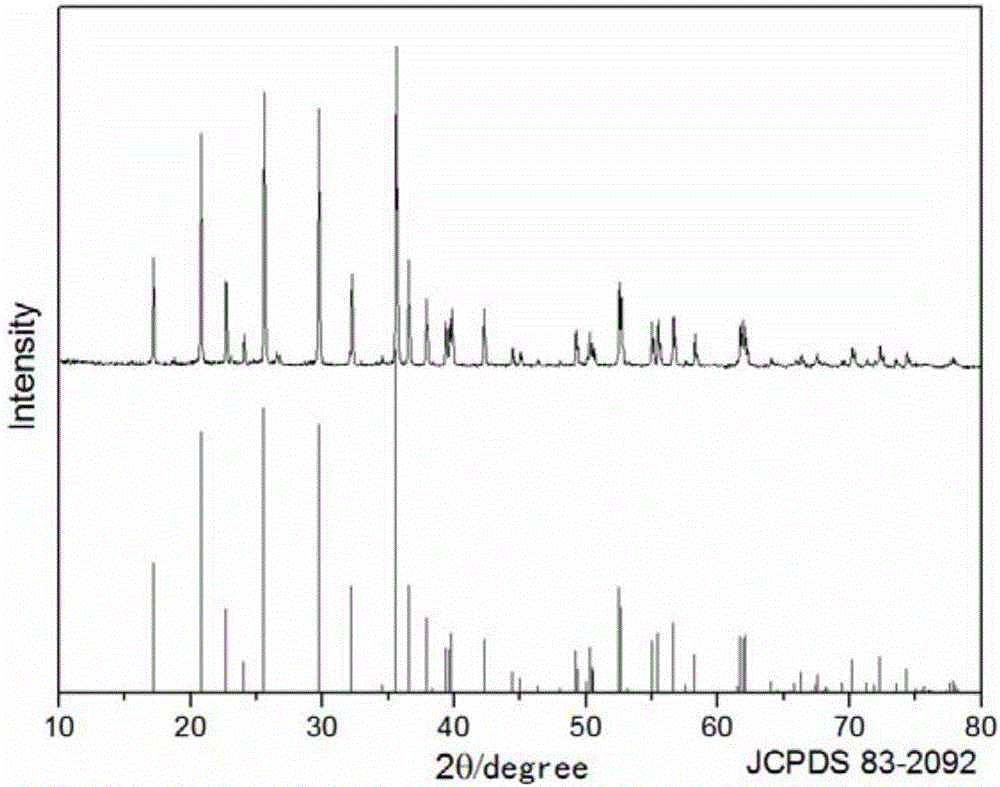
Carbon-coated lithium vanadium fluorophosphate lithium ion battery positive electrode material and preparation method thereof
ActiveCN109755514AHigh phase purityThorough responseCell electrodesSecondary cellsLithium-ion batteryCarbon coated
The invention provides a carbon-coated lithium vanadium fluorophosphate lithium ion battery positive electrode material and a preparation method thereof; the preparation method of the carbon-coated lithium vanadium fluorophosphate lithium ion battery positive electrode material comprises the following steps that a vanadium source, a phosphorus source and a carbon source are added into water to form a solution, and continuous stirring is carried out until a stable viscous solution is formed or rapid solidifying is performed; drying is performed on the viscous solution or the solid obtained in the last step, and heat treatment is performed in a non-oxidizing atmosphere, and then crushing and grinding are carried out to obtain the black carbon coated vanadium phosphate powder; mixing is carried out on the carbon-coated vanadium phosphate powder, lithium fluoride and a fluorine source to obtain precursor powder, and under the non-oxidizing atmosphere, sintering is carried out at 550-750 DEG C for 0.5-10h to obtain the carbon-coated lithium vanadium fluorophosphate material. The method is simple in process route, easy to operate, low in generating cost and capable of realizing large-scale production. The carbon-coated lithium vanadium fluorophosphate lithium ion battery positive electrode material prepared by the method is high in phase purity, uniform in particle sizes and excellent in electrochemical performance.
Owner:大连融科储能集团股份有限公司
Method for selectively leaching lithium from anode material of failed lithium iron phosphate battery
ActiveCN106532172AReduce consumptionEasy to separateWaste accumulators reclaimingProcess efficiency improvementLithium vanadium phosphate batteryLithium iron phosphate
The invention relates to a method for selectively leaching lithium from an anode material of failed lithium iron phosphate battery, and belongs to the technical field of reuse of wastes. In order to overcome the technical shortcomings of low recycle efficiency of ferric phosphate and big impurity of lithium solution in the method of selectively leaching lithium from an anode material of failed lithium iron phosphate battery, the invention discloses a method for selectively leaching lithium from the anode material of failed lithium iron phosphate battery by using H3PO4-H2O2 system. According to the method for selectively leaching lithium from the anode material of failed lithium iron phosphate battery by using H3PO4-H2O2 system, the pH value of the system is 2.0-4.5, thus the effect of selectively leaching lithium is reached, the filtering slag is ferric phosphate and carbon powder. The method can perfectly separate lithium and iron, so that iron can be completely settled in the form of iron phosphate; lithium is dissolved in the leaching solution in the form of lithium dihydrogen phosphate.
Owner:JIANGXI HZONE LITHIUM TECH
Method for estimating SOE (State of Energy) of lithium iron phosphate battery
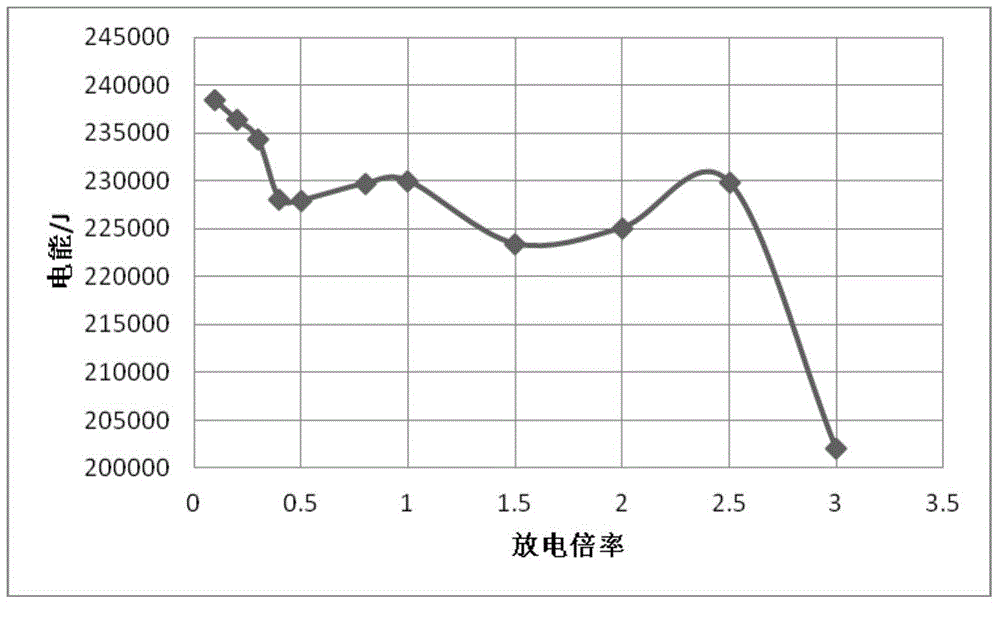
ActiveCN104951662AImprove estimation accuracyPerfect energy loss typeSpecial data processing applicationsElectrical batteryDischarge rate
The invention provides a method for estimating the SOE (State of Energy) of a lithium iron phosphate battery. According to the method, an SOE mathematic estimation model including electric energy and heat energy is established according to the composition form of internal energy of the lithium iron phosphate battery. The external consumed electric energy as well as ohm heat energy, polarization heat energy and entropy generation heat energy in the battery in the battery discharge process are estimated at different discharge rates, the maximum available energy at various discharge rates is acquired, the maximum theoretical total energy and the efficiency function relation at various discharge rates are acquired through simulation, and the total energy of the estimation model is corrected in real time, so that the estimation accuracy of the SOE of the battery is effectively improved. The heat energy form is introduced to perfect types of energy consumption in the battery discharge process, meanwhile, the total release energy in the estimation process is adjusted in real time according to the maximum theoretical total energy and the energy release efficiency, so that the model is closer to the actual working condition, and the method has the advantages of physical concept clearness, high estimation accuracy and the like.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
Method for recovering and recycling waste positive electrode material during production process of lithium iron phosphate battery
InactiveCN106505273AEasy to recycleEasy to fixPhosphatesFinal product manufactureLithium vanadium phosphate batteryEnvironmental resistance
The invention discloses a method for recovering and recycling a waste positive electrode material during production process of a lithium iron phosphate battery. The method comprises the following steps of firstly, placing a lithium iron phosphate positive plate which is obtained by recycling in an alkaline solution, and separating an aluminum foil from the alkaline solution after the aluminum foil is completely separated; secondly, filtering and washing a lithium iron phosphate positive electrode mixed material in the alkaline solution, and then performing drying, ball-milling and sieving; and finally, immersing sieved lithium iron phosphate positive electrode mixed material powder in an organic solvent, performing stirring to remove a binding agent in the mixed material, performing filtering and washing, and performing thermal treatment such as roasting and calcination after drying to obtain a lithium iron phosphate positive electrode material with excellent performance. By the method, waste lithium iron phosphate can be recovered to acquire electrical property equivalent to that of a newly-prepared product, the aluminum foil can be reserved to the greatest extent and is convenient to recycle by an aluminum factory, the thermal treatment temperature required during the whole process is relatively low, and the process is simple and environment friendly.
Owner:XIANGTAN UNIV
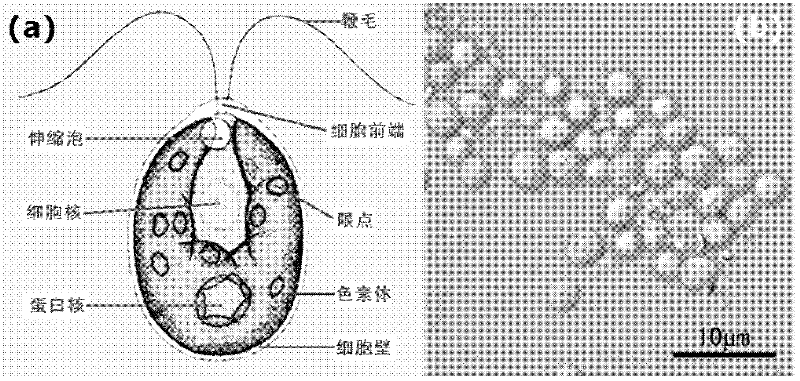
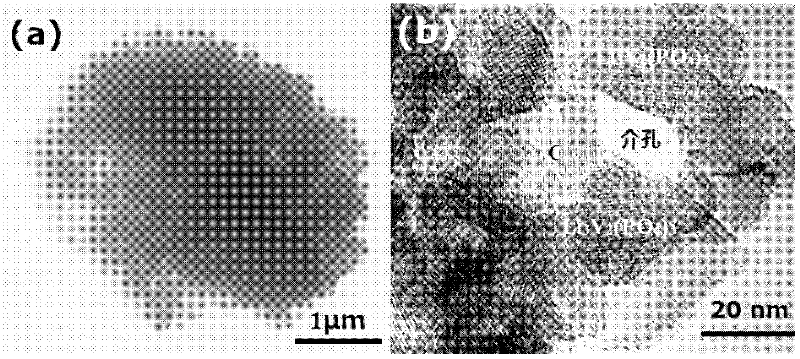
Biomimetic synthesis method of lithium vanadium phosphate/carbon nanometer composite mesoporous microspheres as positive electrode material of lithium ion battery
The invention relates to a preparation method of lithium vanadium phosphate / carbon nanometer composite mesoporous microspheres as positive electrode material of a lithium ion battery. The preparation method comprises the following steps: preparing hungry green algae cell solution from inexpensive green algae cells; adding vanadium oxalate solution into the hungry green algae cell solution dropwise; adding phosphate source and lithium source to obtain gel; drying to obtain lithium vanadium phosphate precursor; grinding lithium vanadium phosphate precursor; heating at about 450 DEG C in the nitrogen atmosphere; and heating to about 750 DEG C and preserving the temperature to obtain black powdered Li3V2(PO4)3 / C nanometer composite mesoporous microspheres. The lithium vanadium phosphate / carbon nanometer composite mesoporous microspheres prepared by the invention can be used as the positive electrode material of the lithium ion battery, and can be used for preparing portable or power lithium ion battery.
Owner:QILU UNIV OF TECH
Power battery using mixed anode material
InactiveCN102117913AGood high temperature performanceGood high temperature cycle performanceFinal product manufactureCell electrodesHigh energyManganese
The invention relates to a power battery using mixed anode material. An anode comprises an active substance, a conductive agent, a binder and a solution, wherein a mixed material of lithium iron phosphate and nickel-manganese-cobalt ternary material is used as the active substance; and the components are as follows in percentage by weight: 5 to 95 percent of lithium iron phosphate, 1 to 20 percent of conductive agent and 2 to 10 percent of binder. The conductive agent comprises acetylene black and carbon black; polyvinylidene fluoride is adopted as the binder; and N-N-dimethyl pyrrole is adopted as the solvent. Compared with a traditional lithium iron phosphate power battery, the power battery has the following advantages: the working voltage ranges from 2.75 to 4.2 volts, and is superior to that of the lithium iron phosphate battery; good high temperature performance is achieved; good low temperature performance is achieved, and discharge capacity is more than 90 percent at 0 DEG C; excellent security feature is achieved, and overcharge resistance is consistent to that of the lithium iron phosphate; and high energy density is achieved: the compacted density of the lithium iron phosphate ranges from 2.0 to 2.1, the compacted density of nickel-manganese-cobalt ternary material ranges from 3.4 to 3.8, and the compacted density of the mixture can range from 2.2 to 2.9.
Owner:ZHUHAI COSMX BATTERY CO LTD
Method for manufacturing and coating anode slurry of lithium iron phosphate battery
ActiveCN102569740AIsolated contactImprove protectionCell electrodesLithium vanadium phosphate batteryLithium iron phosphate
The invention relates to a method for manufacturing and coating anode slurry of a lithium iron phosphate battery. In the process of manufacturing the anode slurry of the lithium iron phosphate battery, a stirring process is carried out in the protection of a high-purity gas; the formulation of the anode slurry comprises the following components in mass percentage: 90-96 percent of lithium iron phosphate, 2-4 percent of conductive agent and 2.5-5 percent of binding agent; in the coating process, the anode slurry is coated on a current collector by several times, so that the adhesion between the anode slurry and the current collector is better and the compacted density is higher; and the binding agent is polyvinylidene fluoride (American Solef5130) with a high molecular weight. The lithium iron phosphate slurry is more uniformly dispersed; the adhesion between powder and the current collector is better; the ratio discharge performance is good; and the circulating performance is good.
Owner:HANGZHOU LIAO TECH
Synthesis of Cathode Active Materials
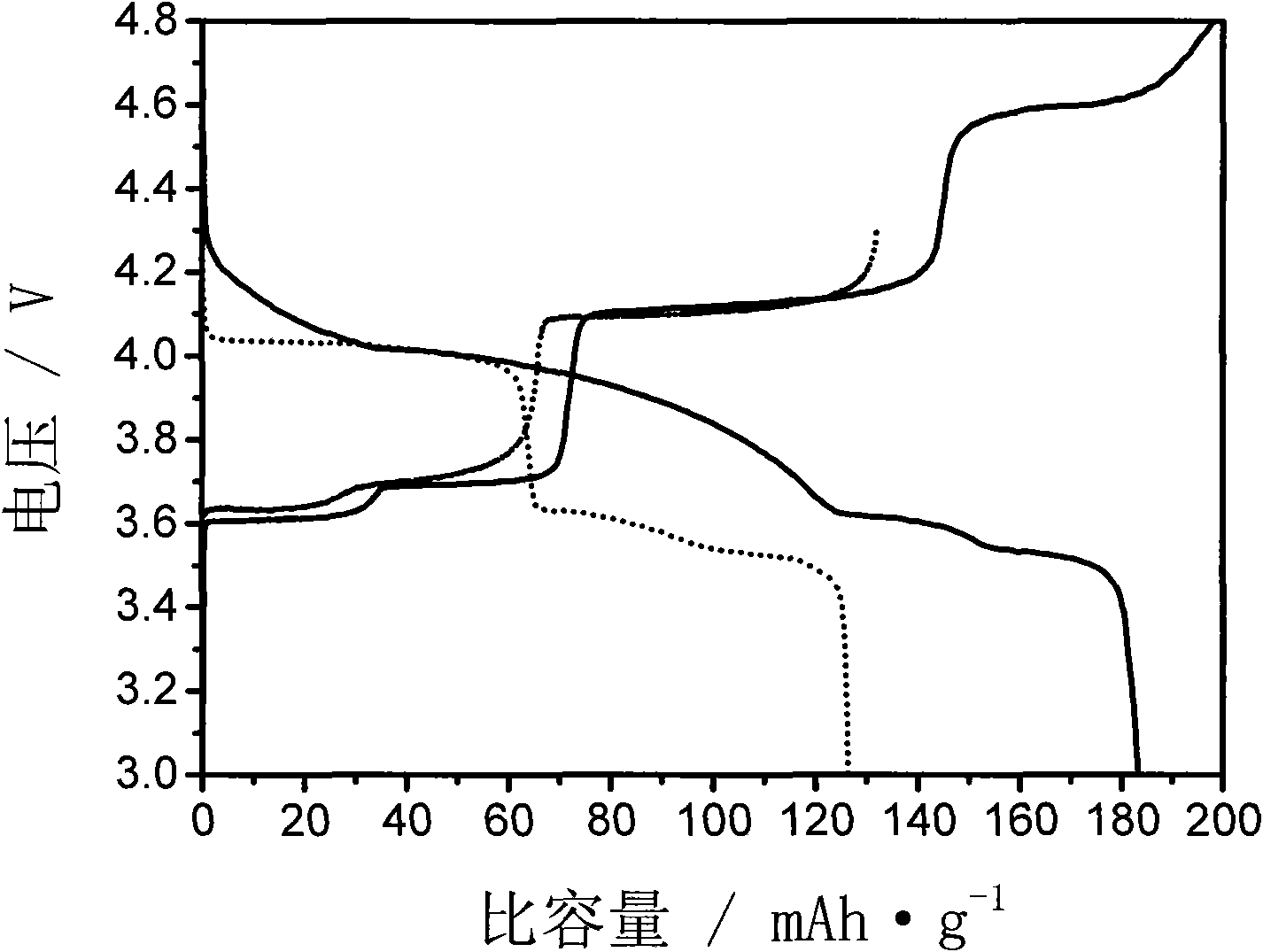
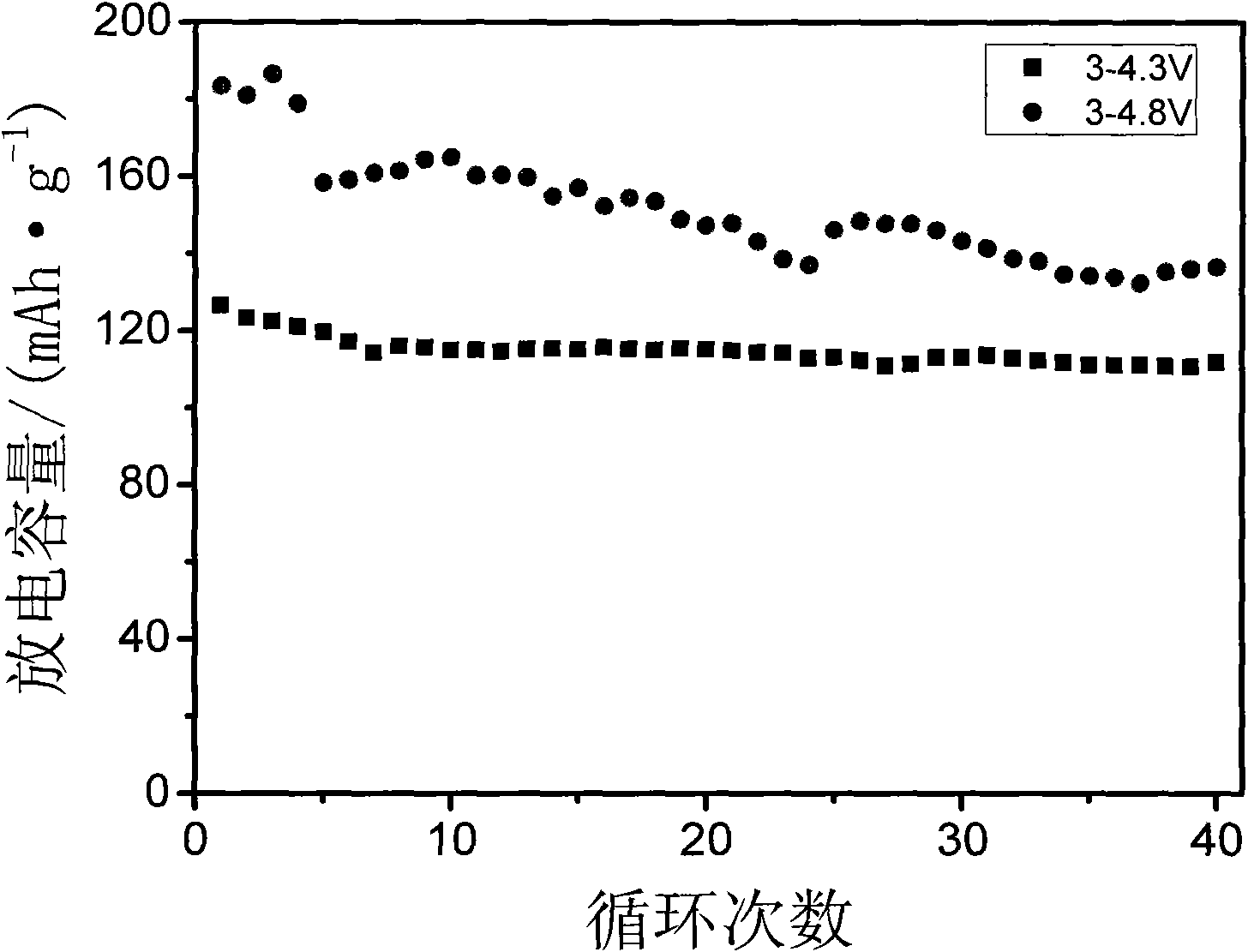
The present invention relates to a method for preparing a lithium vanadium phosphate material comprising forming a aqueous slurry (in which some of the components are at least partially dissolved) comprising a polymeric material, an acidic phosphate anion source, a lithium compound, V2O5 and a source of carbon; wet blending said slurry, spray drying said slurry to form a precursor composition; and heating said precursor composition to produce a lithium vanadium phosphate. In one embodiment the present invention relates to a method for preparing a lithium vanadium phosphate which comprises reacting vanadium pentoxide (V2O5) with phosphoric acid (H3PO4) to form a partially dissolved slurry; then mixing with an aqueous solution containing lithium hydroxide; adding a polymeric material and a source of carbon to form a slurry; wet blending said slurry; spray drying said slurry to form a precursor composition; and heating said precursor composition for a time and at a temperature sufficient to produce a lithium vanadium phosphate compound. In an alternative embodiment the present invention relates to a method for preparing a lithium vanadium phosphate which comprises preparing an aqueous solution of lithium hydroxide; partially dissolving vanadium pentoxide in said aqueous solution; adding phosphoric acid to the aqueous solution; adding a polymeric material and a source of carbon to the solution containing vanadium pentoxide to form a slurry; spray drying said slurry to form a precursor composition; and heating said precursor composition for a time and at a temperature sufficient to form a lithium vanadium phosphate. The electrochemically active lithium vanadium phosphate so produced is useful in making electrodes and batteries.
Owner:SWOYER JEFFREY +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com