Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86 results about "Injectable Lyophilized Powder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medicament composition for water-soluble injection of paclitaxel, preparation method and uses thereof
ActiveCN101366696AOrganic active ingredientsPharmaceutical delivery mechanismFreeze-dryingHydroxystearic Acid
The invention discloses a water-soluble drug composition of taxol for injection. The drug composition comprises taxol as an active component, polyethyleneglycol 15- hydroxy stearic acid ester as a solubilizer, alcohol as a latent solvent and injection water as a solvent. Polysorbate, cyclodextrin derivatives, poloxamer, polyethylene glycol (PEG) and reducing sugar are also added and are favorable for improving the solubility and stability of the taxol in the composition. The composition is prepared to freeze-dried powder injection for injection, can not produce serious anaphylactic reaction, has small simulation, remarkably improves the clinical compliance of patients and has good water solubility and high convenience for medication by clinicians. The powder injection has good stability, can be stored at room temperature and is favorable for storage and transportation. The powder injection is simple in preparation process, easy to control quality, low in production cost and convenient for industrialized production and also greatly reduces the economic burden of the patients for medication. The invention also discloses a method for preparing the drug composition and clinical application thereof.
Owner:姚定全
Phosphatide composition of active skull cap components and its prepn process and prepn
The present invention discloses phosphatide composition of active skullcap components and its preparation. The active skullcap components are prepared through extracting and separating skullcap root, and contain baicalin or wogonin over 50 %. The active skullcap components have low water solubility and fat solubility, solubility increasing with raised solution pH value, and easy chemical degradation in alkaline condition, so that the active skullcap components can not be prepared into injection and oral preparation directly. By means of phosphatide composition technology, the present invention improves the water dispersivity and lipophilicity of the active skullcap components, so as to prepare oral preparation with high bioavailability, freeze dried powder for injection, fat emulsion and mucous membrane administration preparation.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
Hydroypropyl-beta-cyclodextrin inclusion of Rhizoma Zedoariae oil and preparation and preparing method
InactiveCN1457798AImprove stabilityImprove bioavailabilityOrganic active ingredientsAntiviralsOrganic solventExternal application
The hydroxypropyl-beta-cyclodextrin inclusion of Rhizoma zedoariae oil consists of Rhizoma zedoariae oil and hydroxypropyl-beta-cyclodextrin in the ratio of 1 to 1-100. Rhizoma zedoariae oil dissolved in some organic solvent is added into water solution of hydroxypropyl-beta-cyclodextrin via stirring, and the mixture is freeze dried into water soluble inclusion. The included water solution is ultrafiltered, filtered in 0.22 micron porous film and freeze dried to prepare the freeze dried powder for injection. The present invention may be also used for oral taking and external application to result in antiviral and antibacterial effect.
Owner:董英杰
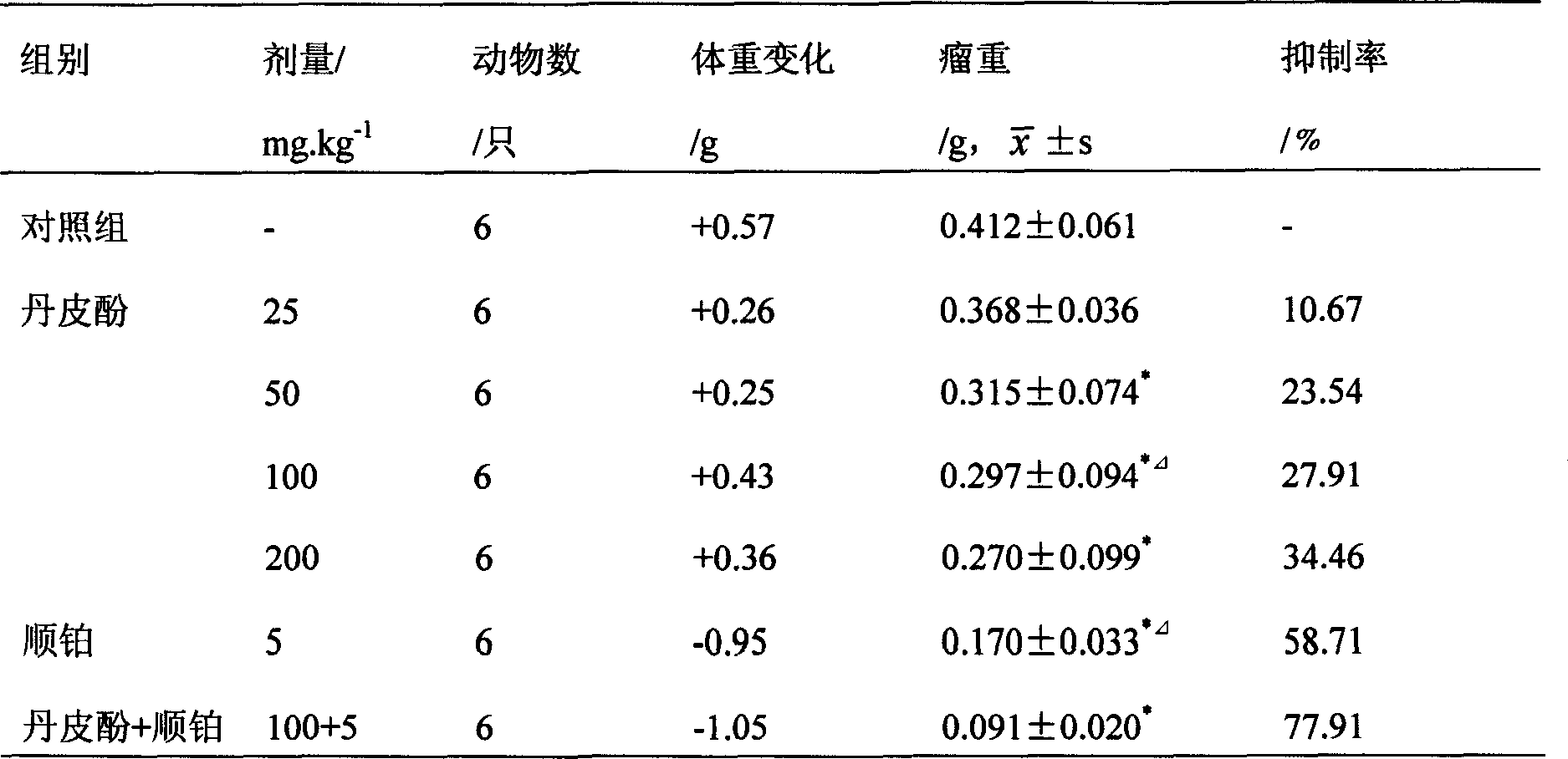
Application of paeonol for preventing and treating fatty liver disease
InactiveCN1919187AEstablish the basis for treatmentLow priceDigestive systemPharmaceutical delivery mechanismFatty liverFluid infusion
Disclosed is the use of paeonol in preparing medicament for the prevention or treatment of fatty liver, and also the use of medicinal combination preparation of paeonol with medicinal carrying agent in preparing medicaments for the prevention and treatment of fatty liver disease, the dose forms of the medicament include soft capsules, drop pills, pills, powders, tablets, capsules, granules, injections, fluid infusions, freeze-dried powders for injection, suppositories, film agents, tiny capsules, tincture, syrup, oral liquids and other pharmaceutically acceptable dose forms.
Owner:戴敏
Pharmaceutical compositions comprising docetaxel and methods for preparation thereof
InactiveUS8044093B2High stability and solubilityEasy to makeOrganic active ingredientsBiocideDocetaxel-PNPInjectable Solution
A pharmaceutical composition of docetaxel comprising an effective amount of docetaxel, a polysorbate (TWEEN® compound) and a co-solvent, wherein the co-solvent is at least one member selected from the group consisting of glycerol and polyethylene glycol. The composition is an injectable solution or a freeze-dried powder for injection. The solubility of decetaxel is improved by adding a polysorbate and a co-solvent. Methods of preparation of the pharmaceutical composition are also disclosed.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
Complex rheum officinale-radix scutellariae extract injecta for treating acute pancreatitis and preparing method thereof
The present invention provides a compound emodin baicalein injection for curing acute pancreatitis and its preparation method. Said invention adopts the combination of ultrasonic wave method and conventional method to respectively extract effective component emodin and baicalein pure products from rhubarb and scutellaria root, then adopts the following steps: adding the emodin and baicalein pure products and required correspondent auxiliary material into hot injection water, using NaOH solution to regulate pH, adding active carbon, heating to foiling, cooling and filtering by using microporous filtration membrane, adding injection water to total quantity, filling and sterilizing for 30 min at 115 deg.C so as to obtain the injection, cold-storing said injection for 1 week, filtering, filling and freeze-drying, sealing so as to obtain the freeze-dried powder injection finished product.
Owner:XI AN JIAOTONG UNIV
A pharmaceutical composition for targeted tumor therapy and its preparation method
The patent of the present invention relates to a pharmaceutical composition containing antitumor antibiotic-dipeptide derivatives that can be used for targeted tumor therapy and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The pharmaceutical composition is an injection composed of antitumor antibiotic-dipeptide derivatives, phospholipids, stabilizers, organic solvents, antioxidants, surfactants, and pH regulators. Dry powder needle. The pharmaceutical composition greatly increases the solubility and stability of the antitumor antibiotic-dipeptide derivatives with targeted antitumor effects in aqueous solution, and can be effectively used for malignant epithelial tumors (such as breast cancer, ovarian cancer, Targeted therapy for lung cancer, colon cancer, pancreatic cancer, skin melanoma) and other solid tumors.
Owner:重庆寰瑞生物技术有限公司
6, 9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5, 10-dione dimaleate and synthesis technology thereof
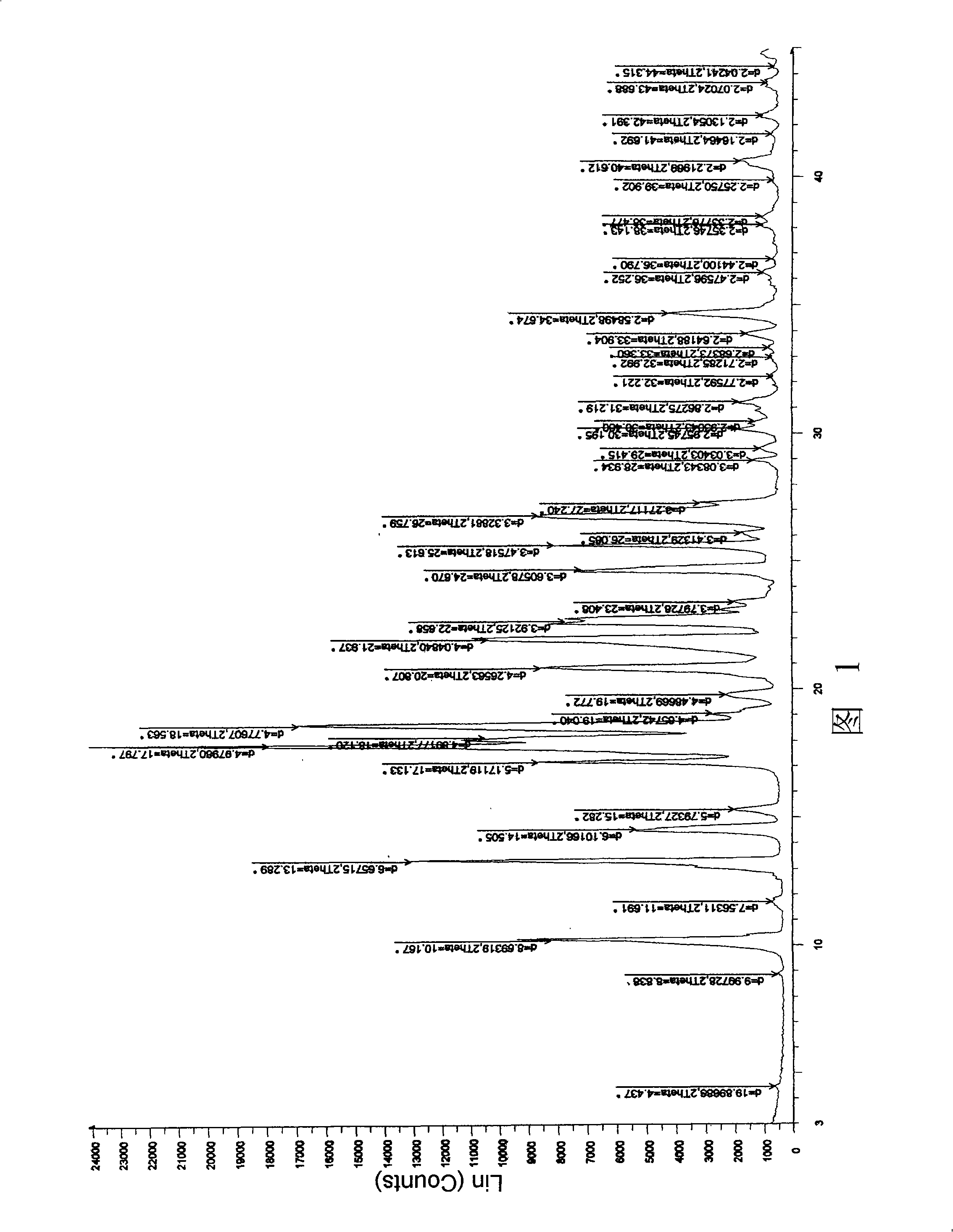
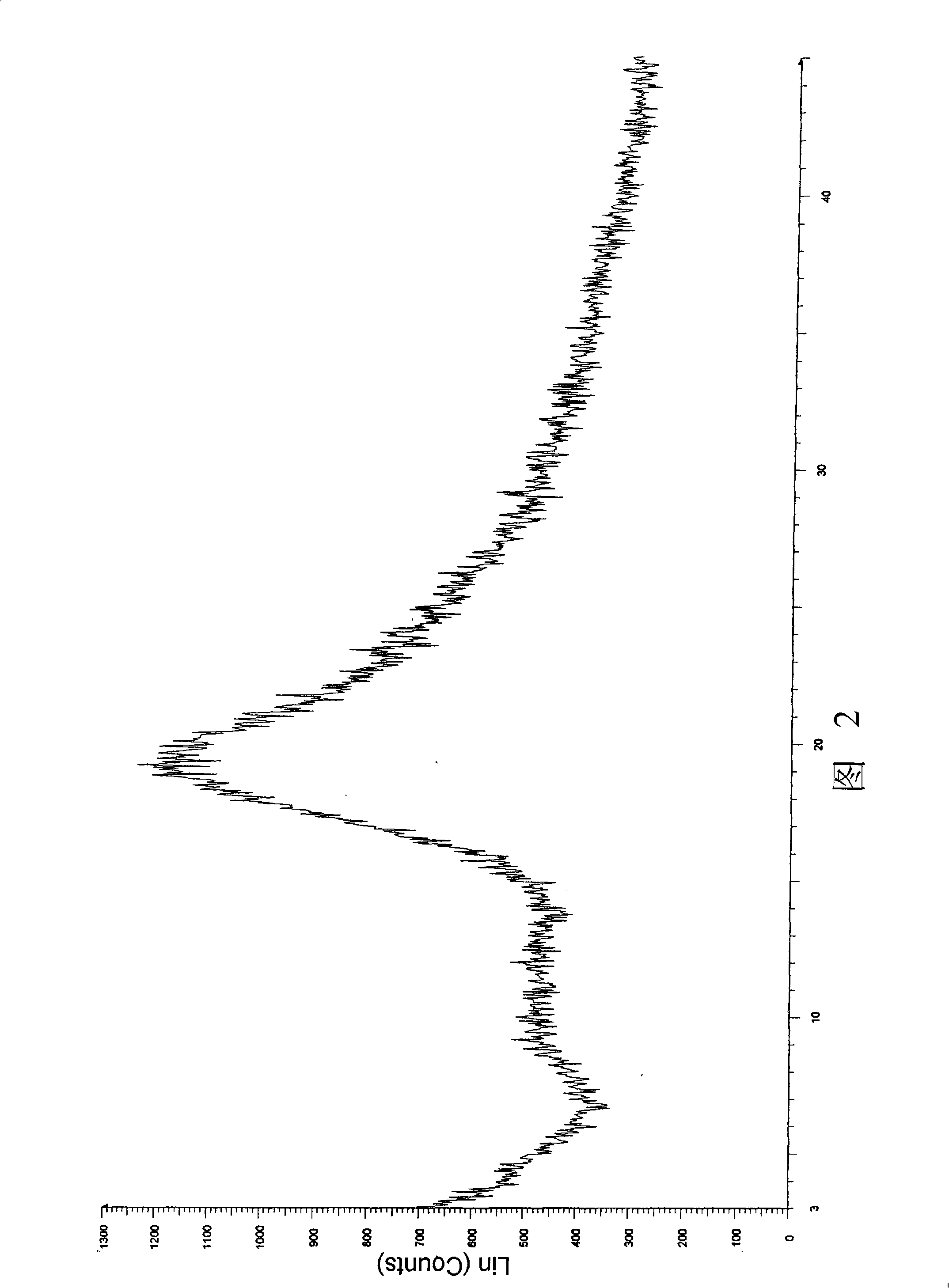
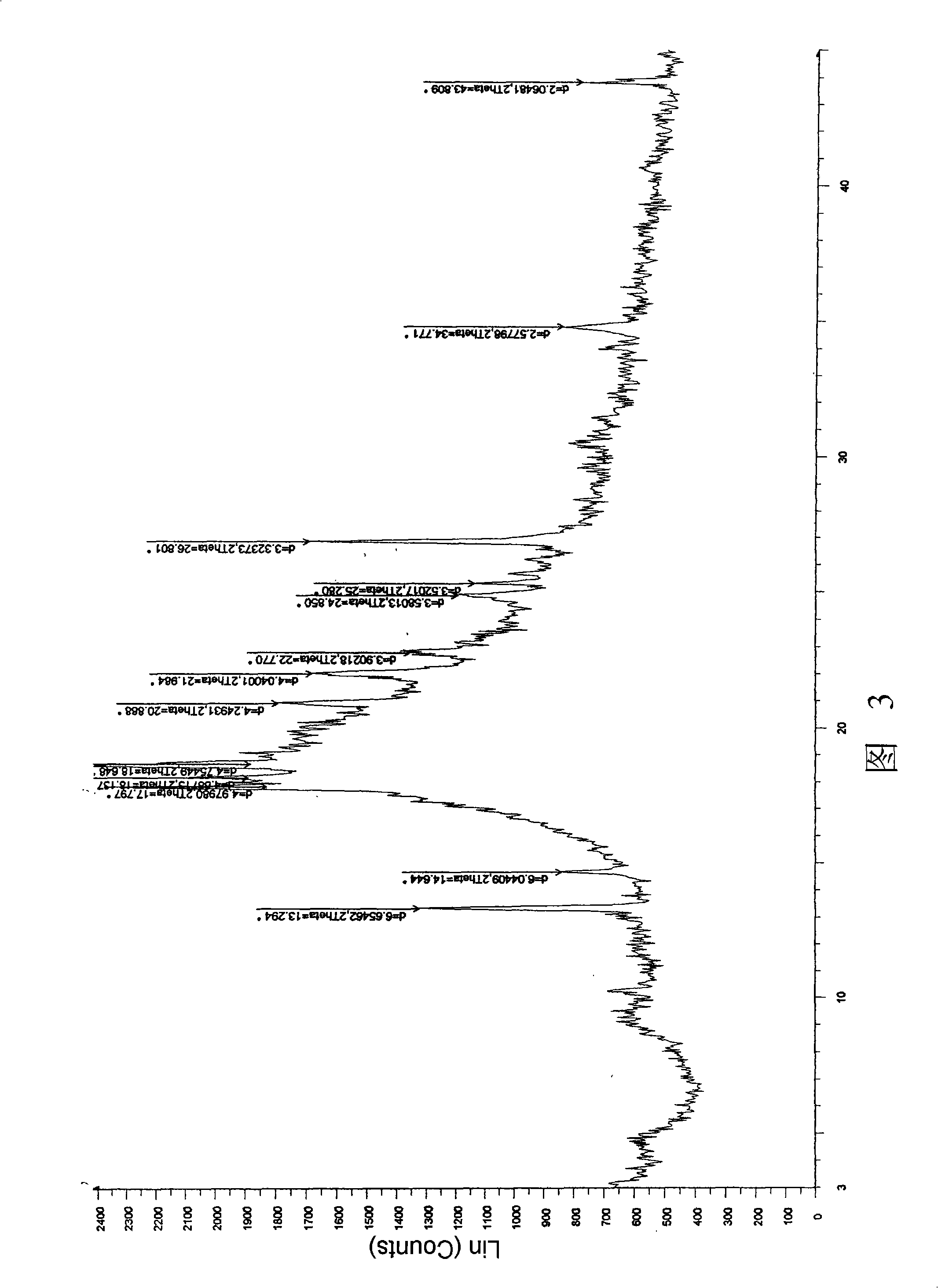
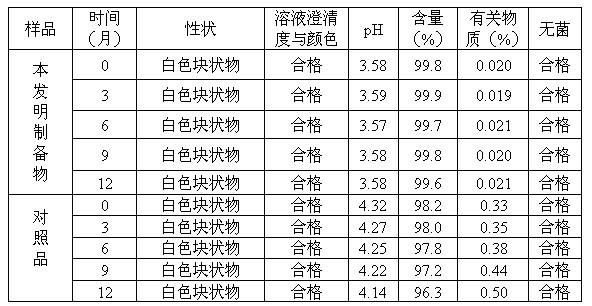
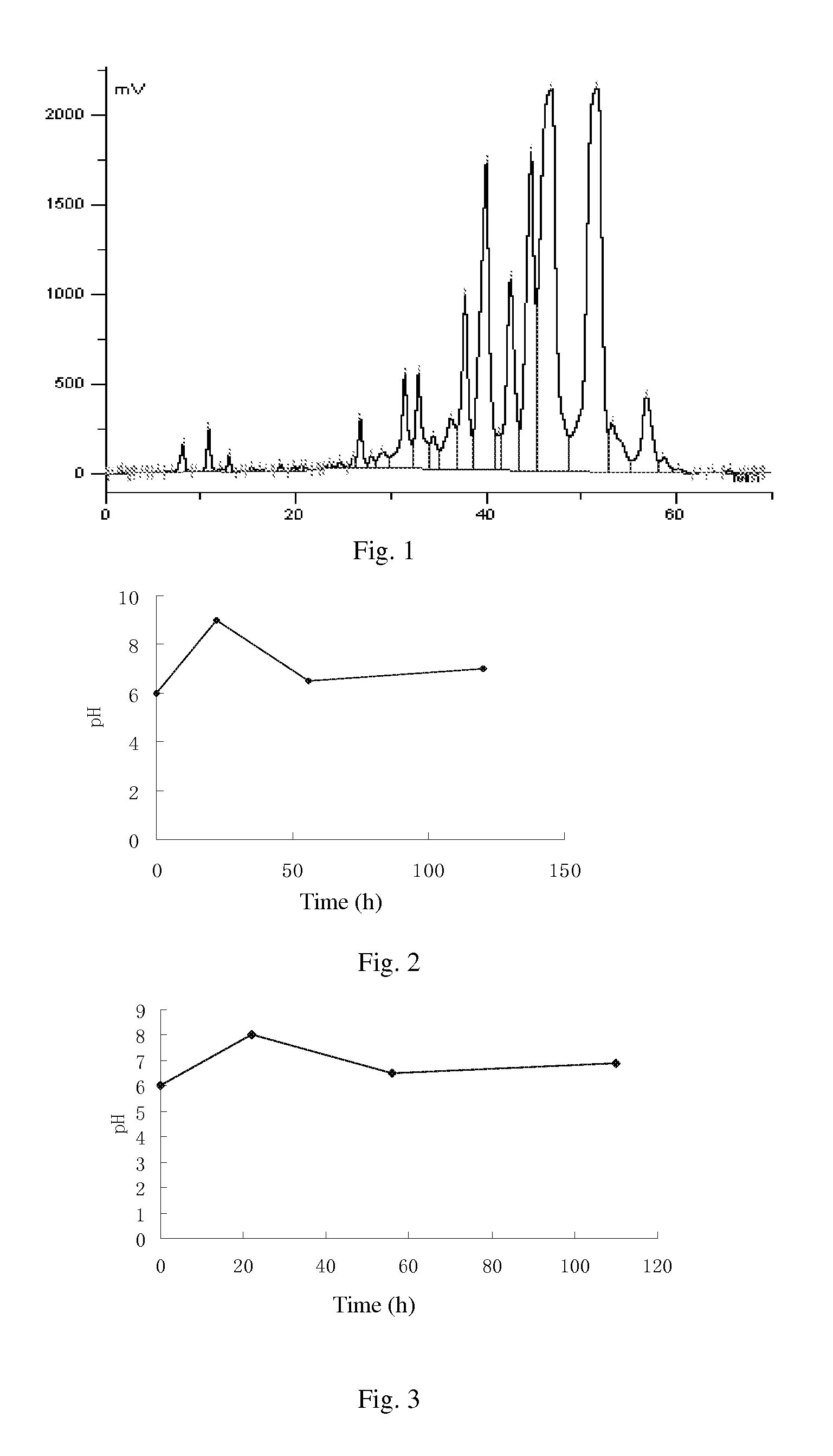
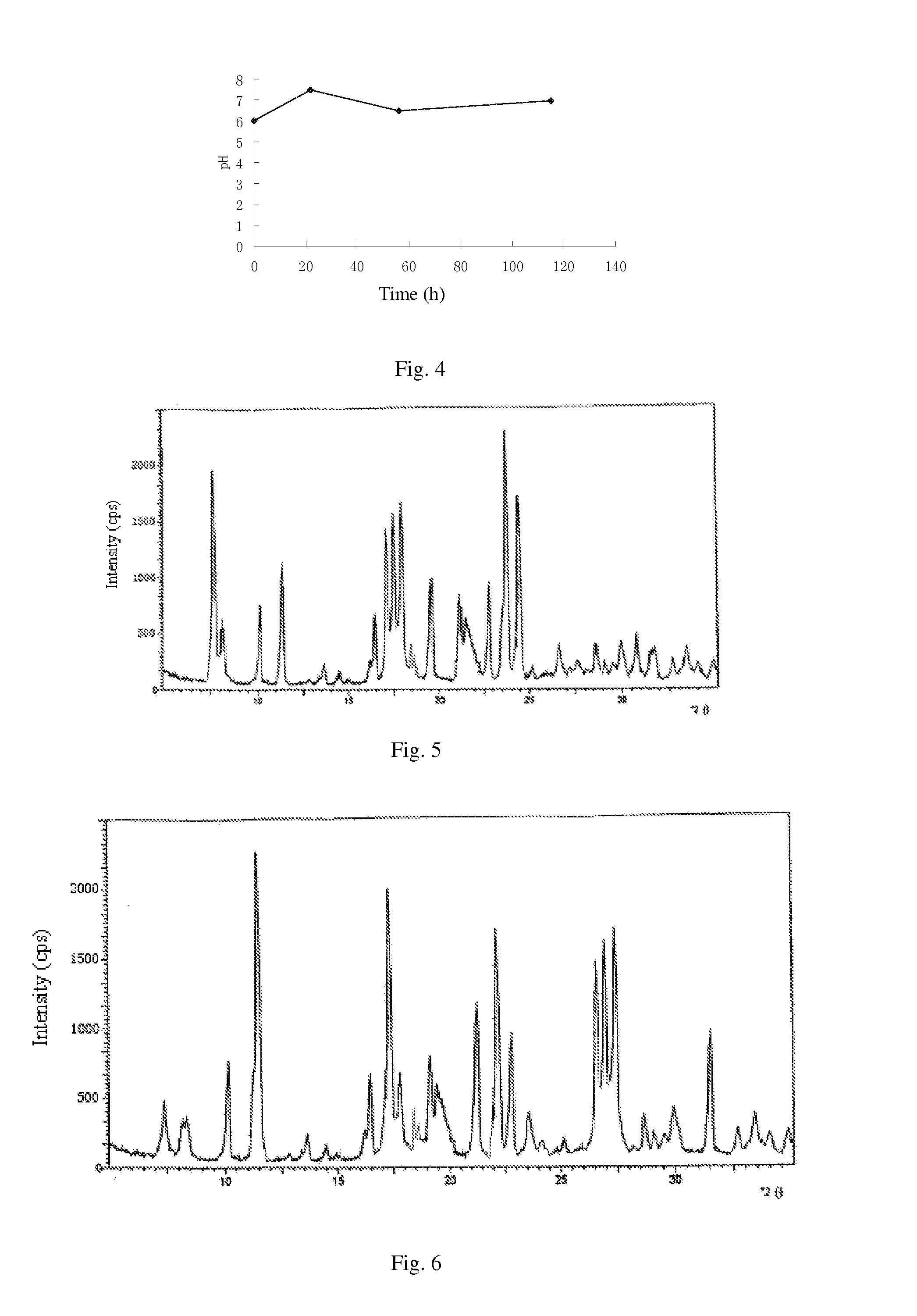
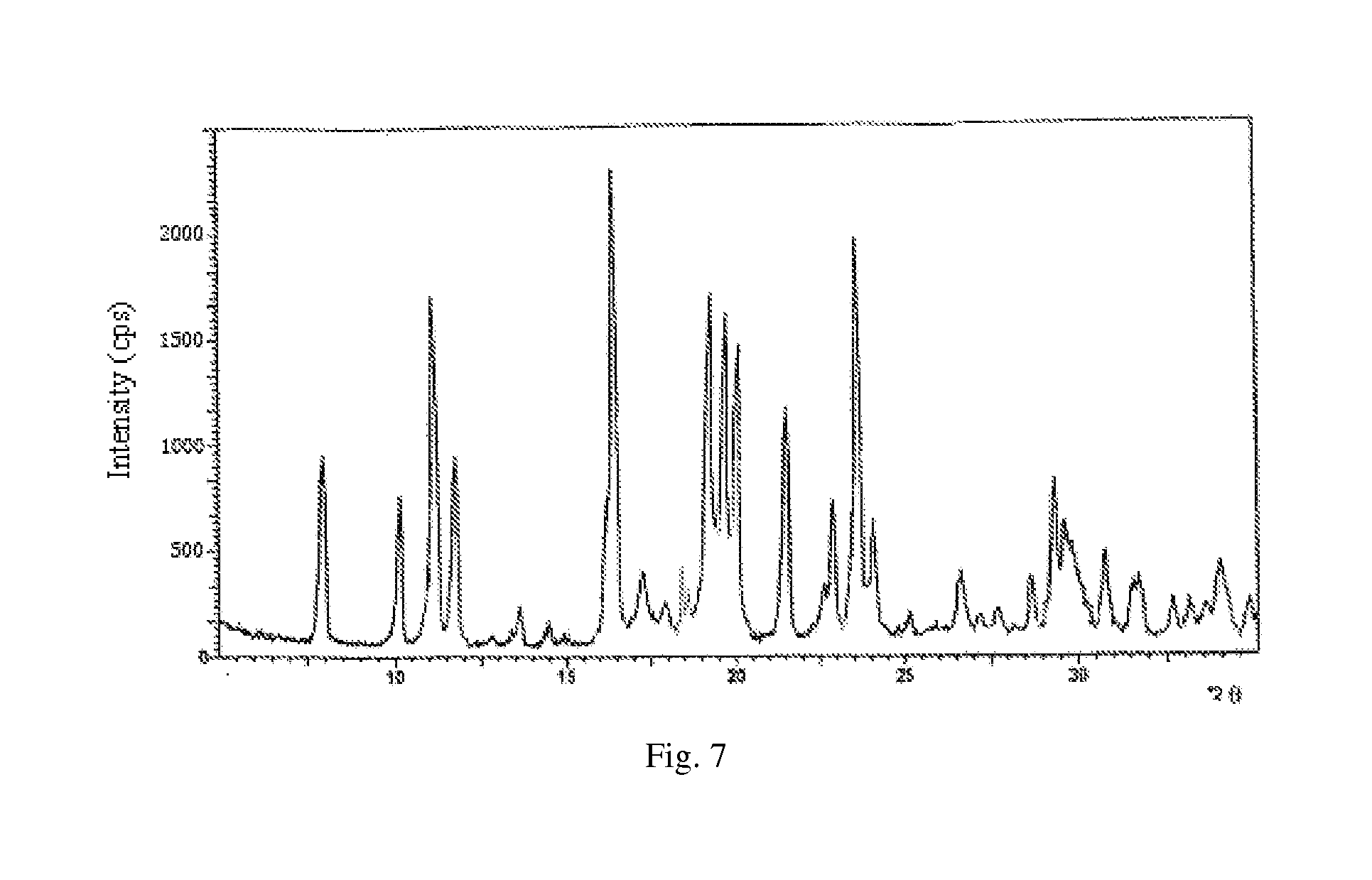
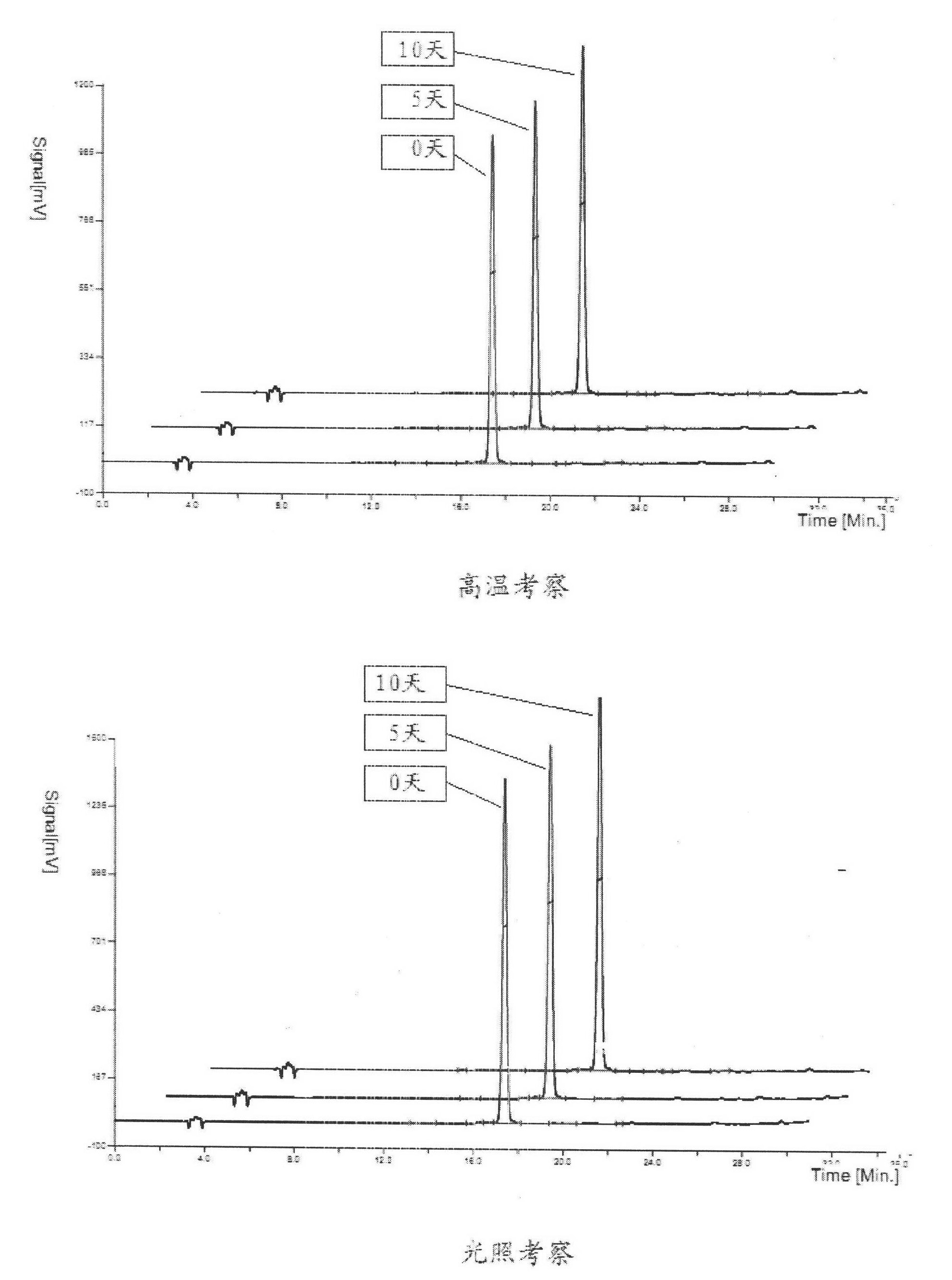
ActiveCN106366036AMild reaction conditionsSimple reaction conditionsOrganic chemistryFreeze-dryingTert-Butyloxycarbonyl protecting group
The invention discloses a synthesis technology of 6, 9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5, 10-dione dimaleate and relates to the technical field of antineoplastic drug synthesis. The synthesis technology comprises that 3, 4-pyridinedicarboxylic anhydride and hydroquinone undergo a reaction in the presence of a catalyst to produce a first intermediate, the first intermediate and N-t-butoxycarbonylethylenediamine undergo a reaction to produce a second intermediate, the second intermediate is subjected to deprotection, and the product and maleic acid undergo a salt forming reaction to produce a product. The prepared product has purity greater than 99.5% and known single impurity and unknown single impurity contents less than 0.1%. The important intermediate of the synthesis technology has stable properties and is convenient for storage. The synthesis technology allows mild reaction conditions, has simple processes, realizes a low cost and is suitable for industrial production. The invention also provides pixantrone dimaleate. The pixantrone dimaleate can be processed to form a freeze-dried powder injection for treating human aggressive non-Hodgkin's lymphoma with easy recurrence and high treatment difficulty.
Owner:HUBEI LIYI PHARM TECH CO LTD
Sulphur butyl ether-beta-cyclodextrin clathrate compound of cinnarizine, formulated product and preparation method thereof
InactiveCN101314045AOrganic active ingredientsMacromolecular non-active ingredientsFreeze-dryingDissolution
the invention belongs to the drug technical field, disclosing a sulphur butyl ether-beta- cyclodextrin(SBE-beta-CD) clathrate compound of cinnarizine, a preparation thereof and a preparation method thereof. The water soluble beta- cyclodextrin derivative SBE-beta-CD is used as a coating material to prepare the cinnarizine clathrate compound. The process comprises the following steps that: the BE-beta-CD is dissolved in proper amount of water, the pH value is adjusted by hydrochloric acid, the cinnarizine is added in the mixed solution and stirred for ultrasonic dissolution, the mixed solutionobtained is added with sodium hydroxide to adjust the pH value, a clathrate solution is obtained, and the clathrate solution is subject to freeze drying or spray drying to form clathrate compound powder; the clathrate compound solution obtained is subject to degerming, pyrogen removal, sterile filling and freeze drying to obtain freeze-dry powder needles for injection; the clathrate compound solution is subject to spray drying, and the obtained powder is directly subject to sterile subpackaging or mixed with proper amount of auxiliary materials for sterile subpackaging; and the clathrate compound powder can be mixed with other auxiliary materials to prepare tablets, capsules and particles, etc. according to requirements.
Owner:SHENYANG PHARMA UNIVERSITY
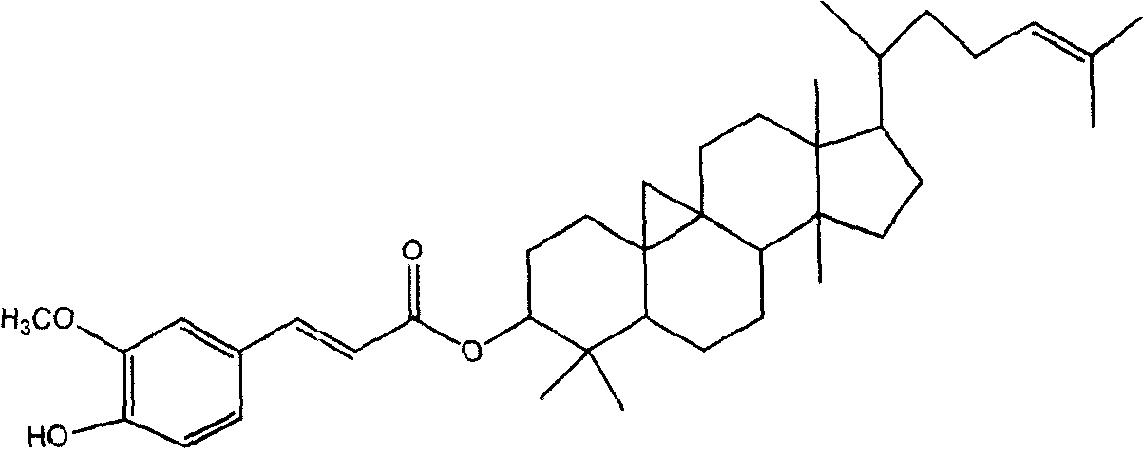
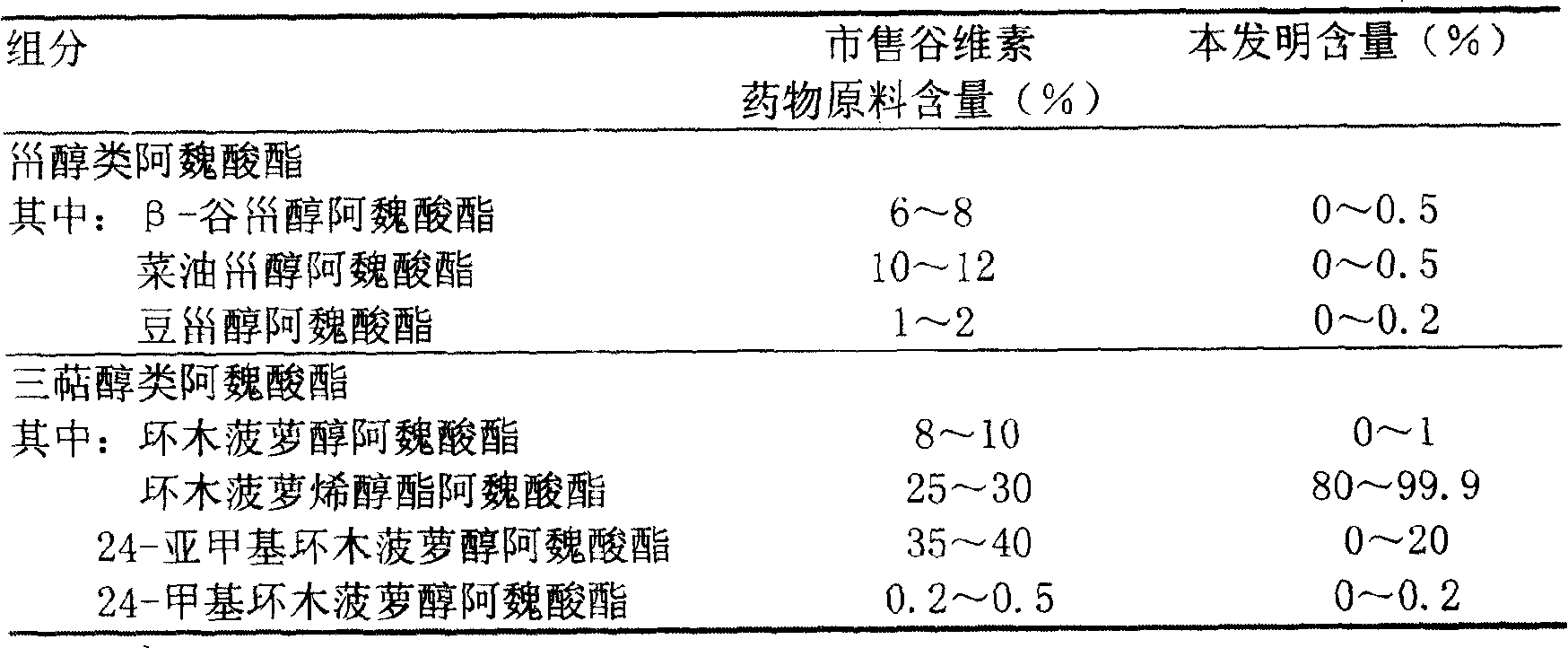
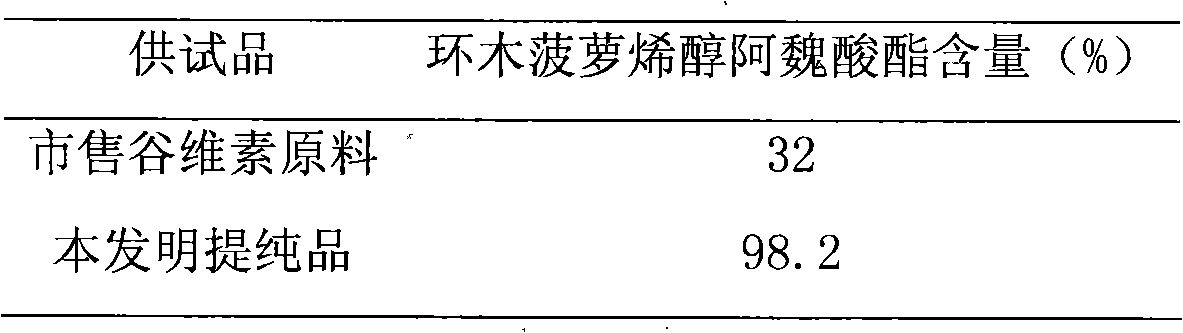
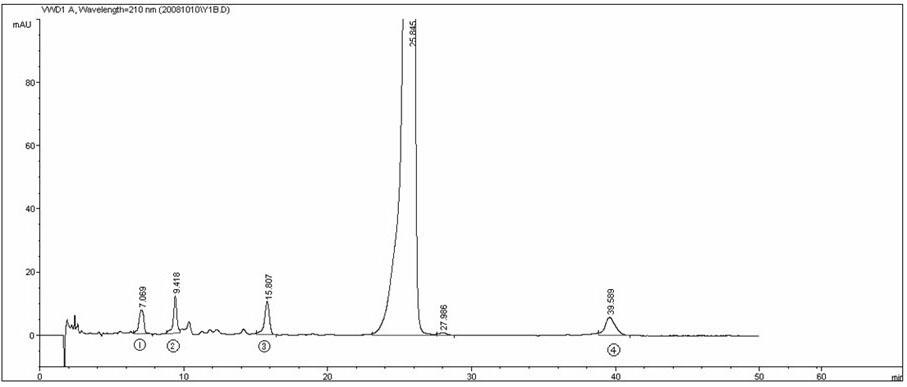
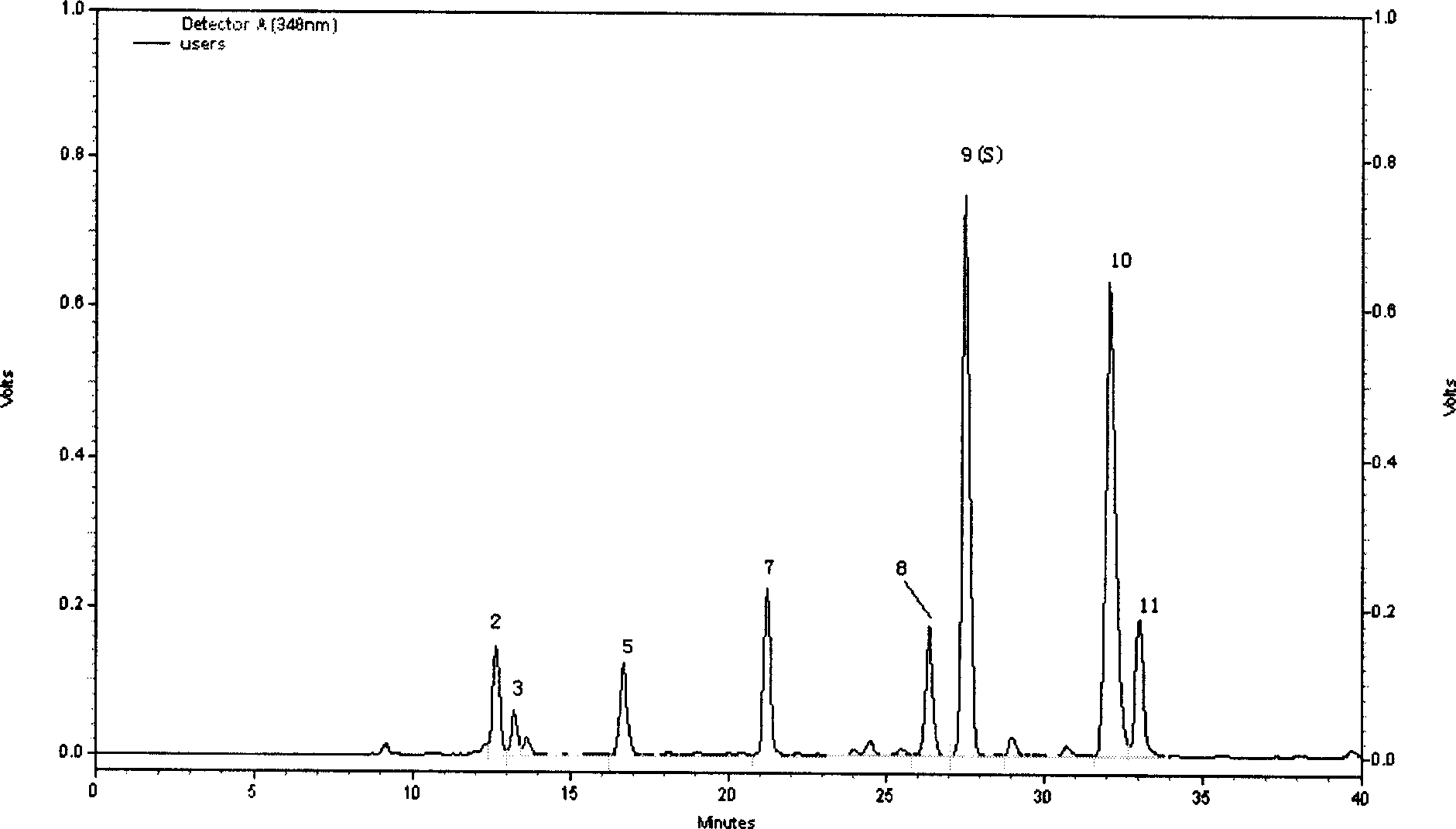
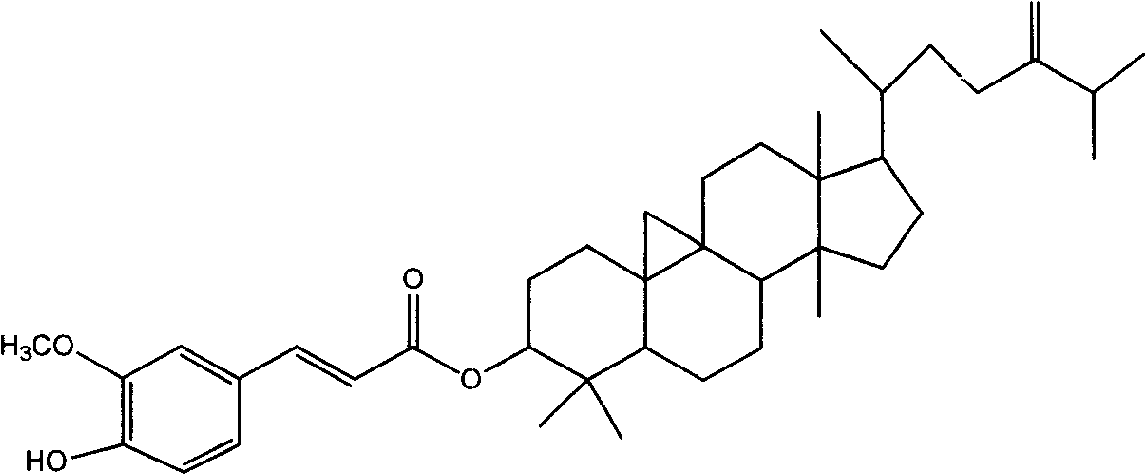
Preparation of compound of cycloartenyl ferulate and purification process thereof
ActiveCN101596200AActivity advantageOrganic active ingredientsNervous disorderCycloartenyl ferulateFreeze-drying
The invention discloses a technical scheme for preparing cycloartenyl ferulate from raw material of oryzanol and further discloses the application of the prepared cycloartenyl ferulate and surfactant to medicinal compound and preparation. Due to the purification process of the cycloartenyl ferulate by recrystallization, the purity of the cycloartenyl ferulate can achieve over 80%. The invention also discloses injection of the compound, particularly freeze-dried powder injection.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
Medicinal composition of trichosanthes rind and its preparation method
InactiveCN1823875AImprove stabilitySolve the real problemPowder deliveryLyophilised deliveryDiseaseFreeze-drying
A freeze-dried powder injection for treating cardiovascular and cerebrovascular diseases is prepared from the extract of trichosanthes bark (5-100 Wt%) and the harmacologically acceptable auxiliary (0-5 Wt%). Its preparing process is also disclosed.
Owner:广东仁泰药业有限公司
Stabilizer of lyophilized powder injection for azithromycin injection
InactiveCN102552918AImprove stabilityReduce contentAntibacterial agentsOrganic active ingredientsAzithromycin InjectionDrug product
The invention belongs to the field of chemical drugs, and specifically relates to a stabilizer of a lyophilized powder injection for azithromycin injection. The stabilizer is characterized in that the stabilizer is prepared by mixing citric acid and sodium hydroxide, and a mass ratio of the main medicine to the stabilizer is 41-73%:26-59%. With the stabilizer of the present invention, the impurity content in the lyophilized powder injection for azithromycin injection can be effectively reduced, the drug stability can be improved, and the drug use safety can be ensured.
Owner:SHANDONG QIDU PHARMA
Gutweed total flavone, its prepn. method, freeze-drying powder injection containing it and quality control method therefor
ActiveCN1868498AReduced activityOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
A general ixeris flavone containing luteolin-7-0-beta-D-phranoglucuronide (C21H18O12), a freeze-dried powder injection prepared from said general ixeris flavone, mannitol and the water for injection, and the preparing process and quality control method of said freeze-dried powder injection are disclosed.
Owner:TONGHUA HUAXIA PHARMA
Pharmaceutical use of paeonol for anti esophageal cancer
InactiveCN1935129ASmall side effectsNo adverse reactionKetone active ingredientsAntineoplastic agentsEsophageal cancerCarcinoma
Owner:孙国平 +2
Milrinone composition for injection and its prepn
InactiveCN1739512AHigh concentration of milrinoneOrganic active ingredientsPowder deliveryHigh concentrationMilrinone
The present invention provides one kind of freeze dried high concentration milrinone powder for injection and its preparation process. The freeze dried milrinone powder for injection contains: milrinone in 2-10 weight portions; pharmaceutically acceptable acid matter, which is L-aspartic acid, tartaric acid, citric acid, glutamic acid or their combination, in the amount of 0.25-4 times weight of milrinone; and pharmaceutically acceptable carrier in 15-100 weight portions. The freeze dried milrinone powder for injection may be dissolved in water to form injection of milrinone in 2-10 mg / ml concentration, and has high stability.
Owner:ZHEJIANG ZHENYUAN PHARMA CO LTD
Human relaxin-2 lyophilized powder preparation for injection
InactiveCN102133200AImprove stabilityFull appearancePowder deliveryPeptide/protein ingredientsDodecyl-beta-D-maltosideMannitol
The invention discloses a human relaxin-2 lyophilized powder preparation for injection, which is composed of human relaxin-2 and medically acceptable medicinal auxiliary materials, wherein the mass ratio of the medically acceptable medicinal auxiliary materials to the human relaxin-2 is (0.2-1.5):1; and the medically acceptable medicinal auxiliary materials are selected from one or more of mannitol, trehalose, polyvinylpyrrolidone, dodecyl-beta-D-maltoside, hydroxypropyl-beta-cyclodextrin, calcium phosphate and zinc carbonate. The inventors of the invention perform a large number of pharmaceutical and animal experiments, and indicated by research, the human relaxin-2 lyophilized powder preparation for injection disclosed by the invention has good stability and full appearance, does not degrade, aggregate or generate conformational change under accelerated test conditions, and has good safety in vivo.
Owner:山东长肽医药科技有限公司
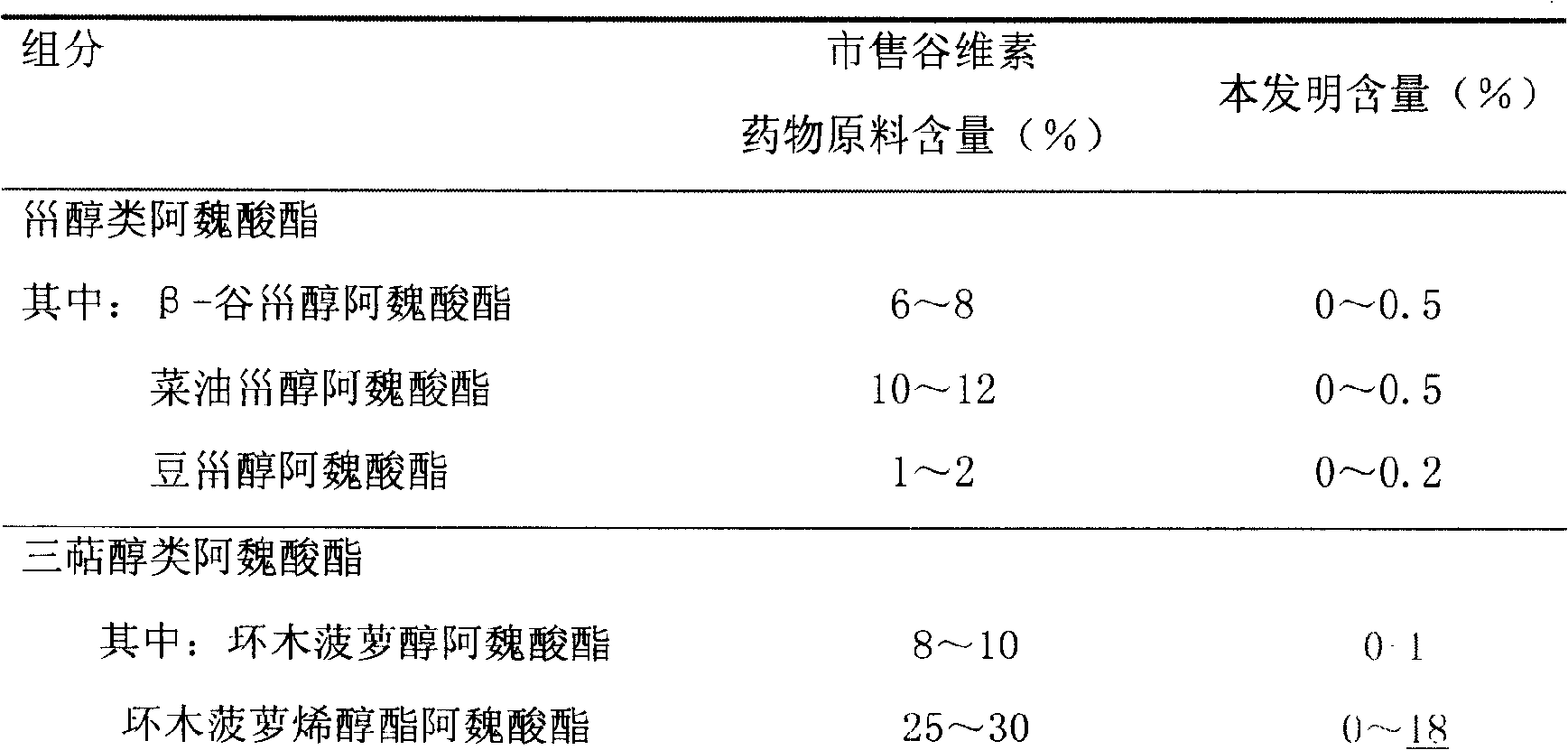
Composition preparation of 24-methylene cycloartenol feruloyl esterase and purification process thereof
ActiveCN101590008AOvercome purityOvercoming the problems of its practical medicinal use etc.Organic active ingredientsNervous disorderPEG 400Freeze-drying
The invention discloses a composition preparation of 24-methylene cycloartenol feruloyl esterase, and a purification process thereof. The purity of 24-methylene cycloartenol feruloyl esterase purified by the purification process can reach more than 80%. The composition disclosed by the invention contains 24-methylene cycloartenol feruloyl esterase and surface active agent, wherein, the surface active agent is selected from Tween 80, polyethylene glycol 400 or mixture thereof. Meanwhile, the invention also discloses an injection of the composition, in particular to freeze-dry powder injection for injection.
Owner:BEIJING CENTURY BIOCOM PHARMA TECH
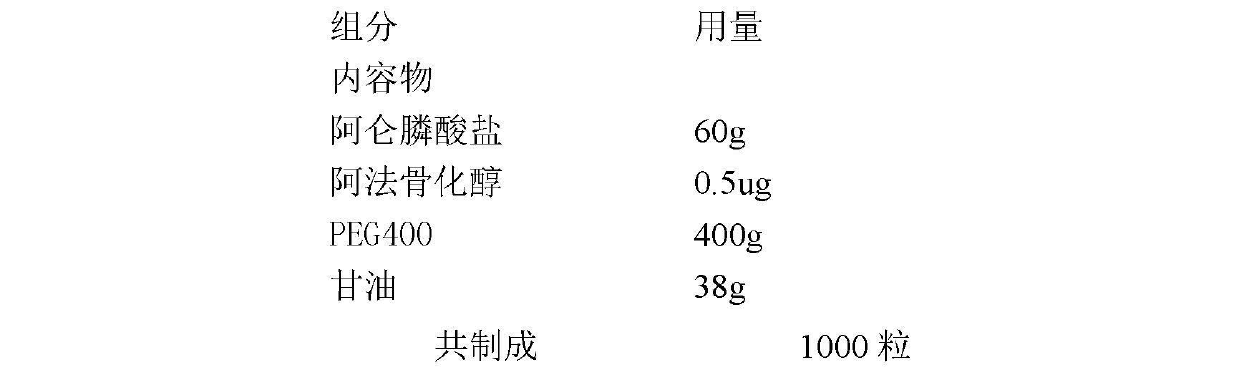
Medicinal composition for preventing and treating osteoporosis
InactiveCN102670627AOrganic active ingredientsSkeletal disorderEffervescent tabletSustained Release Tablet
The invention discloses a medicinal composition for preventing and treating osteoporosis, relates to a medicinal composition of bishosphonates and the derivatives of vitamin D and the like, wherein the medicinal composition can be produced into oral preparations or injection preparations by virtue of being mixed with pharmaceutically acceptable auxiliary materials; the oral preparations comprise soft capsules, capsules, common tablets, chewable tablets, dispersible tablets, orally disintegrating tablets, effervescent tablets, buccal tablets, sustained release tablets, sustained release capsules, oral solution, syrup, and the like; and the injection preparations comprise small-volume injections, large-volume injections, freeze-dried powder for injection, and the like. The medicinal composition can be used for preventing and treating various primary or secondary types of osteoporosis.
Owner:FUKANGREN BIO PHARMA
Freeze dried nimodipine composition and its prepn
InactiveCN1771950AImprove solubilitySimple processOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The present invention discloses one kind of freeze dried nimodipine composition and its preparation. The freeze dried nimodipine composition consists of nimodipine, surfactant, polyglycol as solvent, excipient glycerin and mannitol, etc. The freeze dried nimodipine composition has simple preparation process, high bioavailability, excellent dissolubility and stability, and easy preservation.
Owner:SHENYANG PHARMA UNIVERSITY
Freeze-dried powder and injection preparation of red sage root and safflower, and preparation method
A freeze-dried powder injection is prepared from red sage root and safflower. Its preparing process features that the water-soluble antioxidant is used in hydrothermal extraction, high-speed centrifuge, macroreticular adsorption and ultrafilter by hollow fibre column are used. Its advantages are high curative effect and high quality.
Owner:张正生
Phospholipid complex of natural Baikal skullcap root active ingredients as well as preparation method and preparation thereof
ActiveCN102988484AImprove hydrophilicityImprove lipophilicityAntibacterial agentsDigestive systemSolubilityWater dispersible
The invention discloses a phospholipid complex of natural Baikal skullcap root active ingredients and a preparation method thereof. The natural Baikal skullcap root active ingredients mean an active fraction (the baicalein content is more than 50 percent) or an active ingredient (the baicalein content is more than 90 percent) of baicalein extracted and separated from Baikal skullcap roots serving as a Chinese medicament and an active fraction (the wogomin content is more than 50 percent) or an active ingredient (the wogomin content is more than 90 percent) of wogomin. The water solubility and lipid solubility of the natural Baikal skullcap root active ingredients are poor, the dissolubility of the active ingredients is increased along with rise of the pH value of a solution, and the active ingredients are easily chemically degraded under an alkali condition. Due to the insufficiency of the physical and chemical properties of the natural Baikal skullcap root active ingredients, the active ingredients cannot be prepared into injection, and the bioavailability is low after the active ingredients are orally taken. Through a phospholipid complex technology, the water dispersibility and lipophilic property of the Baikal skullcap root active ingredients are obviously improved, and then the Baikal skullcap root active ingredients can be prepared into high-bioavailability oral preparation, freeze-dried injection or lipid emulsion to meet the requirement for injection or mucosa administration.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
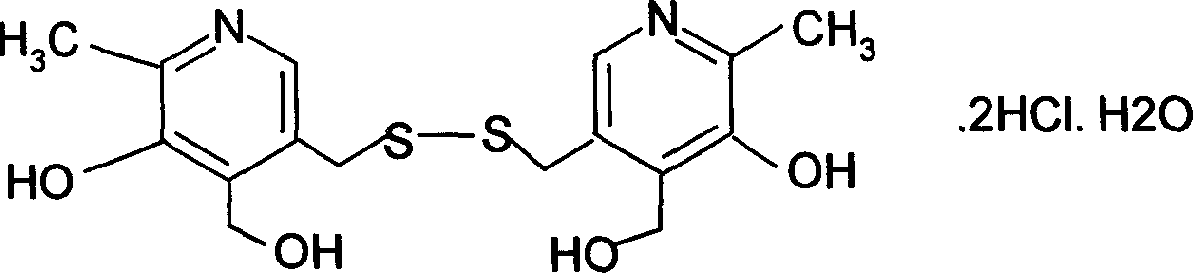
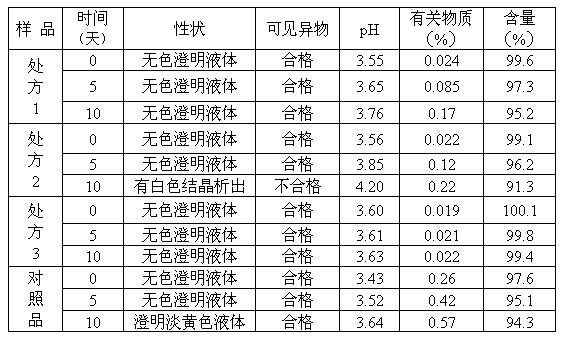
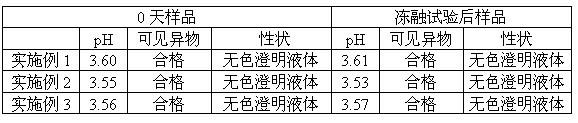
Pyrithioxine hydrochloride freeze-dried power injection and its preparing method
The present invention relates to a kind of freeze dried powder for intravenous injection with pyritinol hydrochloride as main component and its preparation process. The freeze dried powder for intravenous injection with pyritinol hydrochloride as main component is prepared by using water for injection or bacteria-free water as solvent, and mannitol and sodium chloride as supplementary material, and through freeze drying process. The present invention has no limitation of requiring bacteria-free material.
Owner:熊伟
Vinpocetine medicament composition and preparation method thereof
ActiveCN102600143AFew accessoriesImprove stabilityOrganic active ingredientsNervous disorderVinpocetineEthylene glycol
The invention relates to a vinpocetine medicament composition which is prepared from the following raw materials: 10-20g of vinpocetine, 20-40g of L-malic acid, 40-80g of polyethylene glycol400 and 2000ml of WFI (water for injection); and the pH is adjusted to 3.3-3.7. The vinpocetine medicament composition is in the form of injection lyophilized powder or injection. The vinpocetine medicament composition contains less auxiliary materials, is good in stability, and has high safety in clinical use.
Owner:湖北美林药业有限公司
Etoposide freeze-dry powder preparation for injection and preparation method thereof
InactiveCN101422439AConvenient for clinical operationImprove bioavailabilityPowder deliveryOrganic active ingredientsVeinIntramuscular injection
The invention relates to the technical field of medicament and discloses an etoposide freeze-dried powder preparation used for injection, which is convenient to be used in clinic, is high in bioavailability, is low in cost and can cure solid tumors. The medicament can quickly achieve effective curing concentration in a body when being directly injected through vein or muscle; moreover, the first-pass effect of the medicament to a liver is reduced, the bioavailability of the medicament in the body is improved, and the freeze-dried powder preparation used for injection is more convenient to be transported and can be stored for a longer time. The etoposide freeze-dried powder preparation used for injection contains the etoposide or the pharmaceutically acceptable salt thereof and pharmaceutically acceptable accessories used as active components; wherein, the weight percentage of the etoposide or the pharmaceutically acceptable salt thereof can be 1 to 80 percent, preferably be 30 to 70 percent and more preferably be 40 to 60 percent; the content range thereof in the preparation is generally 1 to 1000mg and preferably be 10 to 500mg.
Owner:李铁军
Levoisovalerylspiramycin i, ii or iii, preparations, prepartation methods and uses thereof
ActiveUS20130065848A1Improve antibacterial propertiesImprove pharmacological activityAntibacterial agentsBiocideAdjuvantAntibacterial activity
Disclosed are levoisovalerylspiramycin I, II or III, preparations, preparing methods and uses thereof. The preparations comprise levoisovalerylspiramycin I, II or III and pharmaceutically acceptable carrier and / or adjuvant, wherein the purity of levoisovalerylspiramycin I, II or III is above 90 wt %. The levoisovalerylspiramycin I, II or III has a good antibacterial activity, and the preparations include solution for injection, powder for injection or lyophilized powder for injection.
Owner:SHENYANG FUYANG PHARM TECH CO LTD
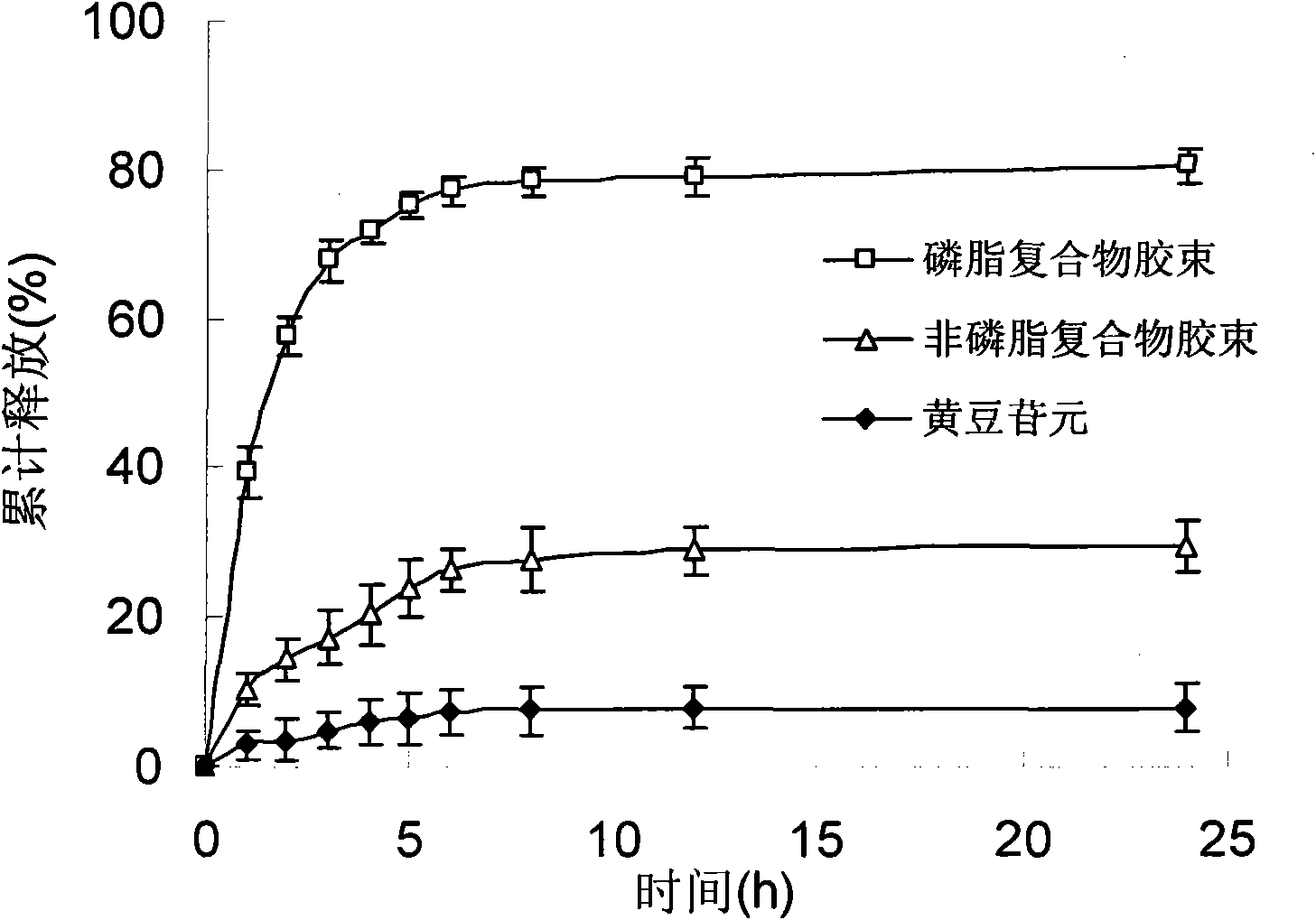
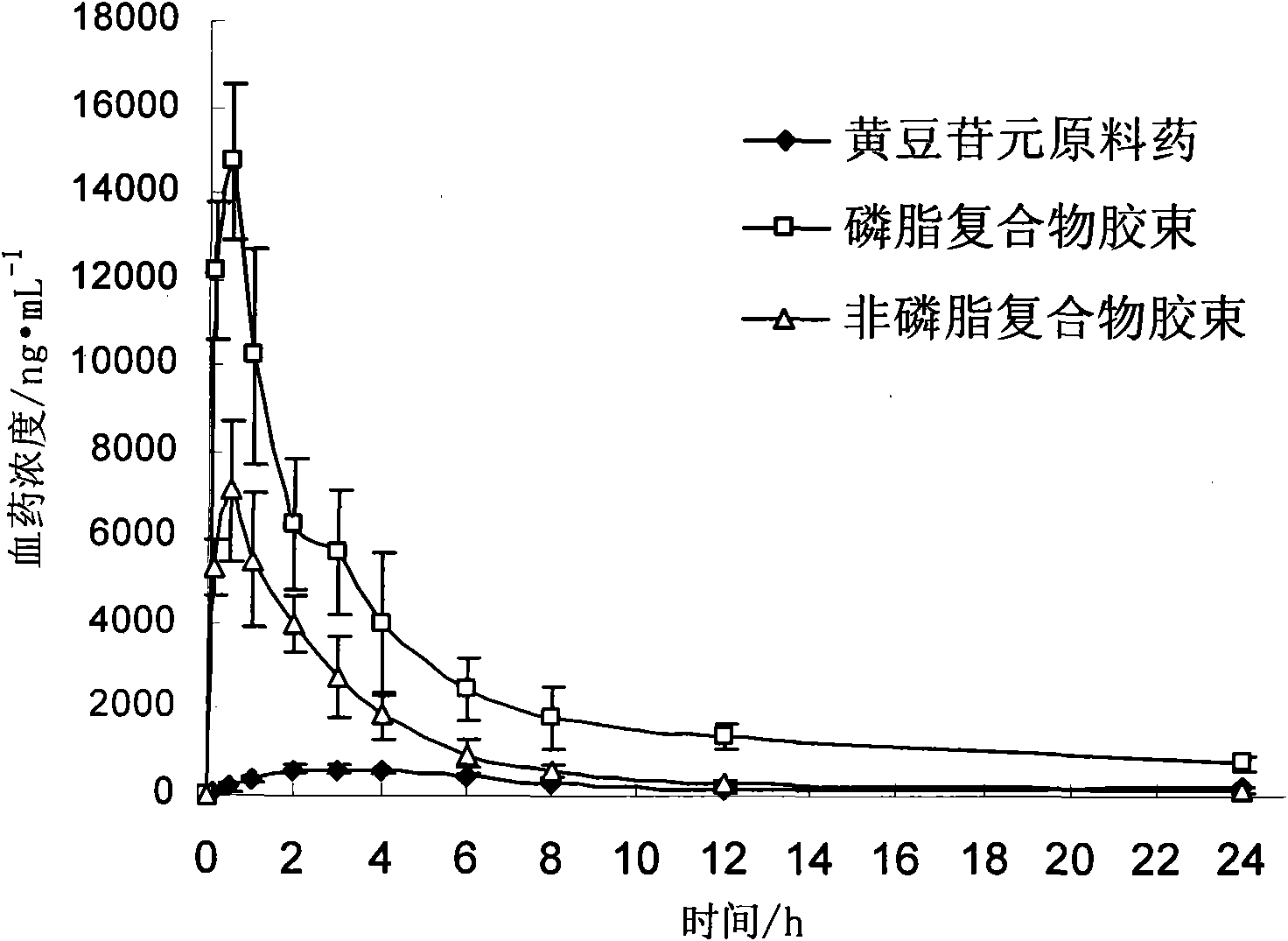
Daidzein micelles and preparation method thereof
InactiveCN102058528AImprove bioavailabilityIncrease route of administrationOrganic active ingredientsPowder deliveryFreeze-dryingDaidzein
The invention relates to daidzein micelles and a preparation method thereof. The daidzein micelles contain 1 part of daidzein, 12 to 30 parts of phospholipid and 1 to 25 parts of additive. The preparation method comprises: preparing a daidzein and phospholipid composite, namely, adding daidzein and 50 to 95 percent of phospholipid into an organic solvent, heating the solution to 40 to 60 DEG C, refluxing under reduced pressure, keeping temperature and stirring for 2 to 10 hours, recovering the organic solvent, drying and crushing to obtain the daidzein and phospholipid composite; and preparing daidzein micelles from the phospholipid composite, namely, dissolving the daidzein and phospholipid composite, the rest phospholipid and the additive in an organic solvent, subjecting the solution to rotary evaporation to form a film, and hydrating at 40 to 60 DEG C to obtain daidzein micelle suspension with opalescence. The average particle size of the daidzein micelles is less than 50 nanometers. The daidzein micelles can be further prepared into oral or injection preparations including capsules, oral mixed suspension, oral liquid, injection, injection freeze-dried powder injection.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Kalii dehydrographolidi succinas freeze-dried powder compound for injection and preparation method thereof
InactiveCN102657673AEasy to processImprove stabilityOrganic active ingredientsPowder deliverySodium bicarbonateFiltration
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Hydroxy propyl-beta-cyclodextrin inclusion compound of anisolene and its preparation and preparation method
InactiveCN1517395AImprove stabilityNo hemolytic reactionMacromolecular non-active ingredientsOrganic solventFreeze-drying
An inclusion compound of anisylene and hydroxypropyl-beta-cyclo-dextrin is prepared through dissolving anisylene in organic solvent, adding it to the aqueous solution of hydroxypropyl-beta-cyclodextrin, stirring, freeze drying to obtain the water-soluble inclusion. It can be used to prepare freeze-drying powder injection and tablet.
Owner:沈阳皓天万嘉医药科技有限公司
Levoleucovorin freeze-dried injection agent and preparation method and pharmaceutical use thereof
The invention provides a freeze-dried sterile injection powder containing an active component named levorotation folinic acid; the invention contains 9-100% levorotation folinic acid, 0-91% excipients acceptable in pharmacy or / and appropriate pH moderator. The pharmaceutical excipients refer to one or a plurality of mannitol, dextran, cane sugar, lactose, gelatin and phosphate. The pH moderator refers to one or a plurality of NaOH, natronite, kalium bicarbonicum, sodium phosphate, etc. The invention also relates to the preparation of the freeze-dried sterile injection powder. The invention avoids decomposition caused by high temperature sterilization in the process of levorotation folinic acid preparation, and is suitable for mass production; the invention is low in water content and stable when placed at room temperature, so the invention is suitable for long-term storage. The sterile injection powder of the invention can be taken as a synergist or attenuation agent in tumor chemotherapy.
Owner:SHANGHAI HUILUN JIANGSU PHARM CO LTD +1
Hydroxypropyl-beta-cyclodextrin clathrate, preparing method and application of radix bupleuri volatile oil
InactiveCN101176741AEasy to storeNot perishableAntibacterial agentsPowder deliveryFiltration membraneFreeze-drying
The invention relates to a hydroxypropyl-Beta-cyclodextrin inclusion compound of bupleurum volatile oil, the preparation method and the application. The bupleurum volatile oil is a fat-soluble component, is hard to dissolve in water, and generally is made into tablet for application, which has low biological utilization degree and influences the curative efficacy. The invention comprises the materials in weight ratio as follows: bupleurum volatile oil: hydroxypropyl-Beta-cyclodextrin = 1:1 to 100; the preparation method is as follow: the bupleurum volatile oil is added into the solution of hydroxypropyl-Beta-cyclodextrin, and then is processed with ultrasonic under the condition of 30 DEG C and 40 KHz, and after cooling and drying, the easily soluble inclusion compound is formed; after inclusion, the solution is remove from the heat source, and filtered with a filtration membrane with 0.22pm micropore, and then is freezed, dried, and made into freeze-dried power injection for injection; excipients are added into the inclusion compound to be made into sublingual tablets.
Owner:HEILONGJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com










![6, 9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5, 10-dione dimaleate and synthesis technology thereof 6, 9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5, 10-dione dimaleate and synthesis technology thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e5e08a16-2b87-4244-92b3-0d9e78e4287d/BDA0001083515110000021.png)
![6, 9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5, 10-dione dimaleate and synthesis technology thereof 6, 9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5, 10-dione dimaleate and synthesis technology thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e5e08a16-2b87-4244-92b3-0d9e78e4287d/BDA0001083515110000031.png)
![6, 9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5, 10-dione dimaleate and synthesis technology thereof 6, 9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5, 10-dione dimaleate and synthesis technology thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e5e08a16-2b87-4244-92b3-0d9e78e4287d/BDA0001083515110000032.png)