Medicinal composition for preventing and treating osteoporosis
A technology of osteoporosis and composition, applied in the field of medicine, can solve the problem of not inhibiting bone resorption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
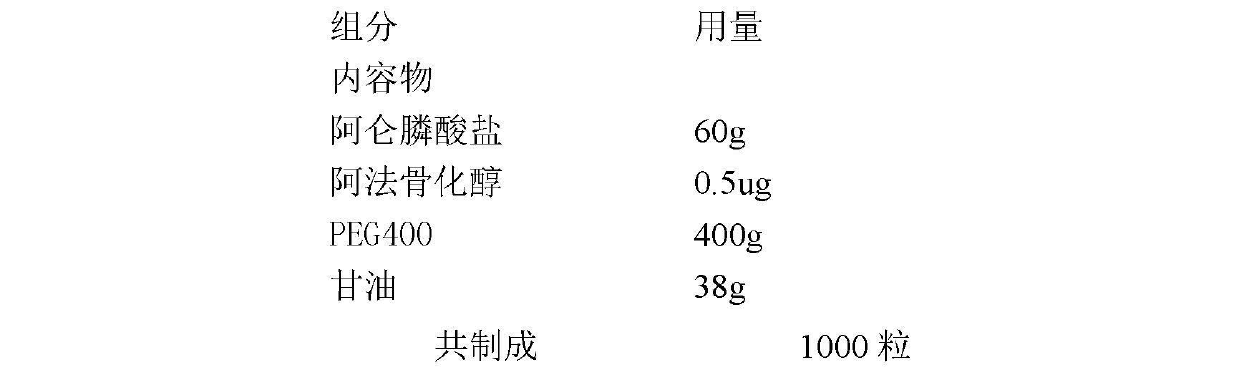
[0015] Alendronate, Alfacalcidol Softgels
[0016] prescription:
[0017]
[0018] Glue: dry gelatin: glycerin: water: titanium dioxide = 1:0.5:0.8:0.05
[0019] Preparation:
[0020] Content preparation: Weigh the prescription amount of alendronate, alfacalcidol, PEG400, glycerin, mix and dissolve and stir evenly, set aside;
[0021] Preparation of glue solution: first add water and glycerin (reserved 0.08%) into the glue tank in proportion to heat and stir, add gelatin when it is heated to 65-75°C, and continue stirring until the gelatin melts in hot water and becomes with glycerin and water After uniform glue solution, vacuumize while stirring until there are no air bubbles; weigh titanium dioxide, mix it with the reserved glycerin, add it to the colloid mill to grind it evenly, add it to the above glue solution, stir until the color is uniform, and the glue is prepared. liquid;
[0022] Pressing pills, shaping, drying: make the above glue into 0.5-1.0mm rubber, put ...
Embodiment 2
[0024] Clodronate, Alfacalcidol Soft Capsules
[0025] prescription:
[0026]
[0027]
[0028] Glue: dry gelatin: glycerin: water: titanium dioxide = 1:0.6:0.9:0.06
[0029] Preparation:
[0030] Contents preparation: Weigh the prescribed amount of clodronate, alfacalcidol, PEG400, glycerin, mix and dissolve, stir evenly, and set aside;
[0031] Preparation of glue solution: first add water and glycerin (reserved 0.08%) into the glue tank in proportion to heat and stir, add gelatin when it is heated to 65-75°C, and continue stirring until the gelatin melts in hot water and becomes with glycerin and water After uniform glue, vacuumize while stirring until there are no air bubbles; weigh silicon dioxide, mix with reserved glycerin, add colloid mill to grind evenly, add to the above glue, stir until the color is uniform, and the preparation is obtained good glue;
[0032] Pressing pills, shaping, drying: make the above glue into 0.5-1.0mm rubber, put the content into t...
Embodiment 3
[0034] Sodium etidronate, paricalcitol soft capsules
[0035] prescription:
[0036]
[0037] Glue: dry gelatin: glycerin: sorbitol: water: titanium dioxide = 1:0.5:0.1:0.7:0.05
[0038] Preparation:
[0039] Contents preparation: Weigh the prescribed amount of etidronate sodium, paricalcitol, and soybean oil, mix and dissolve, stir evenly, and set aside;
[0040] Glue preparation: first add water, glycerin (reserved 0.08%), and sorbitol into the glue pot in proportion to heat and stir, add gelatin when heated to 65-75°C, and continue stirring until the gelatin is melted in hot water and mixed with sorbitol After the alcohol, glycerin, and water become a uniform glue, vacuumize while stirring until there are no bubbles; weigh titanium dioxide, mix it with the reserved glycerin, add it to the colloid mill to grind it evenly, add it to the above glue, and stir until the color is uniform , that is to prepare the glue;
[0041] Pressing pills, shaping, drying: make the abov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com