Sulphur butyl ether-beta-cyclodextrin clathrate compound of cinnarizine, formulated product and preparation method thereof
A technology of cyclodextrin inclusion compound and sulfobutyl ether, which is applied in the field of medicine, can solve the problems of poor thermal stability, low drug concentration in solution injections, and poor drug safety and patient compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
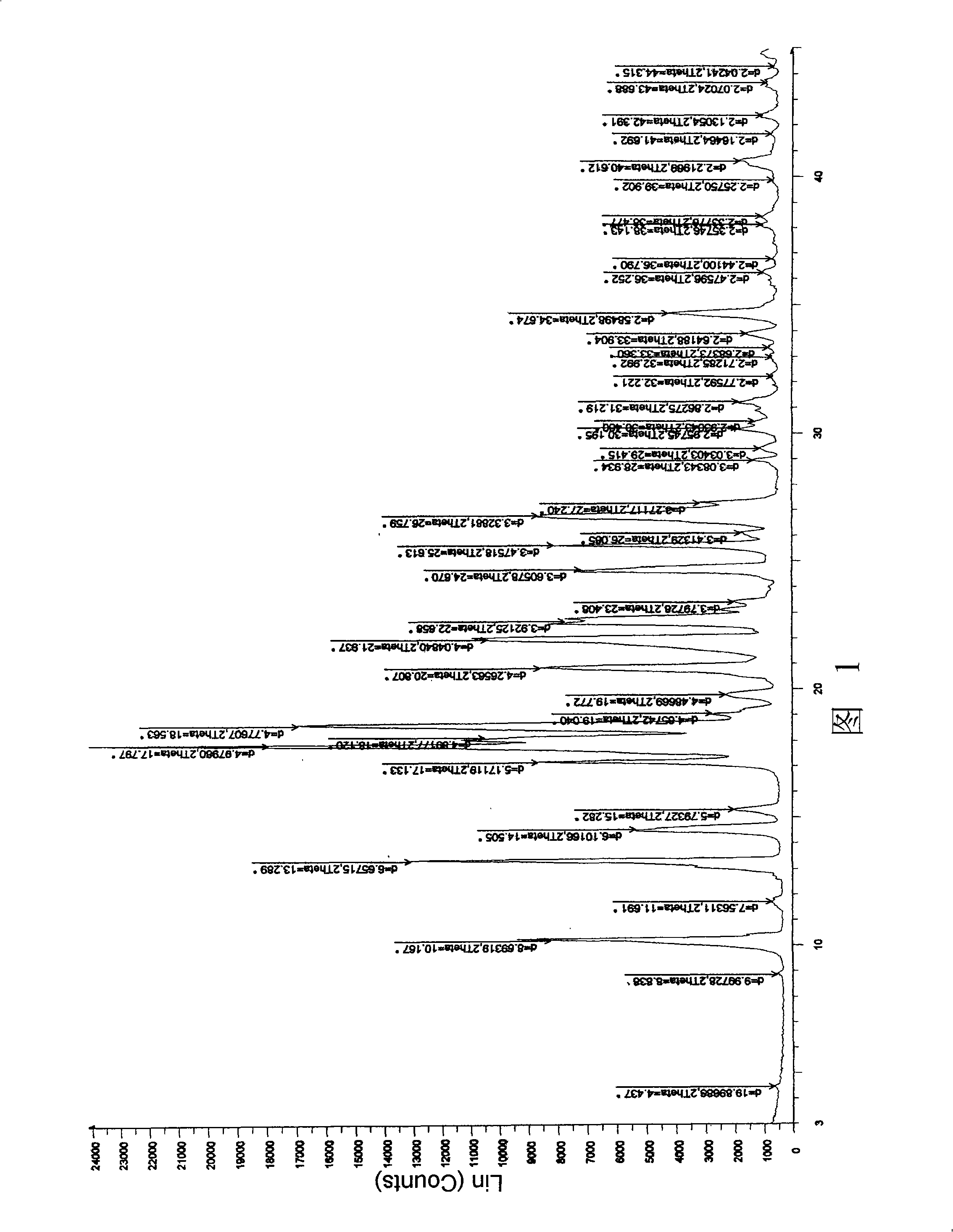
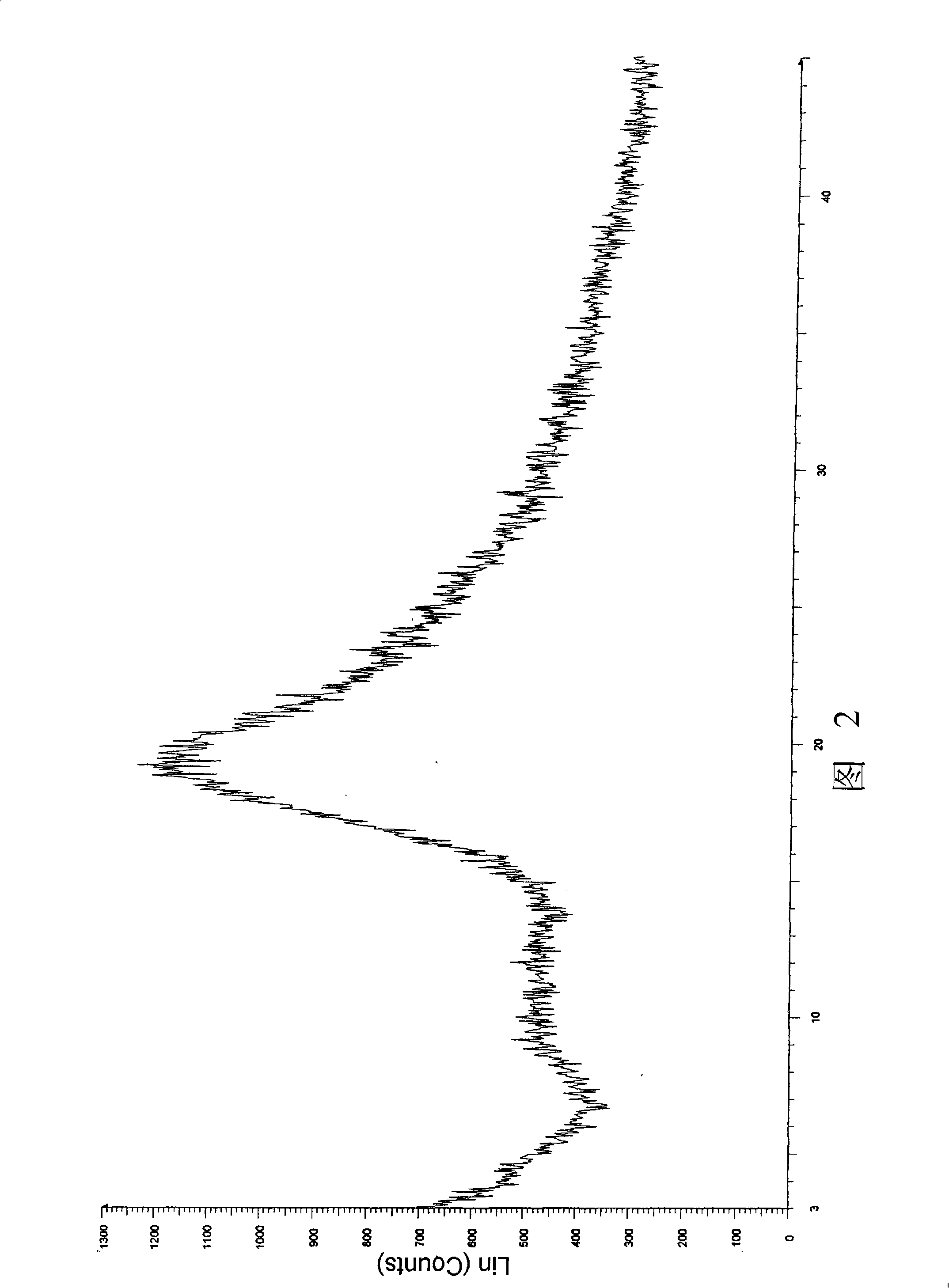
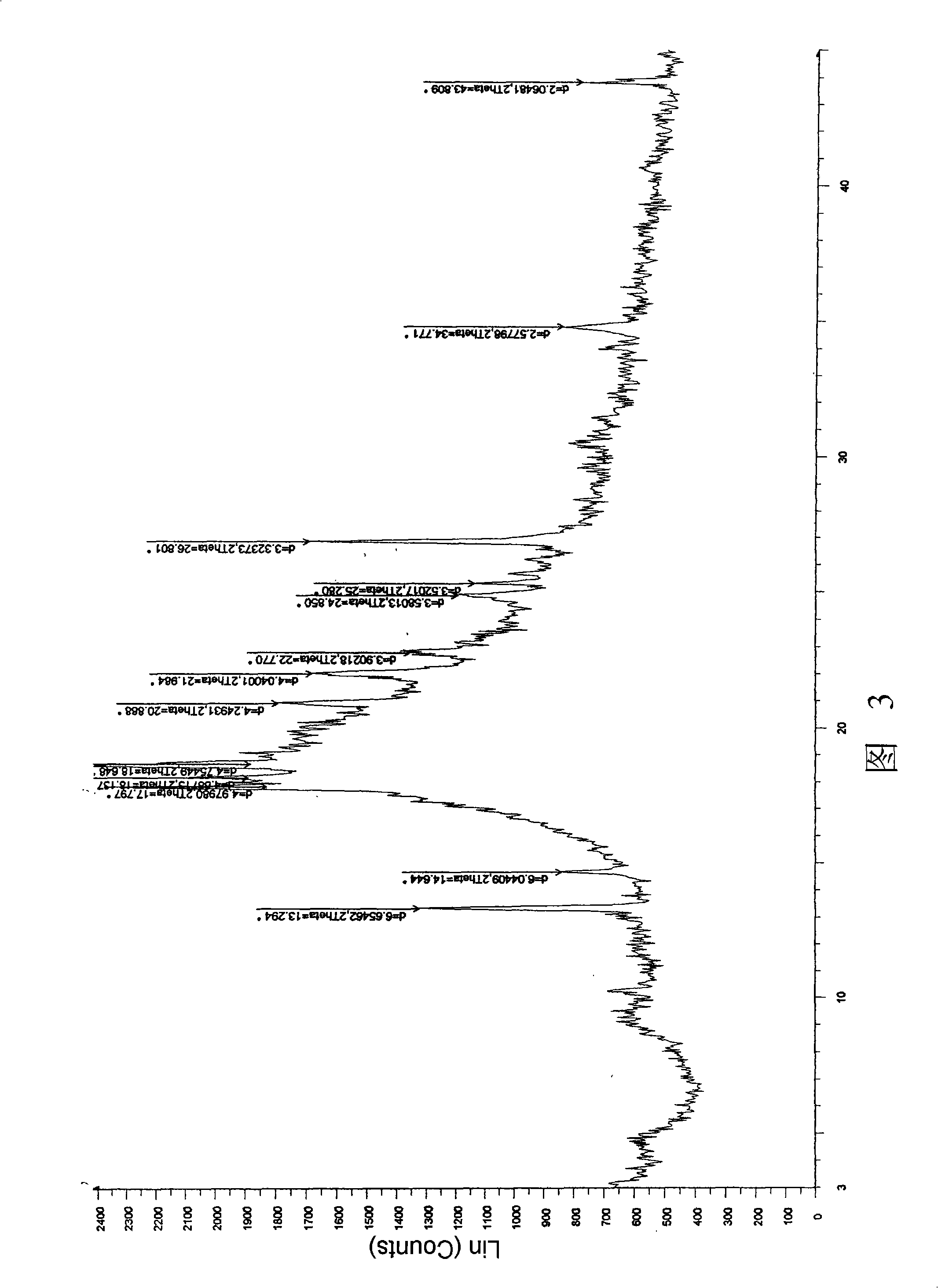
Image
Examples
Embodiment 1
[0078] Preparation of Sulfobutyl Ether-β-Cyclodextrin Inclusion Compound of Cinnarizine
[0079] Ingredients Dosage
[0080] Cinnarizine 2.7×10 -2 mol
[0081] Sulfobutyl ether-β-cyclodextrin 13.4×10 -2 mol
[0082] Hydrochloric acid amount
[0083] Appropriate amount of sodium hydroxide
[0084] Dissolve the prescribed amount of sulfobutyl ether-β-cyclodextrin in about 600 mL of water for injection, and adjust the pH to 1.0-2.0 with hydrochloric acid. Take another prescribed amount of cinnarizine and add it to the above solution, stir and ultrasonically dissolve it, then add sodium hydroxide to adjust the pH to 3.0, which is the clathrate solution. The clathrate solution is freeze-dried to obtain white clathrate powder.
[0085] Note:
[0086] 1. The amount of cinnarizine in the prescription can be increased or decreased according to the specific situation, and the range is not limited.
[0087] 2. In the prescription, the molar ratio of CN:SBE-β-CD can be selected a...
Embodiment 2
[0089] Preparation of Sulfobutyl Ether-β-Cyclodextrin Inclusion Compound of Cinnarizine
[0090] The clathrate solution in Example 1 was spray-dried to obtain white clathrate powder.
Embodiment 3
[0092] Preparation of inclusion compound injection powder (freeze-drying method)
[0093] Add appropriate amount of proppants or excipients (such as mannitol, sorbitol, xylitol) to the clathrate solution in Example 1, any one or any of isotonic regulators (sodium chloride, citric acid, etc.) Mixture of two or more), stir or (and) ultrasonic to make into a homogeneous solution. After adding an appropriate amount of activated carbon and stirring to remove pyrogens, filter to remove carbon. Then filter with a 0.22μm microporous membrane, and add water for injection to 1000mL. Aseptically fill in vials, 2mL / bottle, freeze-dry, stopper and cap to obtain freeze-dried powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com