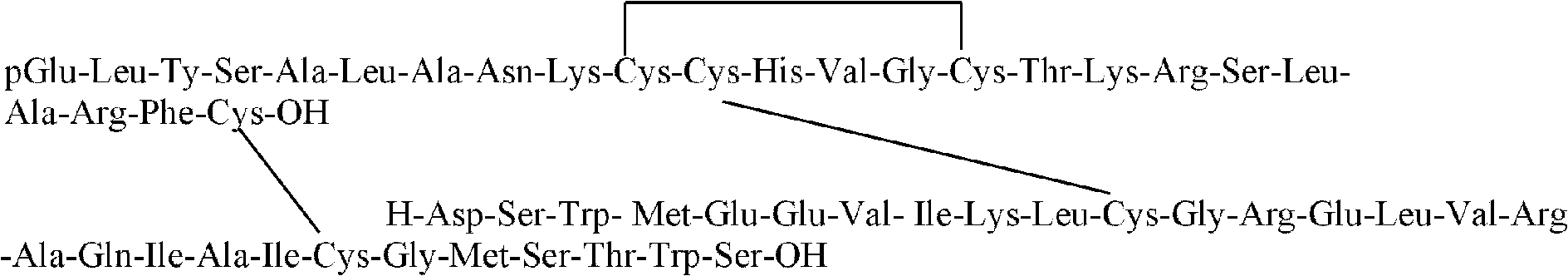Human relaxin-2 lyophilized powder preparation for injection
A technology for relaxin and injection, which is applied in the field of pharmaceutical preparations of human relaxin-2, and can solve problems such as easy collapse, destruction, and poor stability of ordinary injection preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of the lyophilized powder preparation for injection of human relaxin-2
[0025] The formula is as follows:
[0026] Human Relaxin - 2 5g
[0027] Trehalose 3g
[0029] The preparation method is as follows:
[0030] 1) Dosing: mix human relaxin-2 with excipients, add 1000ml of water for injection, stir to dissolve at room temperature, and adjust the pH value to 4.0-6.0 with phosphate buffer;
[0031] 2) Decolorization: Add 0.03 g of activated carbon for needles to the solution prepared in step 1) according to the ratio of 0.03 g of activated carbon for needles per 100 ml of solution, stir at room temperature for 20 minutes for decolorization, filter to remove carbon, measure the pH value or further adjust the pH value , ensure that it is at 4.0-6.0, add water for injection to 1500ml, then filter with a 0.22μm microporous membrane, pack in vials, and half stopper;
[0032] 3) freeze-drying:
[0033] a. Pre-freezi...
Embodiment 2
[0036] Example 2 Preparation of the lyophilized powder preparation for injection of human relaxin-2
[0037] The formula is as follows:
[0038]
[0039] The preparation method is as follows:
[0040] 1) Dosing: mix human relaxin-2 with excipients, add 1000ml of water for injection, stir to dissolve at room temperature, and adjust the pH value to 4.0-6.0 with phosphate buffer;
[0041] 2) Decolorization: Add 0.03 g of activated carbon for needles to the solution prepared in step 1) according to the ratio of 0.03 g of activated carbon for needles per 100 ml of solution, stir at room temperature for 15 minutes for decolorization, filter to remove carbon, measure the pH value or further adjust the pH value , ensure that it is at 4.0-6.0, add water for injection to 1500ml, then filter with a 0.22μm microporous membrane, pack in vials, and half stopper;
[0042] 3) freeze-drying:
[0043] a. Pre-freezing: quickly freeze the solution in the vials to -40°C, and keep freezing fo...
Embodiment 3
[0046] Example 3 Preparation of Injectable Freeze-dried Powder Preparation of Human Relaxin-2
[0047] The formula is as follows:
[0048] Human Relaxin - 2 5g
[0049] Trehalose 3g
[0050] Calcium phosphate 1.5g
[0051]The preparation method is as follows:
[0052] 1) Dosing: mix human relaxin-2 with excipients, add 1000ml of water for injection, stir to dissolve at room temperature, and adjust the pH value to 4.0-6.0 with phosphate buffer;
[0053] 2) Decolorization: Add 0.03 g of activated carbon for needles to the solution prepared in step 1) according to the ratio of 0.03 g of activated carbon for needles per 100 ml of solution, stir at room temperature for 20 minutes for decolorization, filter to remove carbon, measure the pH value or further adjust the pH value , ensure that it is at 4.0-6.0, add water for injection to 1500ml, then filter with a 0.22μm microporous membrane, pack in vials, and half stopper;
[0054] 3) freeze-drying:
[0055] a. Pre-freezing: qui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com