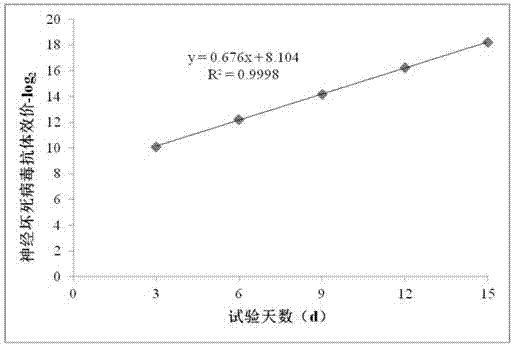
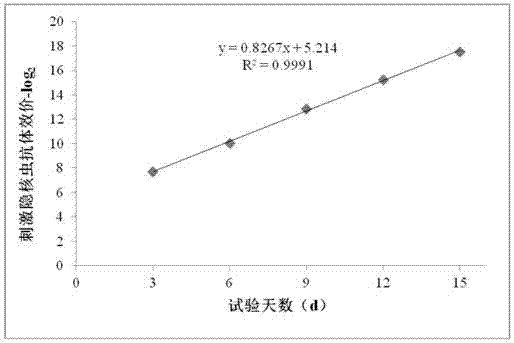
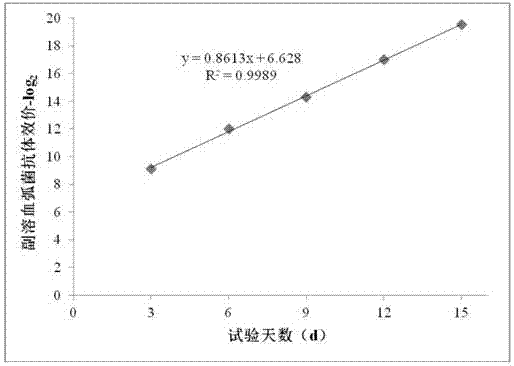
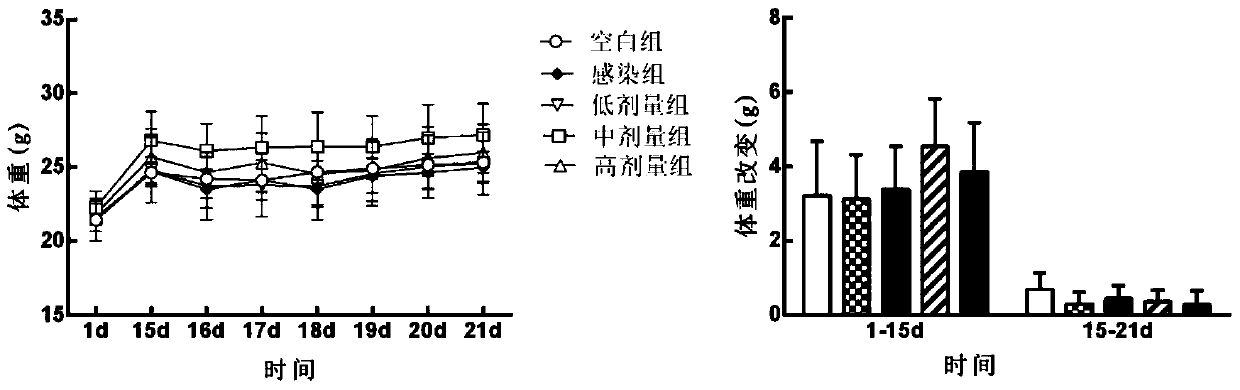
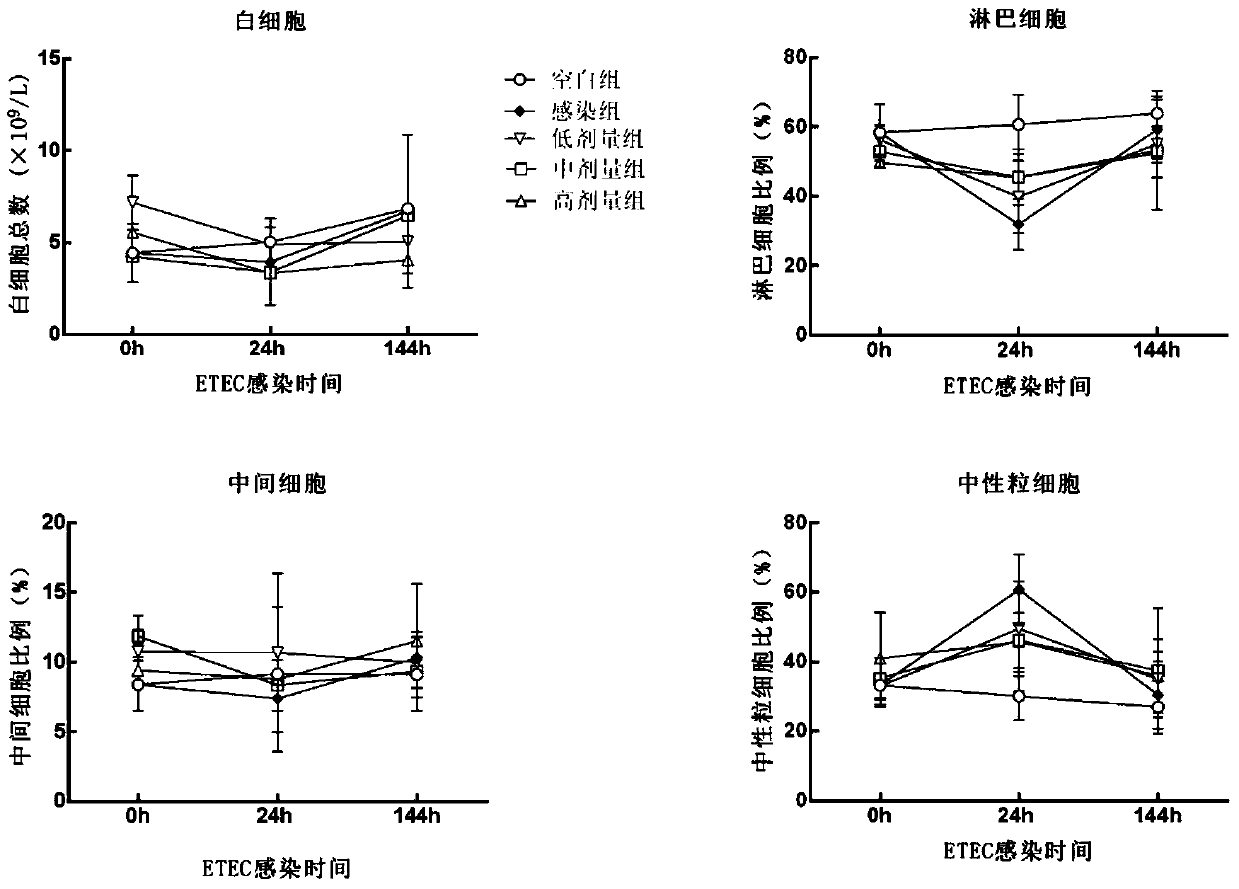
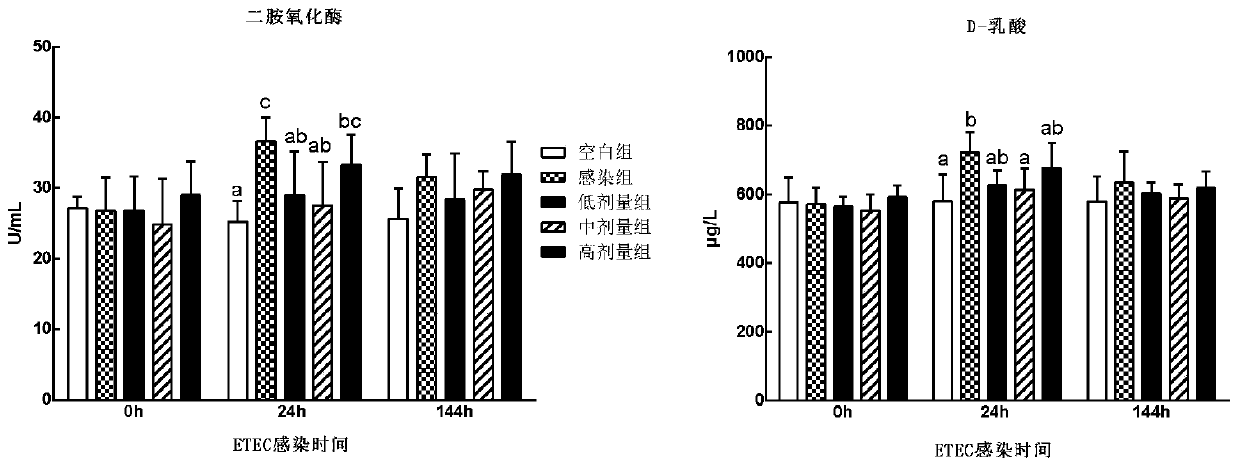
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Immunoglobulins / Antibodies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunoglobulins (Antibodies) are proteins that are found in blood or other bodily fluids of vertebrates, and are used by the immune system to identify and neutralize foreign objects, such as bacteria and viruses.
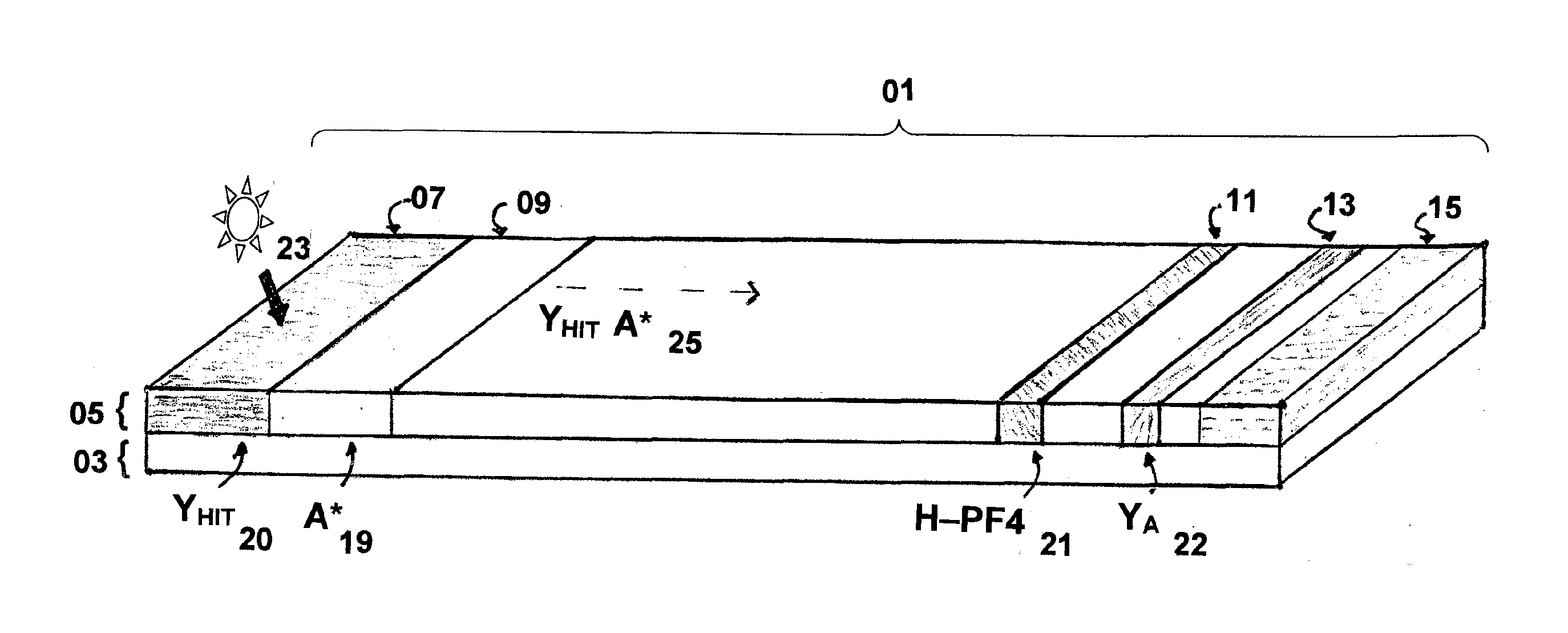
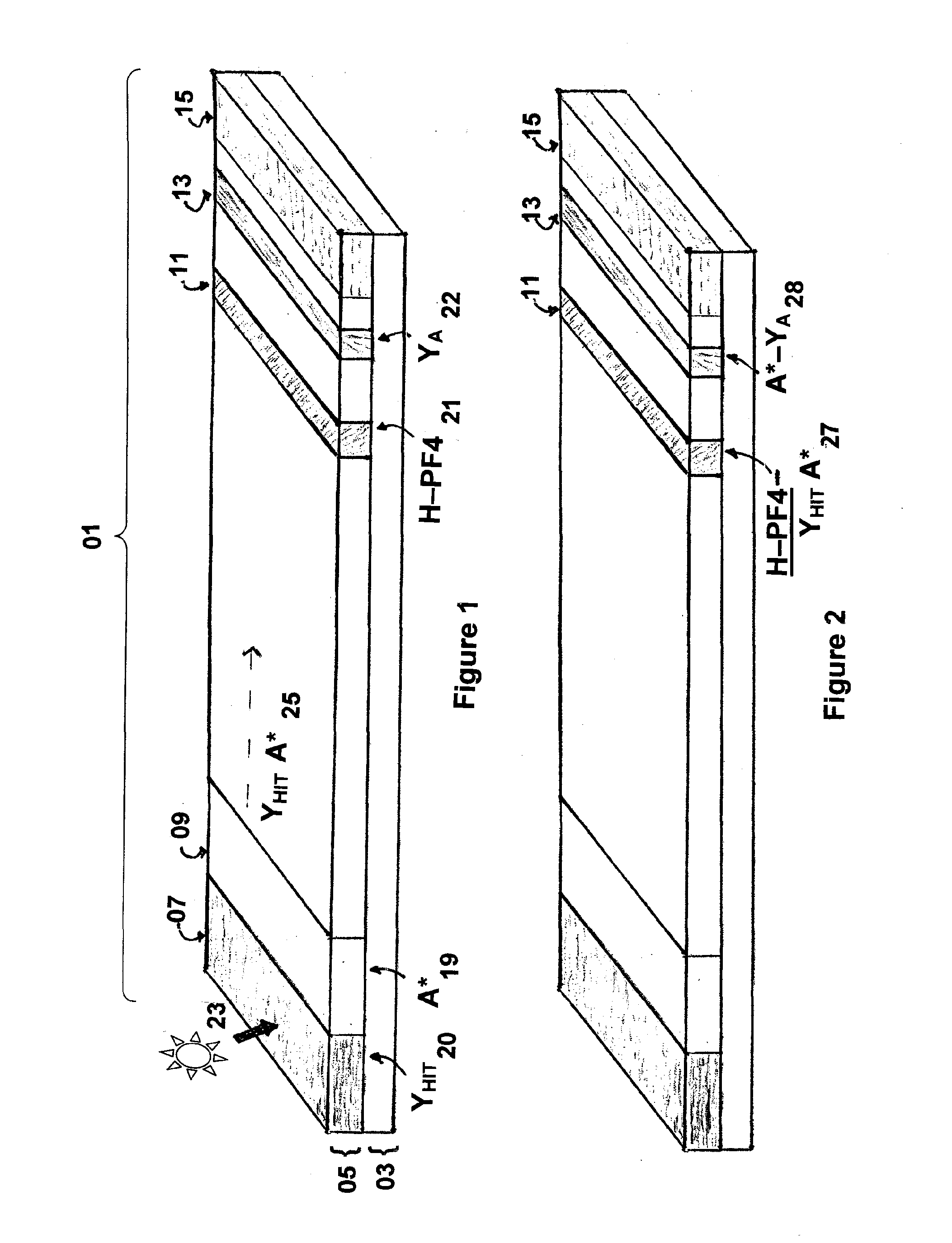
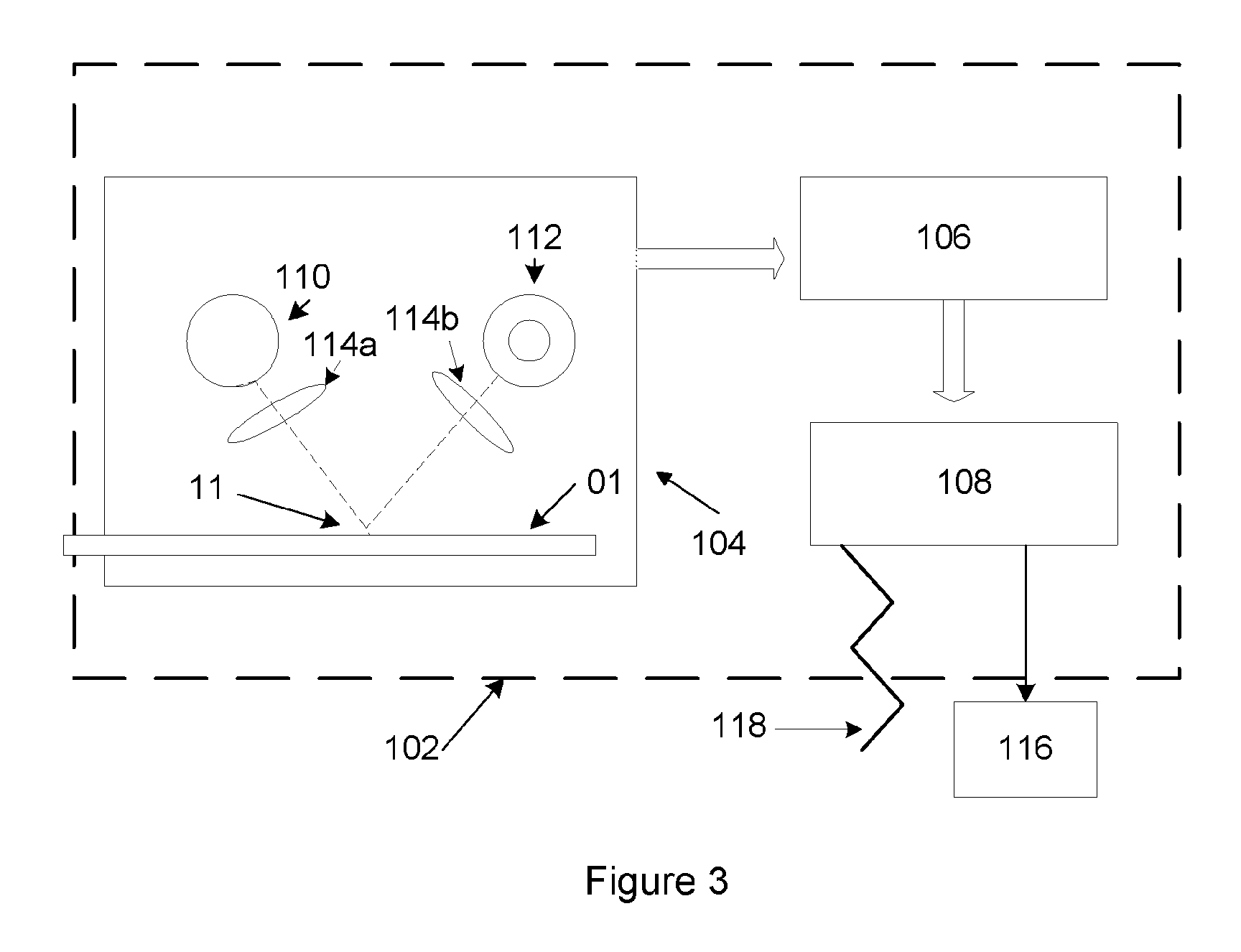
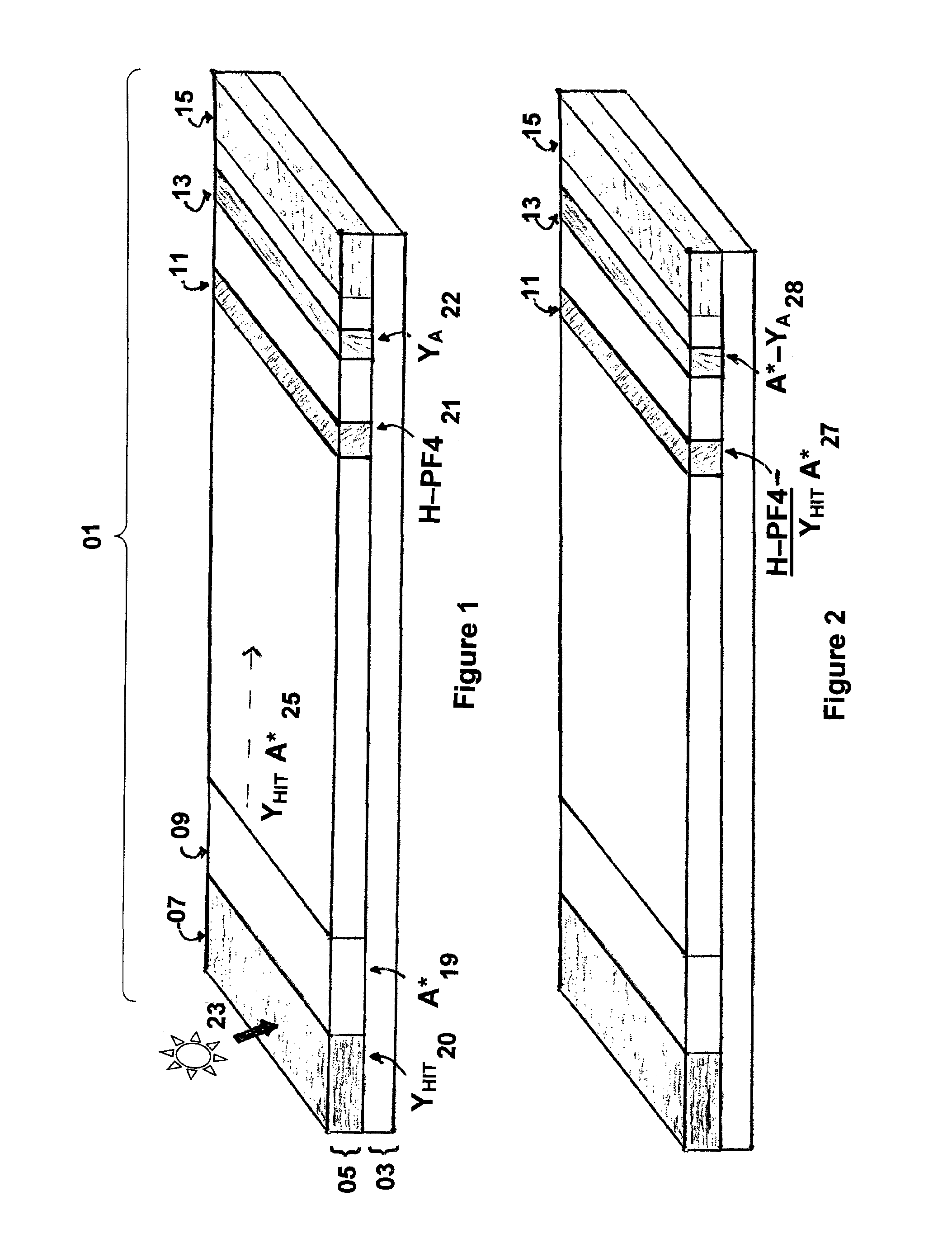
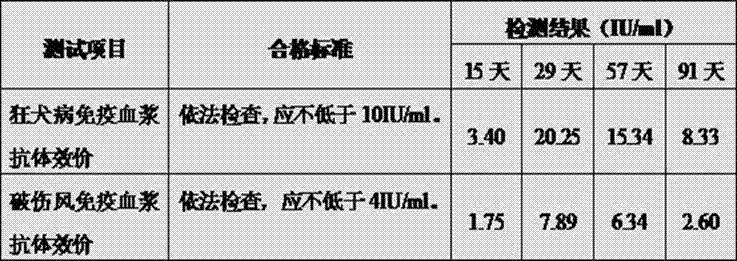
Rapid and sensitive method for quantitative determination of the level of heparin - pf4 complex induced immunoglobulin antibodies
ActiveUS20100255510A1Bioreactor/fermenter combinationsBiological substance pretreatmentsImmuno assayQuantitative determination
Disclosed herein is a lateral flow immuno-assay system capable of rapidly, cost effectively, and quantitatively detecting and assessing the level of HIT antibodies in body fluids of a patient. Also taught are methods for employing the system to assist in diagnosis of HIT, and for screening or detecting a changing titer of HIT antibodies in the body fluids of a patient to determine susceptibility toward HIT.
Owner:BIOMEDOMICS
Method of detecting surrogate markers in a serum sample
ActiveUS20130137598A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCompetitive bindingCholesterol
The invention provides a method of detecting surrogate markers for active tuberculosis in a serum sample. The surrogate markers are selected from serum mycolic acid antigen, serum anti-mycolic acid antibodies or both. The method includes the steps of combining the serum sample with a labelled monoclonal immunoglobulin antibody or fragment thereof to mycolic acids to produce a combined serum sample, the antibody or fragment thereof not substantially cross-reacting with cholesterol and the label being selected so that binding of the labelled antibody to immobilized mycolic acid antigen of mycobacterial origin produces a detectable signal and combining a blank sample with the labelled monoclonal immunoglobulin antibody or fragment thereof to mycolic acid to produce a combined blank sample. The method includes exposing both samples to immobilised mycolic acid antigen of mycobacterial origin or a synthetic analogue or analogues thereof so that the labelled immunoglobulin antibodies or fragments thereof in each sample bind to the immobilised antigen to produce detectable signals. If the surrogate markers are present, the signal produced by the blank sample will be stronger than that produced by the serum sample because of inhibition of binding of the labelled antibody in the serum sample arising from prior binding of the labelled antibody with the mycolic acid antigen in the serum sample or by competitive binding of serum anti-mycolic acid antibodies in the serum sample to the immobilised mycolic acid antigen or both.
Owner:UNIVERSITY OF PRETORIA
Quick detection method for newcastle disease virus and its immune colloidal gold test card
The invention provides a method for quickly detecting Newcastle disease virus. The method comprises the following steps that: a Newcastle disease virus monoclonal antibody, a goat-anti-Newcastle disease virus polyclonal antibody and a goat-anti-Balb / C mouse immunoglobulin antibody are established; the treatment of a combination pad and NC membrane is completed; after a to-be-detected sample is added on a sample pad and filtered, the NDV in the sample is combined with the gold-labeled antibody in the combination pad; the gold-labeled antibody is firstly combined with the Ab2 on the NC membrane through chromatography to form a visible combining line, and an uncombined gold-labeled antibody undergoes chromatography continuously and is combined with Ab3 to form a second combining line; finally, a step of result observation, during which, two combining lines are formed when the result is positive, while one quality control line is formed when the result is negative. The method has the characteristics of simple operation, quickness, high sensitivity and excellent specificity, etc.; moreover, the method is low in cost and is suitable for Newcastle disease virus detection at grass root or clinic; in addition, the method can detect the Newcastle disease virus in bird blood and dejection and can obtain a result within 10 min with the sensitivity reaching to 10ng / ml. The invention also provides a Newcastle disease immune colloidal gold test card for realizing the method.
Owner:CHONGQING UNIV OF TECH
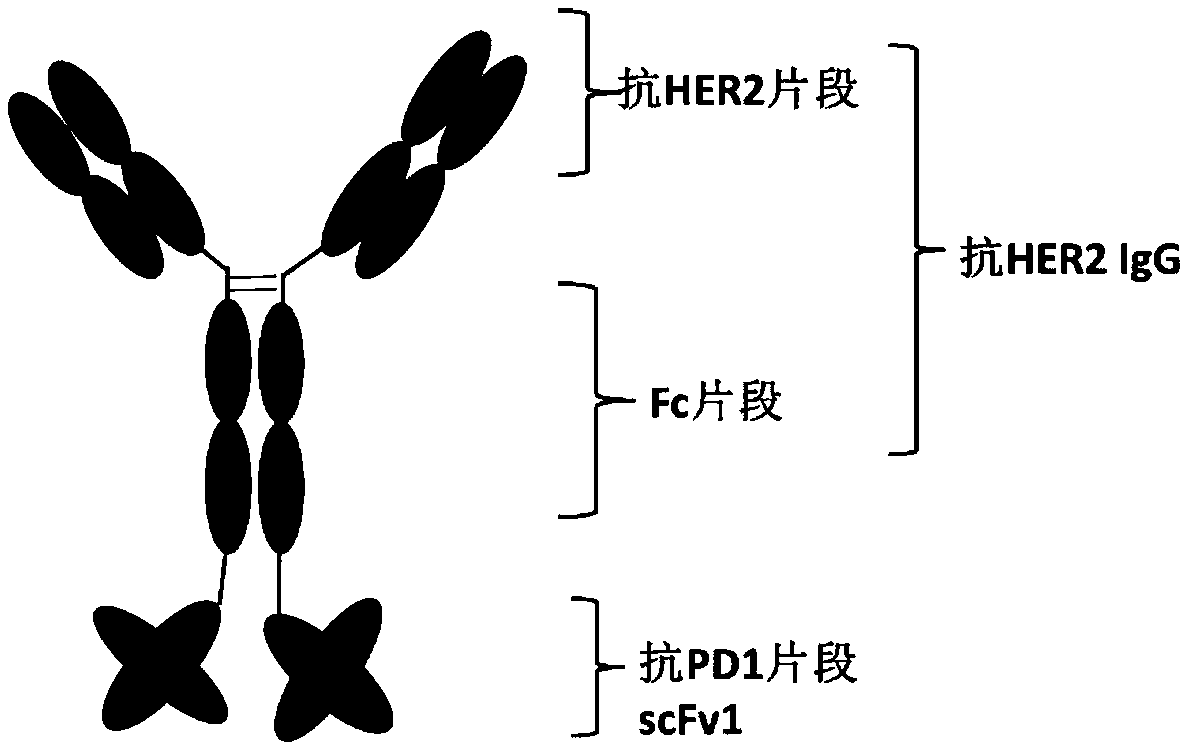
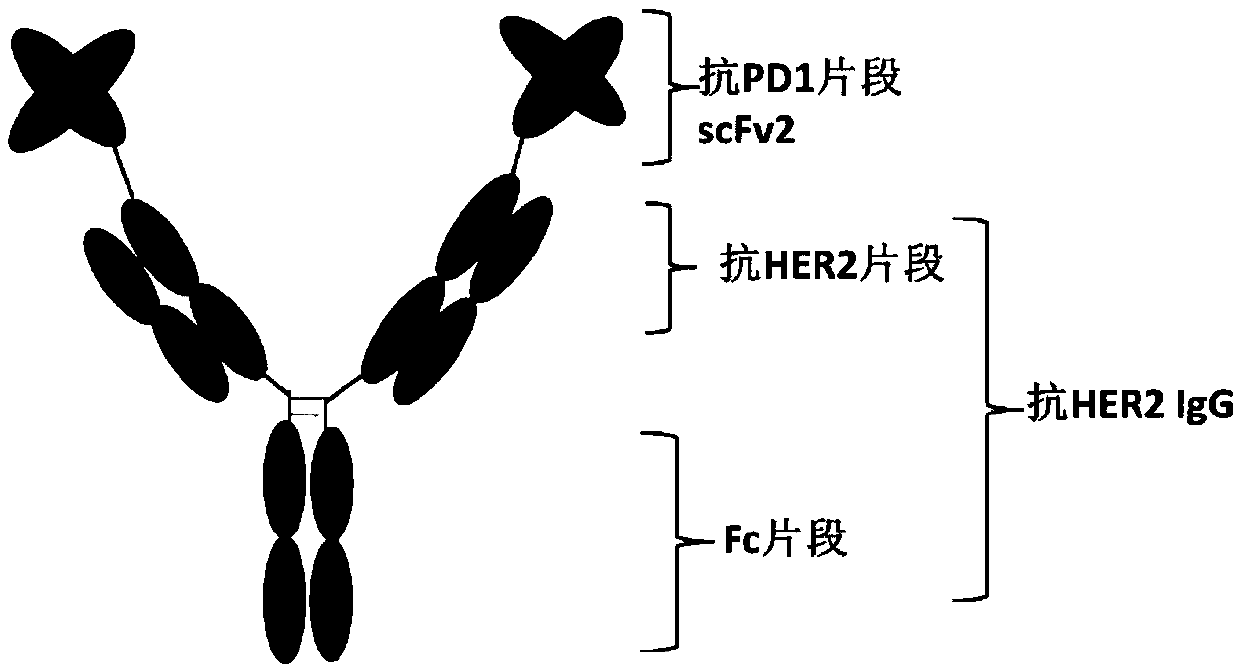
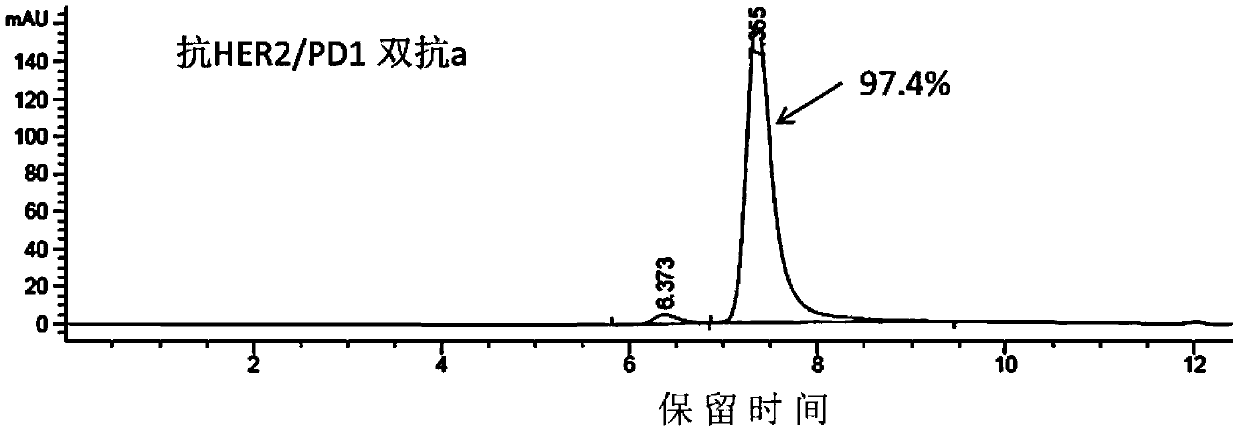
Anti-HER2/PD1 bispecific antibody
InactiveCN111196856AImprove stabilityInhibition killHybrid immunoglobulinsAntibody ingredientsTumor therapyIntravenous gammaglobulin
The invention belongs to the field of tumor treatment, and discloses an anti-HER2 / PD1 bispecific antibody, a preparation method and an application in tumor resistance. Specifically, the single-chain variable fragment scFv and the immunoglobulin antibody IgG are connected through a peptide joint to obtain a bispecific antibody, and the bispecific antibody can target a tumor cell surface molecule HER2 and a T lymphocyte surface molecule PD1. The experimental results show that the bispecific antibody provided by the invention can inhibit the growth of tumor cells, activate T lymphocytes and promote the killing of the T cells to the tumor cells.
Owner:SUNSHINE GUOJIAN PHARMA (SHANGHAI) CO LTD
Method for preparing anti-A type botulinus toxin immunoglobulin antibody
ActiveCN101497909AHigh purityHigh titerImmunoglobulins against bacteriaMicroorganism based processesSerum igeAntitoxin
The invention discloses a method for preparing an anti-type A botulinum neurotoxin immunoglobulin antibody, which comprises the steps of preparing and obtaining the anti-type A botulinum neurotoxin immunoglobulin antibody through taking type A botulinum neurotoxin recombinant protein rAHc as immunogen to immunize animals. The method for preparing the anti-type A botulinum neurotoxin immunoglobulin antibody eliminates a Fc segment in the antibody which can cause side effect and obtains the horse anti-type A botulinum neurotoxin immunoglobulin F(ab')2 antibody; after blood serum obtained by pentalogy hyper-immune and hexalogy hyper-immune are mixed, the content (purity) of F(ab')2 of the antibody can achieve 80.2 percent in a semi-finished product obtained through further purification; the titer of the antibody can achieve 8000 IU / ml; the antibody has good specificity and sensitivity to type A botulinum neurotoxin; and the purity and the specific activity of the antibody are higher than the antitoxin prepared from the traditional toxin immunity horses. Moreover, the invention has the good stability and safety.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
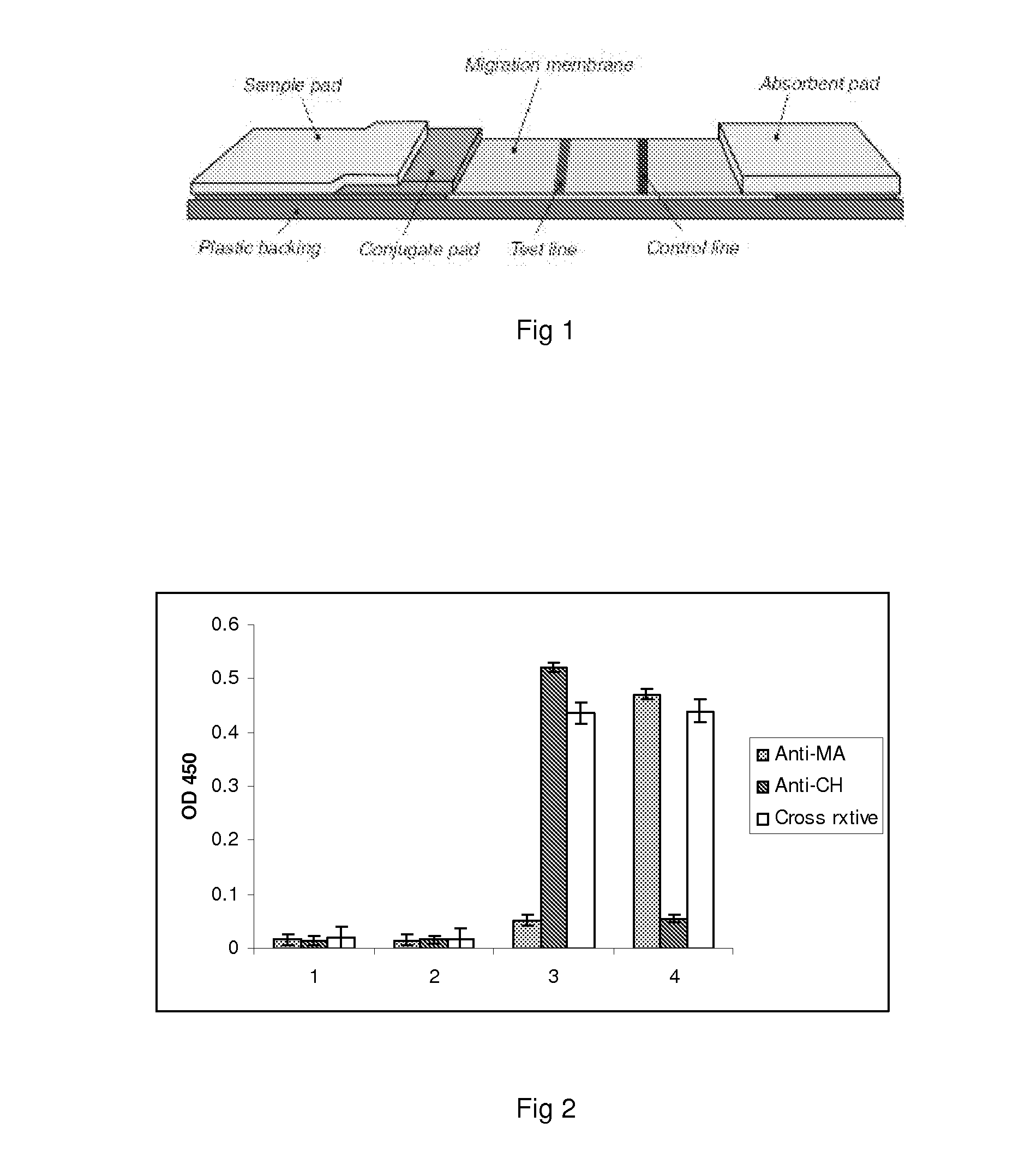
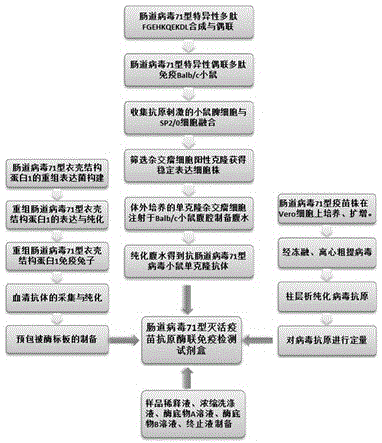
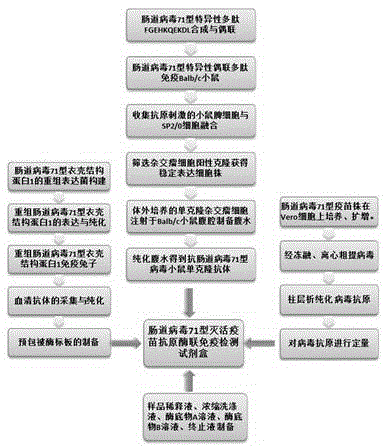
ELISA (enzyme-linked immunosorbent assay) kit for EV (enterovirus) 71 inactivated vaccine antigen
The invention relates to an ELISA (enzyme-linked immunosorbent assay) kit for an EV (enterovirus) 71 inactivated vaccine antigen. The detection kit comprises an EV71 pre-coated polyclonal antibody ELISA plate, a sample diluent, a second antibody, an enzyme-labeled antibody, a concentrated cleaning solution, an enzyme substrate solution and a stop buffer, wherein the ELISA plate is pre-coated with a polyclonal antibody prepared by taking recombinant EV71 structural protein 1 as an immune source; the second antibody adopts a monoclonal antibody prepared by taking keyhole limpet hemocyanin coupled polypeptide sequence FGEHKQEKDL as the immune source; the enzyme-labeled antibody adopts a horse radish peroxidase labelled goat anti-mouse immunoglobulin antibody; and an antibody standard is placed in the kit. The ELISA kit has better sensitivity when measuring the titer of the EV71 inactivated vaccine antigen, and has better linearity in the range of 5.9-750 ng / ml, and the linearly dependent coefficient r2 is larger than 0.99. The kit for measuring the titer of the EV71 inactivated vaccine antigen simply and conveniently has good specificity, accurate quantification, high sensitivity and good repeatability.
Owner:ZHEJIANG PUKANG BIOTECH
Immunologic assay for detection of autoantibodies to folate binding protein
InactiveUS20060115860A1Improve processingDisease diagnosisBiological testingFolate-binding proteinFluorescence
The present invention is directed to an assay that detects autoantibodies to folate receptor and can be used in the clinical diagnostic testing of these autoantibodies in humans. Although there are other methods that exist to detect these autoantibodies, the assay described in the present invention has several features that offer advantages over the existing methods. Some of these features include adaptability to high-throughput processing, the use of an immunoglobulin antibody to bind autoantibodies bound to folate receptor or the use of enzyme-labeled folic acid to bind folate binding protein and use of fluorescence or chemiluminescence for detection. This assay thereby avoids the use of radioactivity and can be automated and scaled to process hundreds of samples safely and simultaneously.
Owner:CABRERA ROBERT M +1
Immunologic assay for detection of autoantibodies to folate binding protein
The present invention is directed to an assay that detects autoantibodies to folate receptor and can be used in the clinical diagnostic testing of these autoantibodies in humans. Although there are other methods that exist to detect these autoantibodies, the assay described in the present invention has several features that offer advantages over the existing methods. Some of these features include adaptability to high-throughput processing, the use of an immunoglobulin antibody to bind autoantibodies bound to folate receptor or the use of enzyme-labeled folic acid to bind folate binding protein and use of fluorescence or chemiluminescence for detection. This assay thereby avoids the use of radioactivity and can be automated and scaled to process hundreds of samples safely and simultaneously.
Owner:CABRERA ROBERT M +1
Method for separating immune globulin antibody
InactiveCN103936851ALarge adsorption capacityChromatographic separation efficiency is highMilk immunoglobulinsPeptide preparation methodsIon exchangeGradient elution
The invention discloses a method for separating an immune globulin antibody. The method comprises the following steps: 1, diluting a raw material containing immune globulin by using a buffer solution of which the pH value is 4.0-8.0 and deionized water to obtain a loading fluid; 2, sampling the preprocessed loading fluid to a crystal gel column at the flow rate of 0.1-20cm / min to carry out adsorption; 3, with the deionized water or the buffer solution of which the pH value is 4.0-8.0 as the washing fluid, flushing un-adsorbed impurities inside the crystal gel column; and 4, with the buffer solution of which the pH value is 4.0-8.0 and contains 0.01-4M of alkali metal salt as an eluent, carrying out gradient elution, and collecting an eluting peak containing the immune globulin to obtain the immune globulin. According to the method for separating immune globulin, which is disclosed by the invention, the used anion exchange type crystal gel medium has ultra-large pores, realizes convective diffusion inside the pores and high-purity IgG (immunoglobulin G) separation, and is high in mass transfer efficiency; the method disclosed by the invention is simple in craft process, good in adaptability and low in extraction cost.
Owner:SHIHEZI UNIVERSITY
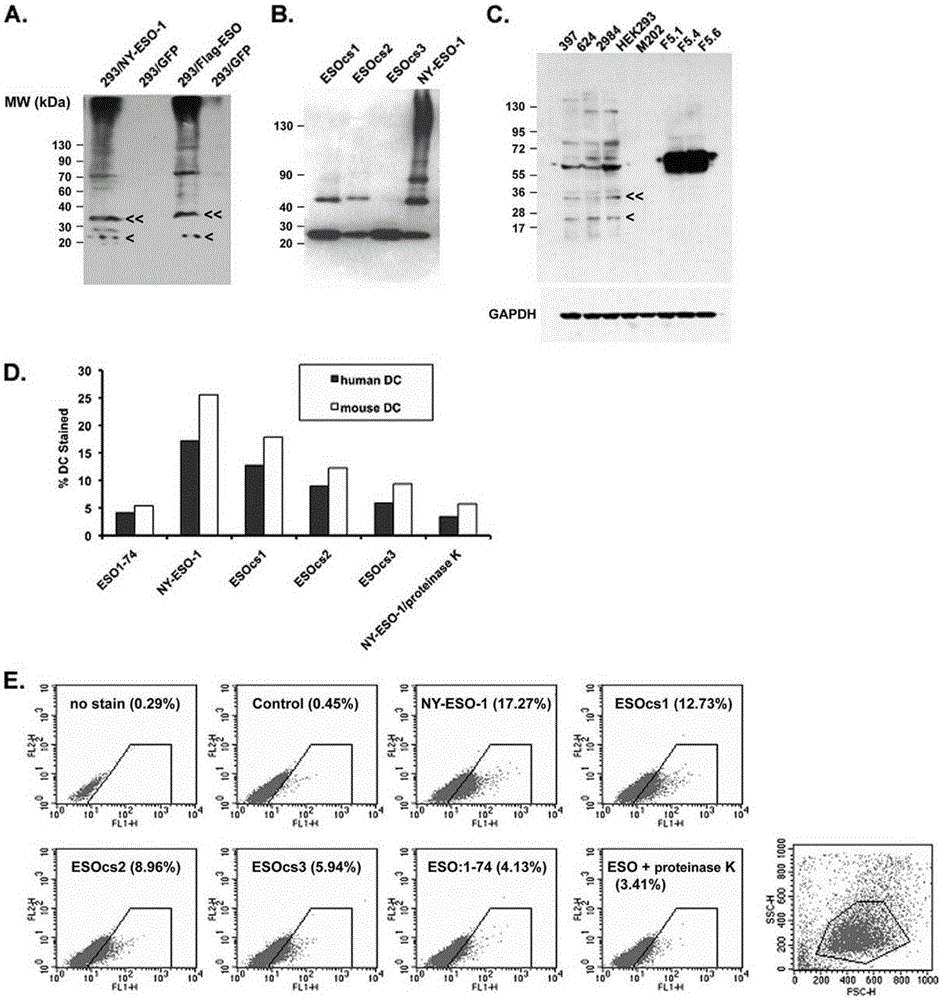
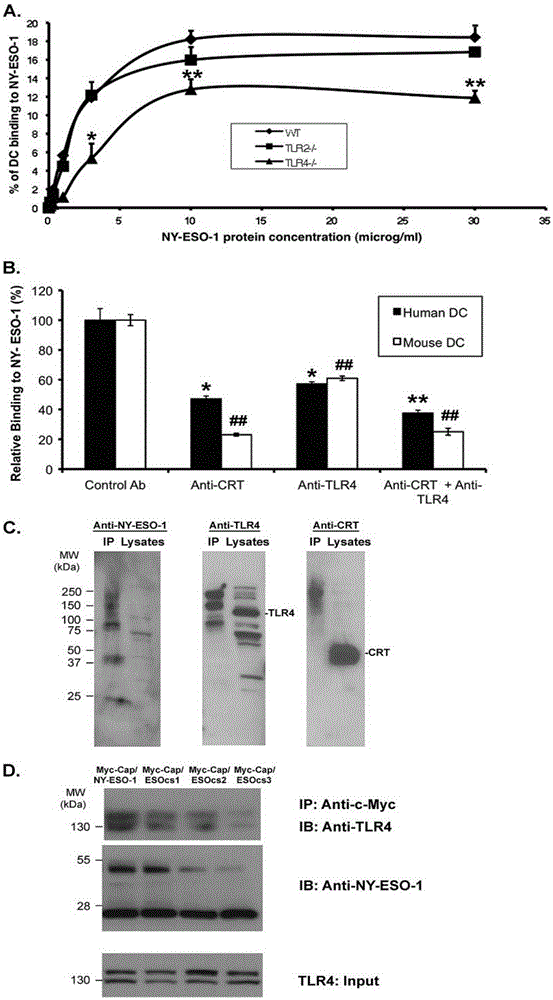
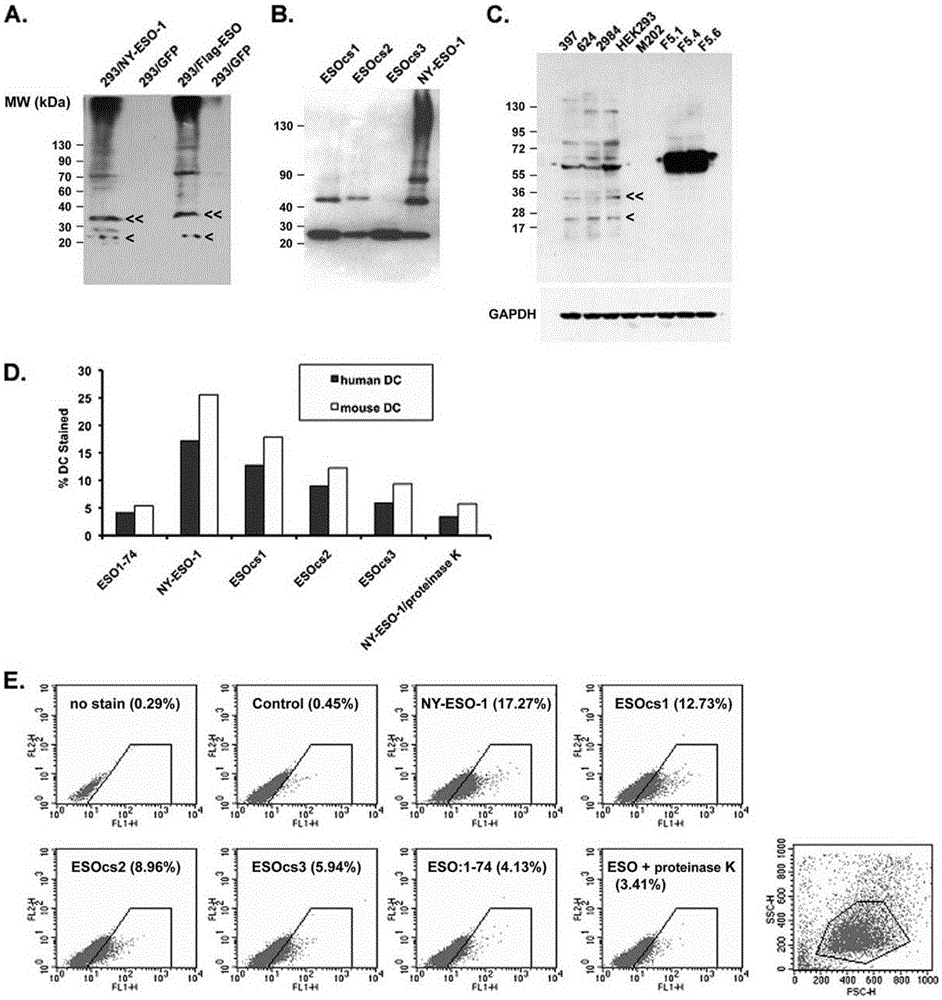
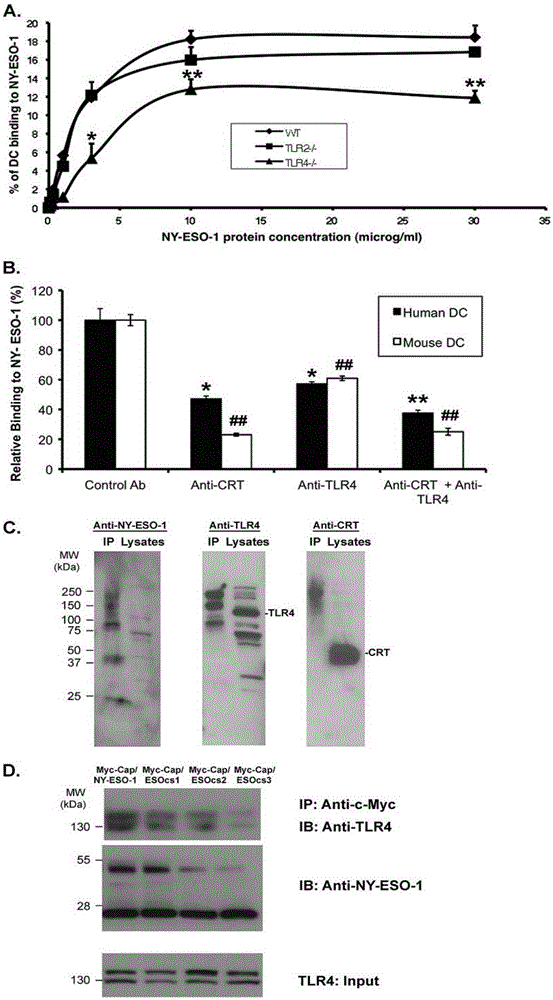
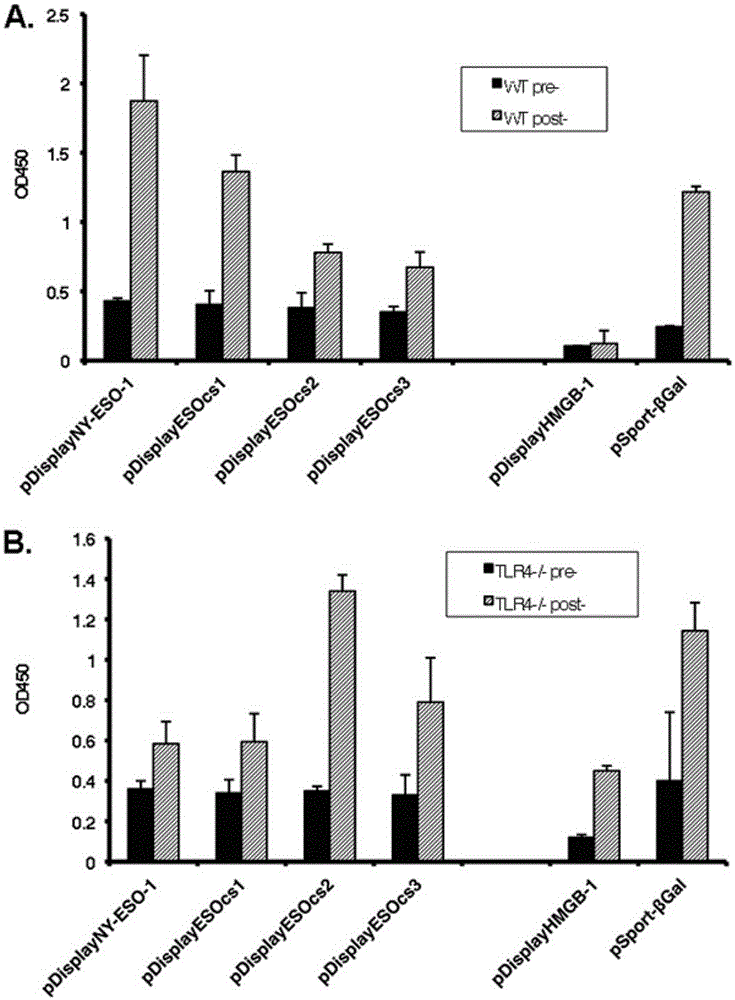
Applications of NY-ESO-1 as molecule adjuvant in enhancement of Art v1 (wtArt) allergen immune reaction
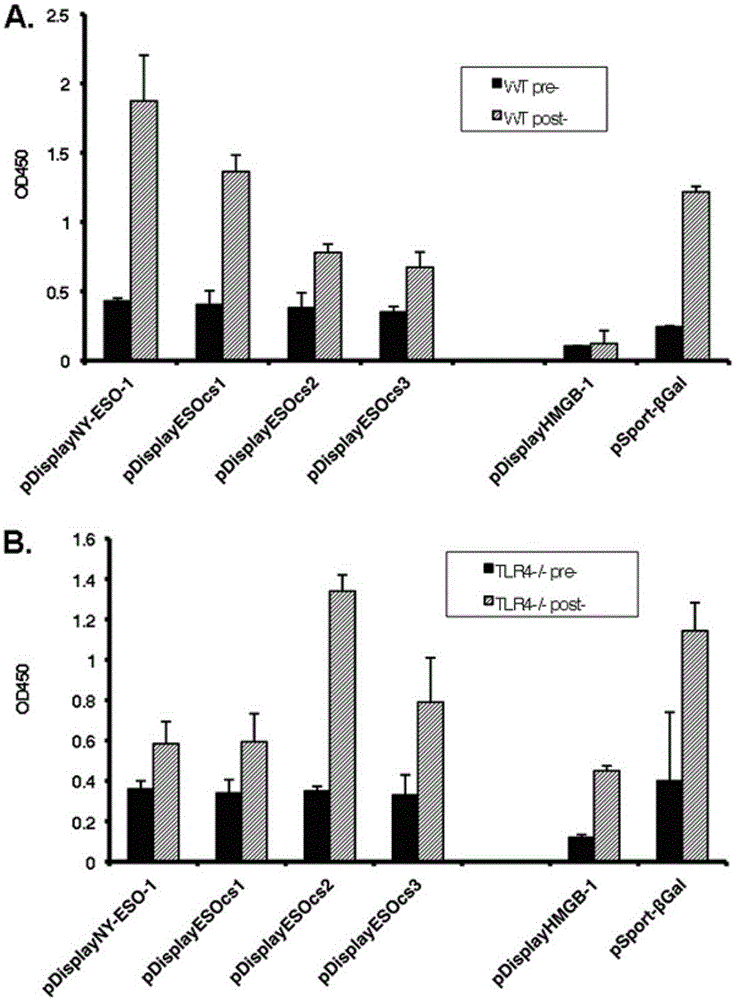
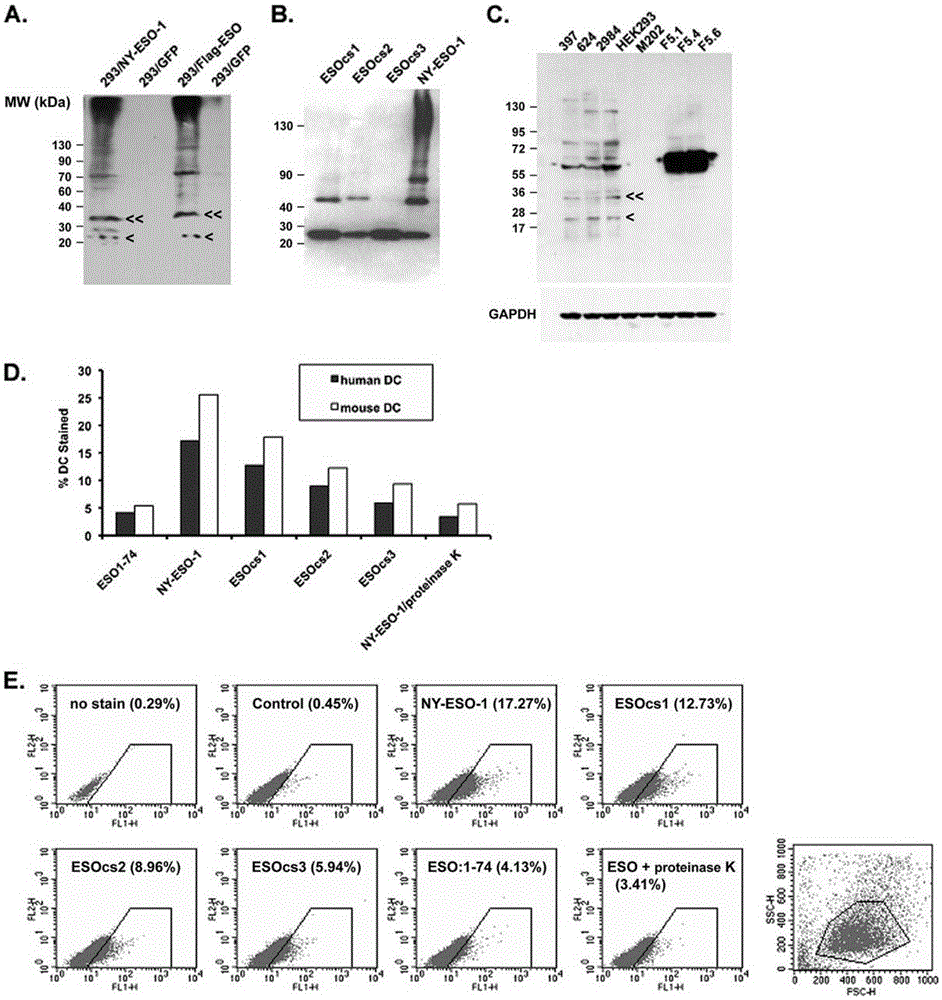
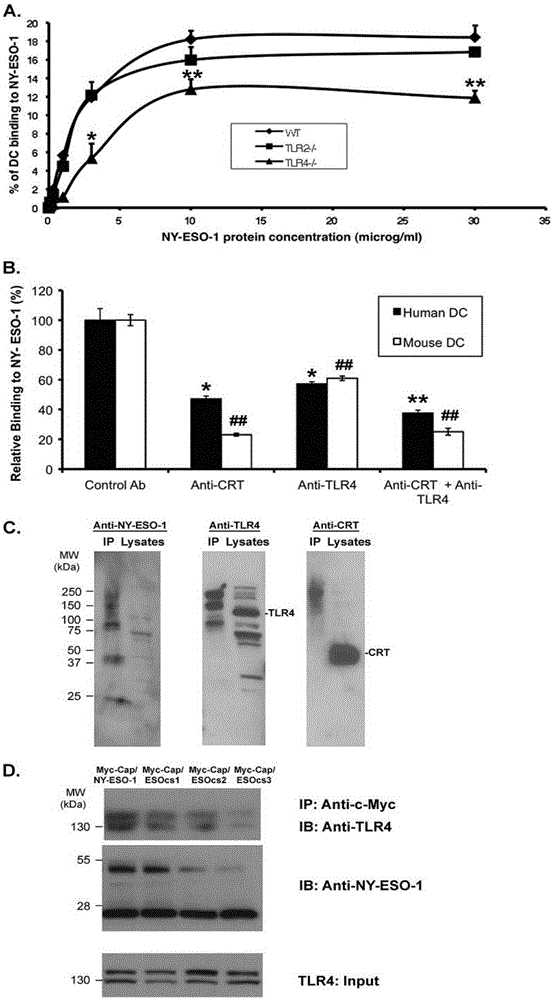
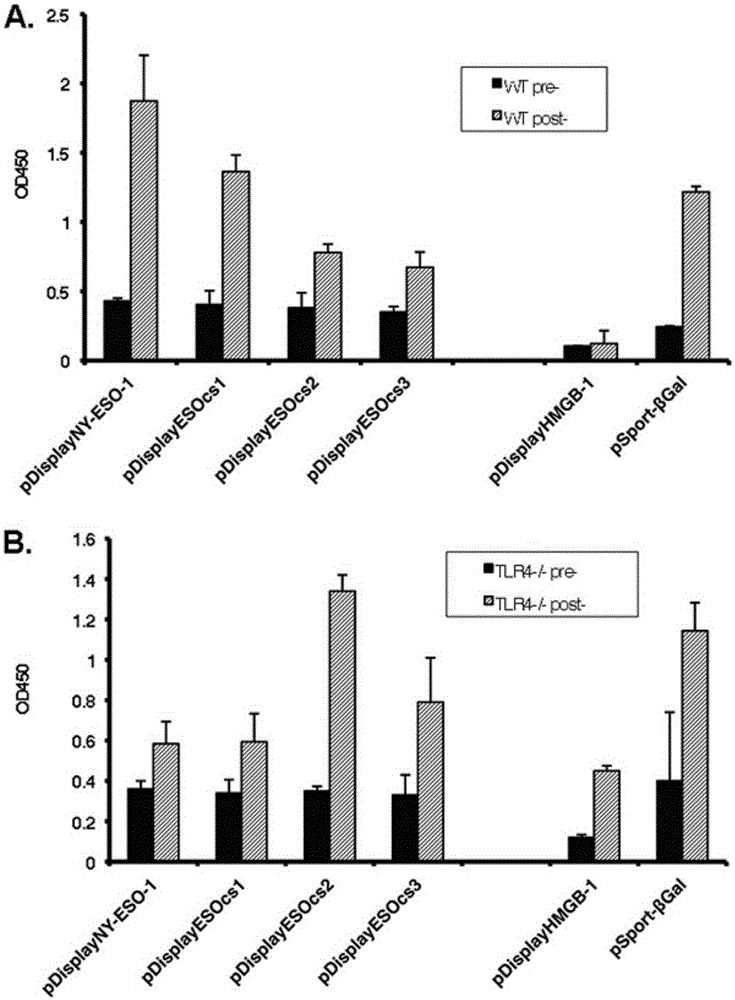
The present invention relates to the field of biomedicine, particularly to applications of NY-ESO-1 as a molecule adjuvant in enhancement of an Art v1 (wtArt) allergen immune reaction. According to the present invention, it is explored that the NY-ESO-1 protein can form a poly-structure even in a loading buffer containing beta-mercaptoethanol having a conventional concentration, the polymerization reaction of the NY-ESO-1 is mediated by the intermolecular disulfide bond, the binding of the NY-ESO-1 and the human / murine immature dendritic cells in vitro is related to the polymerization structure of the NY-ESO-1, TLR4 affects the in vitro binding of the NY-ESO-1 and the DC cells in bone marrow, the binding of the NY-ESO-1 and the human / murine immature dendritic cells can be performed through the action of calreticulin, the polymer oligomerization reduces the binding of TLR4 and NY-ESO-1, the polymer structure of the NY-ESO-1 and TLR4 in host participate into an immunoglobulin antibody reaction, and the improving of the art V1 (wtArt) and CA9 gene immunogenicity depends on the NY-ESO-1 gene fusion expression; and the test results prove that the NY-ESO-1 can be adopted as the molecule adjuvant in enhancement of the Art v1 (wtArt) allergen immune reaction.
Owner:宁波美丽人生医药生物科技发展有限公司
Preparation method and application of rabbit anti-grouper serum immunoglobulin HRP-labeled antibody
ActiveCN107389921AHigh purityIncrease enzyme activityColor/spectral properties measurementsAntibody conjugateViral disease
The invention discloses a preparation method and application of a rabbit anti-grouper serum immunoglobulin HRP-labeled antibody, and belongs to the technical field of antibody preparation. The rabbit anti-grouper serum immunoglobulin HRP-labeled antibody is an enzyme antibody conjugate comprising HRP and a rabbit anti-grouper serum immunoglobulin antibody, the rabbit anti-grouper serum immunoglobulin HRP-labeled antibody can be specifically bound with grouper serum immunoglobulin for color development of a HRP substrate. The HRP-labeled antibody prepared by the method has high purity, high enzyme activity, suitable conjugate labeling rate, great dilution degree for performing ELISA tests, and high sensitivity, and can be used for detecting viral diseases, parasitic diseases and bacterial diseases of grouper.
Owner:FISHERIES RES INST OF FUJIAN
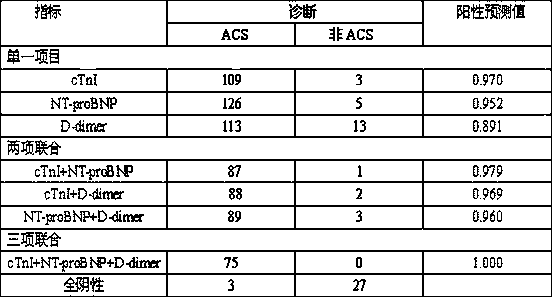
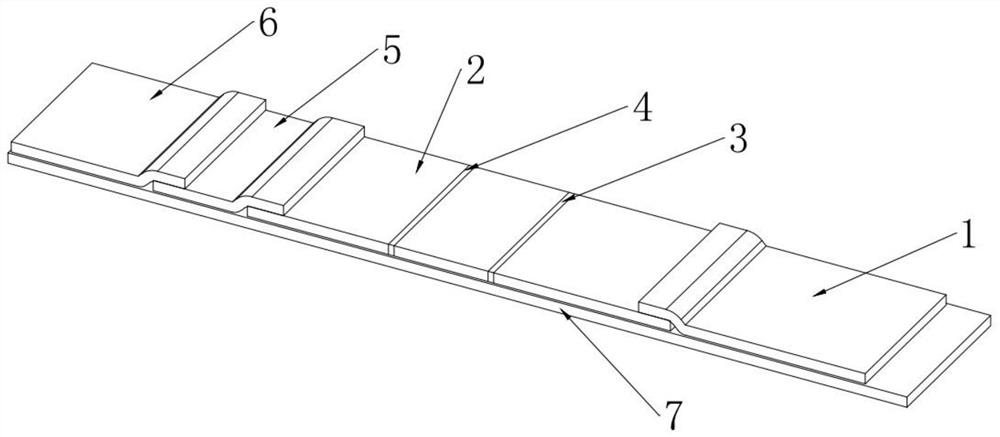
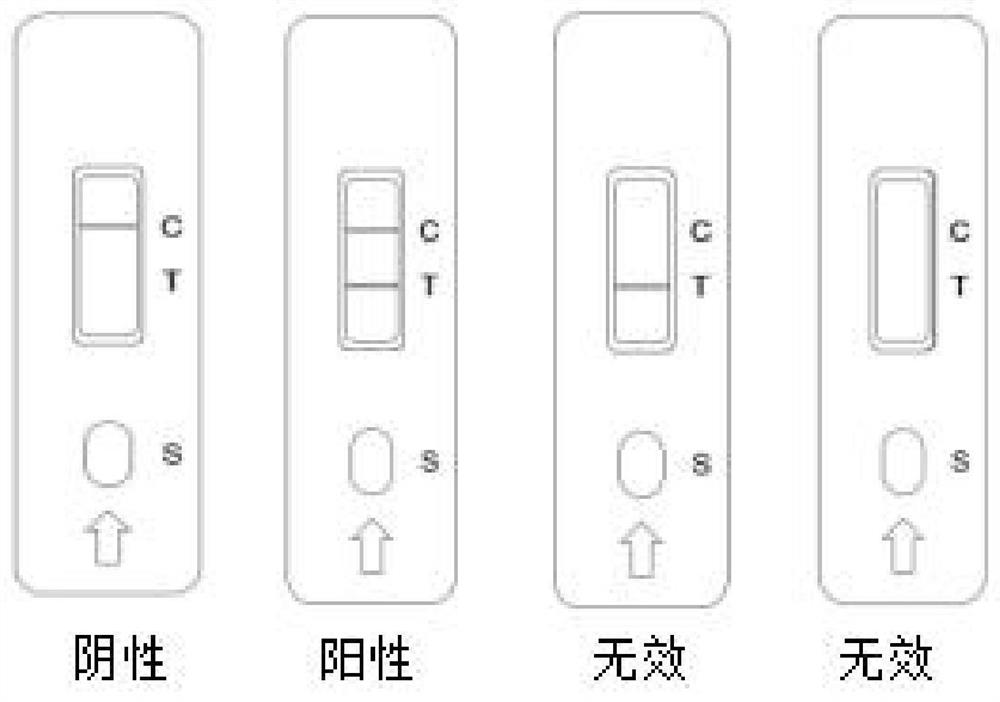
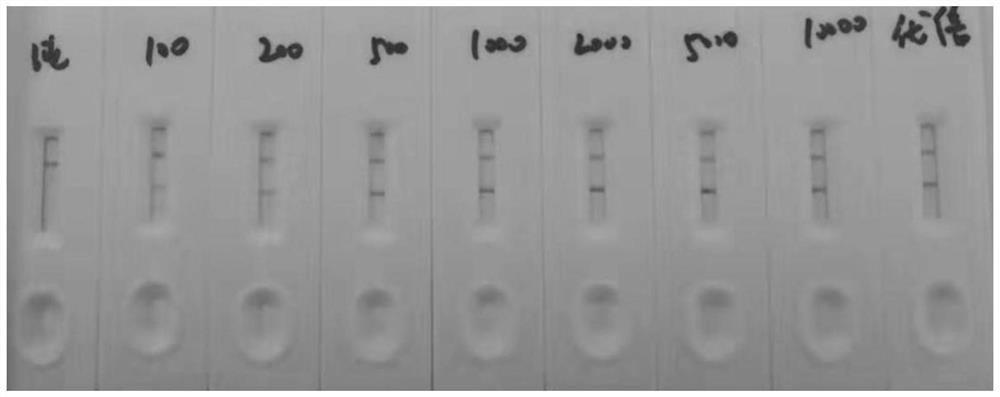
Preparation method of cTnI, NT-proBNP and D-dimer chest pain triple colloidal gold immunostrip
PendingCN109061197AEarly diagnosisEarly identificationBiological material analysisBiological testingMonoclonal antibodyColloid
The invention relates to a preparation method of a cTnI, NT-proBNP and D-dimer chest pain triple colloidal gold immunostrip. The detection problem of cTnI, NT-proBNP and D-dimer can be effectively solved. The preparation method comprises the following steps: respectively preparing an anti-cTnI protein conjugate monoclonal antibody, an anti-NT-proBNP protein conjugate monoclonal antibody and an anti-D-dimer protein conjugate monoclonal antibody for colloidal gold marking and a coating detection line; respectively preparing colloidal gold markers of the anti-cTnI protein conjugate monoclonal antibody, the anti-NT-proBNP protein conjugate monoclonal antibody and the anti-D-dimer protein conjugate monoclonal antibody, drying and curing the colloidal gold markers, sequentially coating the anti-cTnI protein conjugate monoclonal antibody, the anti-NT-proBNP protein conjugate monoclonal antibody and the anti-D-dimer protein conjugate monoclonal antibody for the coating detection line on a nitrocellulose membrane from left to right to form a detection line, and coating an anti-mouse immunoglobulin antibody on the nitrocellulose membrane to form a quality control line of the coating membraneso as to prepare the cTnI, NT-proBNP and D-dimer chest pain triple colloidal gold immunostrip. The preparation method provided by the invention is simple in process and is novel and unique, and various acute chest pains can be diagnosed and identified quickly, conveniently and effectively at the early stage.
Owner:HENAN ACAD OF SCI INST OF BIOLOGY LIABILITY
Anti-PD1-CD19 bispecific antibody, pharmaceutical composition thereof and application thereof
PendingCN111944051ASmall molecular weightEnhanced non-specific killingHybrid immunoglobulinsAntibody ingredientsSingle-Chain AntibodiesComplementarity determining region
The invention relates to the field of antibody drugs, and particularly relates to an anti-PD1-CD19 bispecific antibody, a pharmaceutical composition thereof and application thereof. The antibody comprises a heavy chain and a light chain, wherein the heavy chain and the light chain both comprise variable regions, and the variable regions comprise complementary determining regions. The antibody further comprises an immunoglobulin antibody IgG and a variable fragment scFv, wherein the scFv comprises a variable region VH and a variable region VL. According to the antibody, two specific single-chain antibodies are connected in series by using a molecular biological technology, one end of an anti-epidermal growth factor receptor is used for recognizing tumor cells, and the other end of the anti-epidermal growth factor receptor is used for recognizing and activating T lymphocytes by using an anti-PD1 single-chain antibody, so that the effect of removing the tumor cells is achieved.
Owner:SHUNHAO CELL BIOTECHNOLOGY (TIANJIN) CO LTD
Bovine immunoglobulin (IgG) double-antibody sandwich colloidal gold immunochromatographic test strip, preparation method, kit and detection method
InactiveCN111896750ARapid Quantitative DetectionQuick screeningBiological testingReagent stripIntravenous gammaglobulin
The invention provides a bovine immunoglobulin (IgG) double-antibody sandwich colloidal gold immunochromatographic test strip, a preparation method, a kit and a detection method. The colloidal gold immunochromatography test strip is characterized by comprising a bottom plate, and a water absorption pad, a chromatographic membrane, a conjugate release pad and a sample pad which are arranged on thePVC bottom plate in an overlapping manner. The conjugate release pad is coated with a colloidal gold labeled bovine immunoglobulin antibody a compound, the chromatographic membrane is provided with adetection line and a quality control line, the detection line is coated with an anti-bovine immunoglobulin antibody b, and the quality control line is coated with goat anti-mouse IgG. The invention also provides a preparation method of the chromatographic test strip, a kit comprising the reagent strip, and a detection method of the kit. According to the technical scheme, the kit is prepared basedon the principles of double-antibody sandwich colloidal gold color development and gold-labeled immunoassay analyzer quantification, and can be used for rapidly and quantitatively detecting the content of bovine immunoglobulin (IgG).
Owner:HANGZHOU BINGUO INFORMATION TECH CO LTD
Rapid and sensitive method for quantitative determination of the level of heparin—PF4 complex induced immunoglobulin antibodies
ActiveUS8940495B2Bioreactor/fermenter combinationsBiological substance pretreatmentsImmuno assayQuantitative determination
Disclosed herein is a lateral flow immuno-assay system capable of rapidly, cost effectively, and quantitatively detecting and assessing the level of HIT antibodies in body fluids of a patient. Also taught are methods for employing the system to assist in diagnosis of HIT, and for screening or detecting a changing titer of HIT antibodies in the body fluids of a patient to determine susceptibility toward HIT.
Owner:BIOMEDOMICS
Method for preparing specific protein composition
InactiveCN107096026AEasy to produceLow costAntibacterial agentsImmunoglobulins against virusesWhole blood productSpecific immunity
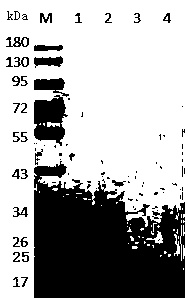
The invention provides a method for synchronously preparing a specific protein composition from human hydrophobia immunoglobulin and human tetanus immunoglobulin. The method has the key points: firstly, immunizing the same blood plasma donor with a human tetanus vaccine and a human hydrophobia vaccine according to respective immunization schedules; collecting blood plasma after the last-time immunization; preparing the specific protein composition from the obtained specific immune blood plasma by adopting a blood-product immunoglobulin production method; adjusting the concentration of the specific protein composition in a manner that the valence of a human hydrophobia immunoglobulin antibody is not lower than 10IU / ml and the valence of a human tetanus immunoglobulin antibody is not lower than 4IU / ml. The method has the positive effects that the production process is simpler, and the cost is lower.
Owner:苏文全
Mite allergen specific yolk immunoglobulin antibody and anti-allergic preparation thereof
InactiveCN108191975ANo adverse reactionHigh antibody activityEgg immunoglobulinsImmunoglobulins against animals/humansYolkMite allergen
The invention relates to a mite allergen specific yolk immunoglobulin antibody and an anti-allergic preparation thereof. The mite allergen specific yolk immunoglobulin antibody is mainly prepared by the following method: extracting a mite allergen, carrying out supplementary immunization on chickens, preparing crude extract, finely extracting and purifying. The mite allergen specific yolk immunoglobulin antibody and the preparation thereof, provided by the invention, have the advantages of high antibody activity and specific defending effect on local sensitization caused by mites, and have a good rejection effect on the local sensitization caused by sensibiligen; the mite allergen specific yolk immunoglobulin antibody has no immunogenicity, no adverse effect on skin mucous membranes of human bodies and high safety performance.
Owner:广州益琪琳生物科技有限公司
Immunoglobulin antibody against SARS-CoV and its preparing method
The present invention relates to anti-SARS-CoV immune globulin F(ab' )2 antibody for treatment, prevention and diagnosis of SARS-CoV. The present invention features that the antibody is horse anti-SARS-CoV immune globulin F(ab' )2 antibody. The present invention also discloses the preparation process of the antibody. The antibody of the present invention can kill SARS-CoV specifically, and has excellent effect of preventing and treating SARS.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Preparation method and application of immunoglobulin antibody with high sialic acid content
InactiveCN101358225BReduce doseRaise the ratioAntipyreticAnalgesicsEnzymatic synthesisImmunologic disorders
The preparation method and application of an immunoglobulin antibody with high sialic acid content relate to an immunoglobulin antibody, in particular to a method for increasing the sialic acid content on an immunoglobulin antibody by catalyzing a glycosyltransferase and its application. Provided are a preparation method and application of an immunoglobulin antibody with high sialic acid content. Cytidine-5'-phosphate-sialic acid synthase catalyzes the reaction of sialic acid with cytidine-5'-triphosphate to generate cytidine-5'-phosphate-sialic acid; β1,4-galactosyltransferase converts uridine diphosphate -Galactose as a substrate, catalyzes the connection of galactose to the terminal N-acetylglucosamine of immunoglobulin sugars; sialyltransferase catalyzes the connection of sialic acid to cytidine-5'-phosphate-sialic acid On the galactose at the glycosyl-terminus of immunoglobulins. The prepared immunoglobulin antibody with high sialic acid content can be used to prepare medicines for treating autoimmune diseases, especially rheumatoid arthritis.
Owner:XIAMEN UNIV
Hybridoma cell line capable of secreting monoclonal antibody against bovine immunoglobulin IgG and its application
InactiveCN104099300BHigh sensitivityStrong specificityImmunoglobulins against animals/humansMicroorganism based processesScreening methodImmunoglobulin IgG
The invention relates to a screening method for a hybridoma cell line able to secrete an anti-bovine immunoglobulin IgG monoclonal antibody for bovine immunoglobulin IgG detection, the hybridoma cell line, the monoclonal antibody secreted thereby and application in the field of bovine immunoglobulin IgG detection. The hybridoma cell is preserved in China Center For Type Culture Collection, Wuhan University at Luojiashan, Wuchang, Wuhan City in Hubei Province, and the preservation number is CCTCC No:2013183. The anti-bovine immunoglobulin IgG monoclonal antibody secreted by the hybridoma cell has the advantages of strong specificity, large affinity and high titer and the like, and can be widely applied in the detection reagent or detection equipment field of bovine immunoglobulin IgG. A bovine immunoglobulin IgG rapid detection card built based on the immunochromatography principle can be used for detection of bovine immunoglobulin IgG in colostrum and its products, bovine blood and other samples, and has significant advantages compared with conventional detection methods in the aspects of specificity, sensitivity and detection efficiency, etc.
Owner:SHENYANG AGRI UNIV
A kind of preparation method and application of rabbit anti-grouper serum immunoglobulin HRP-labeled antibody
ActiveCN107389921BHigh purityIncrease enzyme activityColor/spectral properties measurementsAntibody conjugateViral disease
The invention discloses a preparation method and application of a rabbit anti-grouper serum immunoglobulin HRP-labeled antibody, and belongs to the technical field of antibody preparation. The rabbit anti-grouper serum immunoglobulin HRP-labeled antibody is an enzyme antibody conjugate comprising HRP and a rabbit anti-grouper serum immunoglobulin antibody, the rabbit anti-grouper serum immunoglobulin HRP-labeled antibody can be specifically bound with grouper serum immunoglobulin for color development of a HRP substrate. The HRP-labeled antibody prepared by the method has high purity, high enzyme activity, suitable conjugate labeling rate, great dilution degree for performing ELISA tests, and high sensitivity, and can be used for detecting viral diseases, parasitic diseases and bacterial diseases of grouper.
Owner:FISHERIES RES INST OF FUJIAN
In-vitro binding of immature dendritic cells mediated by NY-ESO-1 via polymeric structure domain
InactiveCN106754710ABlood/immune system cellsAnimals/human peptidesEpidermal Dendritic CellsVaccine Immunogenicity
The invention relates to the field of biological medicine, in particular to in-vitro binding of immature dendritic cells mediated by NY-ESO-1 via a polymeric structure domain. The inventor of the invention discovers that NY-ESO-1 protein can form a poly structure even in a loading buffer with the conventional concentration of beta-mercaptoethanol; a polymerization reaction of NY-ESO-1 is mediated by an intermolecular sulfide bond of NY-ESO-1; the in-vitro binding of NY-ESO-1 with the immature human and mouse dendritic cells is related to a polymerization structure of NY-ESO-1; TLR4 influences in-vitro binding of NY-ESO-1 with DCs in marrow; the binding of NY-ESO-1 with the immature human and mouse dendritic cells can also be achieved by a calprotectin effect; the oligomerization of a polymer reduces the binding capacity of TLR4 and NY-ESO-1; a polymer structure of NY-ESO-1 and TLR4 in a host participate in immunoglobulin antibody response; and Art v1 (wtArt) and CA9 gene immunogenicity is improved by NY-ESO-1 gene fusion expression. NY-ESO-1 is confirmed to mediate the in-vitro binding of the immature dendritic cells via the polymeric structure domain.
Owner:宁波美丽人生医药生物科技发展有限公司
A gel for blood transfusion cross-matching, preparation method and experimental combiner
InactiveCN105548581BThe operation of blood transfusion and cross matching is convenient and quickReliable resultsBiological testingGel preparationPhosphate
Owner:LUOHE MEDICAL COLLEGE
A kind of enterovirus type 71 inactivated vaccine antigen ELISA detection kit
ActiveCN104569428BStrong specificityAvoid non-specific reactionsBiological testingAbzymeMicrotiter plate
Owner:ZHEJIANG PUKANG BIOTECH
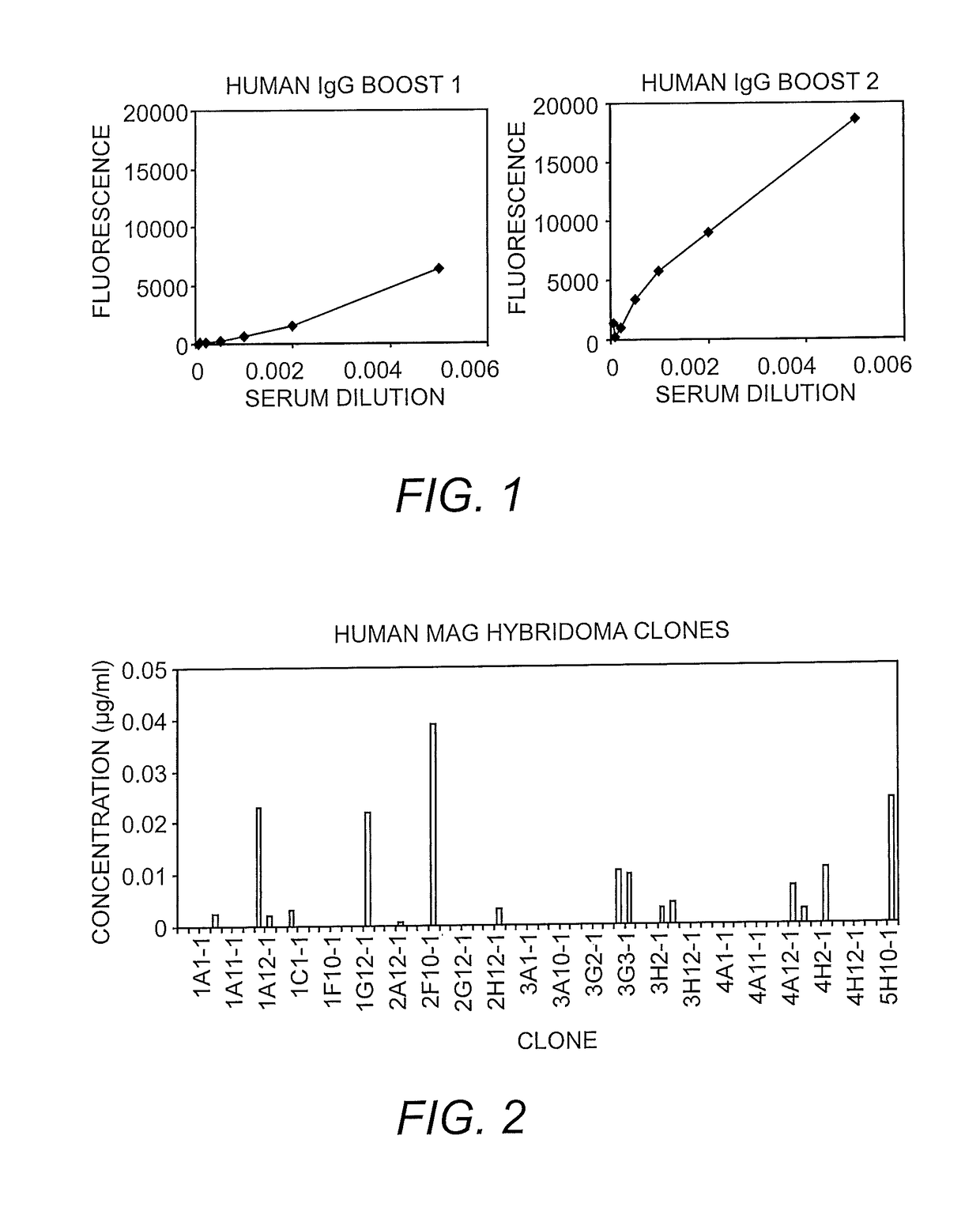
Method for producing human monoclonal immunoglobuling antibodies
The present invention a method for producing a human immunoglobulin G (IgG) antibody using a prime-boost regime in a Bone Marrow Liver Thymic (BLT) mouse.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Correlation of TLR4 with in-vitro binding of NY-ESO-1 and DC
The invention relates to the field of biological medicine, in particular to a correlation of cell surface TLR4 and in-vitro binding of NY-ESO-1 and a DC (dentritic cell). The inventor of the invention discovers that NY-ESO-1 protein can form a poly structure even in a loading buffer with the conventional concentration of beta-mercaptoethanol; a polymerization reaction of NY-ESO-1 is mediated by an intermolecular sulfide bond of NY-ESO-1; the in-vitro binding of NY-ESO-1 with immature human and mouse dendritic cells is related to a polymerization structure of NY-ESO-1; TLR4 influences in-vitro binding of NY-ESO-1 and the DC in marrow; the binding of NY-ESO-1 with the immature human and mouse dendritic cells can also be achieved by a calprotectin effect; the oligomerization of a polymer reduces the binding capacity of TLR4 and NY-ESO-1; a polymer structure of NY-ESO-1 and TLR4 in a host participate in immunoglobulin antibody response; and Art v1 (wtArt) and CA9 gene immunogenicity is improved by NY-ESO-1 gene fusion expression. The cell surface TLR4 is confirmed to be correlated with in-vitro binding of NY-ESO-1 and the DC.
Owner:宁波美丽人生医药生物科技发展有限公司
Test strip with structured substrate for multiplex lateral flow assay for disease diagnostics
Methods and kits for diagnosis of confirmatory diagnosis of a tick-borne infection, such as Lyme disease, is provided. The methods and kits include one or more multi-channel lateral flow immunoassay test strips. In an embodiment, the test strip comprises at least five individual, discrete fluid flow channels where each of the at least five channels includes one or more binding members (antigens) for detection of immunoglobulin antibodies against a Borrelia bacterium in a blood sample. The binding members (antigens) are, in one embodiment, selected from a group of immunoglobulin antibodies consisting of p18 (decorin-binding protein), p23, p28, p30, p39, p41 (flagellin), p45, p58, p66, p93, VlSE / C6 and p31. The kits include external controls selected from a container with a positive control, a container with a negative control, and both of a container with a positive control and a container with a negative control.
Owner:QUIDEL
A rapid detection test strip of Fasciola large fasciola immunochromatography and its preparation method
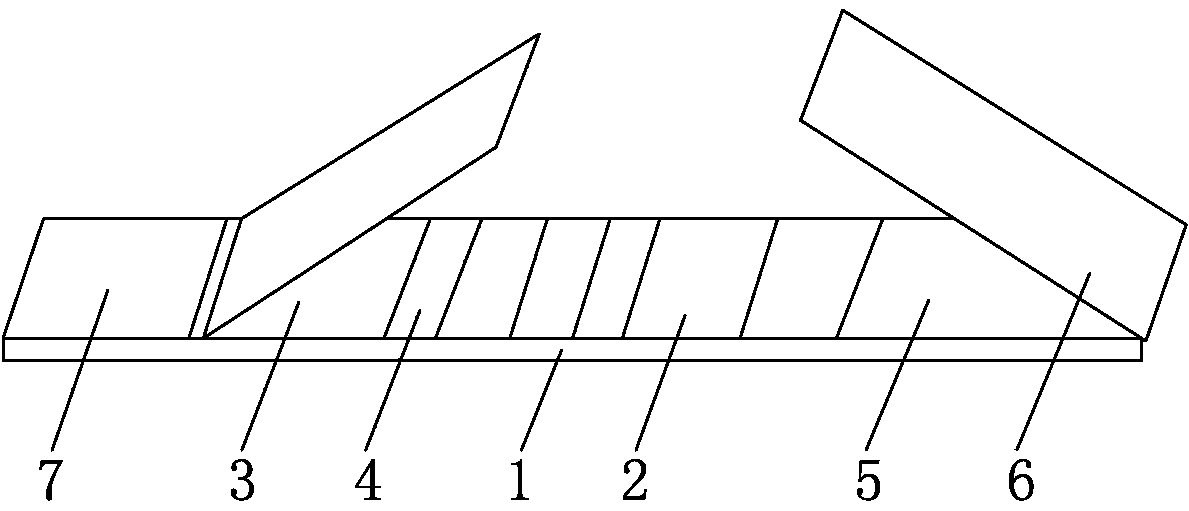
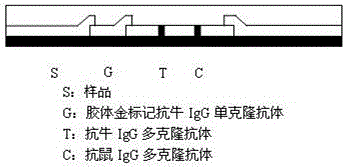
The invention discloses a rapid detection test strip of flax fluke immunochromatography, which relates to the technical field of biological detection, and includes a support liner, on which a coating film, a marker pad and a sample pad are sequentially pasted, on the support liner The hand-held end of the liner is pasted with a water-absorbing pad; the marker pad is coated with the 7D1 monoclonal antibody against the ES antigen of Fasciola macula, the front part of the coating film is provided with a detection line, and the rear part of the coating film is provided with a detection line. Quality control line, the detection line is coated with the 7D4 monoclonal antibody against the ES antigen of Fasciola large, and the quality control line is coated with rabbit anti-mouse immunoglobulin antibody. Compared with the prior art, the present invention The test strip is easy to operate, does not require professionals in the field and professional equipment and instruments, can meet the needs of personnel and departments at different levels, especially the needs of grassroots units, and has the advantages of rapidity, accuracy, sensitivity, specificity, and low price.
Owner:GUANGXI UNIV
A kind of plant lactobacillus derived from giant panda and its application
InactiveCN106434411BProtection from damageReduce percentageAntibacterial agentsBacteriaEscherichia coliIncreased serum IgA
Owner:SICHUAN AGRI UNIV +1
Feed additive with effect of improving of nitrogen stress resistance of tilapia mossambica
InactiveCN107874019AImprove integrityGuaranteed functionAnimal feeding stuffAccessory food factorsNitrogen stressToxicant
The invention relates to the technical field of aquatic feed additives, in particular to a feed additive for improving the nitrogen stress tolerance of tilapia. Specifically, the feed is added with potato juice as a culture substrate, and after inoculated with brewer's yeast and undergoes four-stage fermentation treatment, It is obtained after high-temperature sterilization and spray drying; it is added to tilapia feed as an additive, and acts synergistically to supplement non-enzyme antioxidant substances in tilapia, capture free radicals in the body, exert antioxidant effects, and protect phospholipid membranes Unsaturated fatty acids, maintain the normal function of the membrane, maintain the integrity of the cell membrane, and help to accelerate metabolism, combine with toxic substances in the body, convert them into non-toxic substances and excrete them from the body, and can also improve the body's stress ability, promote immunoglobulins and antibodies form.
Owner:安徽省家牧动物营养科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com