Anti-HER2/PD1 bispecific antibody
A bispecific antibody, antibody technology, applied in the direction of antibodies, specific peptides, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1. Construction of anti-HER2 / PD1 double antibody molecule
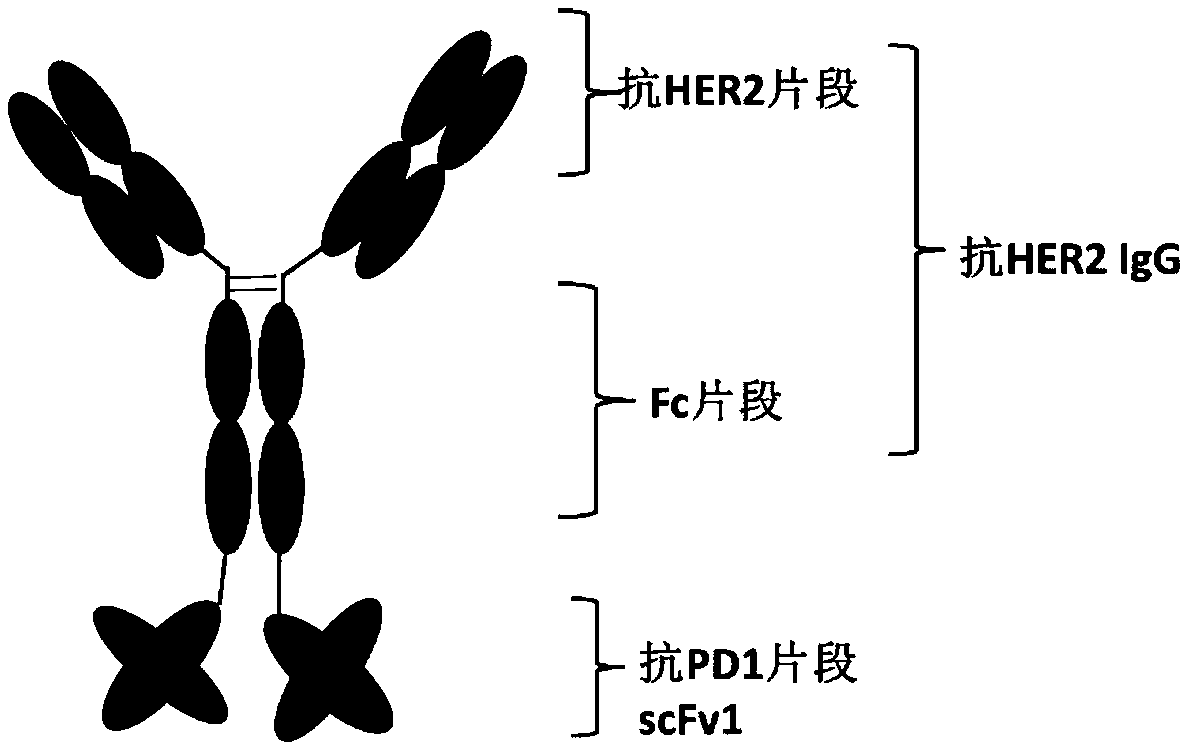
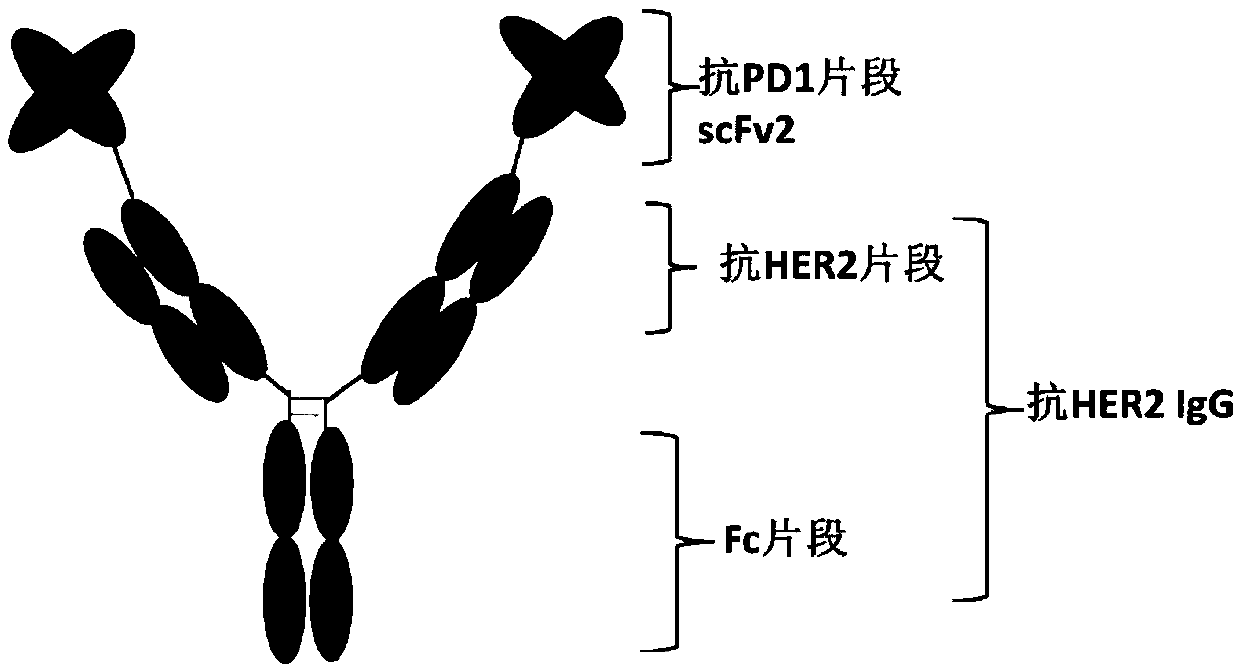
[0116] In the present invention, the anti-HER2 / PD1 bispecific antibody a is constructed by using the HER2 monoclonal antibody IgG and the scFv of the PD1 monoclonal antibody in series. The light chain variable region VL (SEQ ID NO: 14) and the heavy chain variable region VH (SEQ ID NO: 13) of the anti-PD1 monoclonal antibody were connected through a peptide linker L1 (SEQ ID NO: 17) to obtain anti-PD1 The single-chain antibody fragment VL-L1-VH, that is, the anti-PD1 fragment scFv1 (SEQ ID NO: 19). Using L2 (SEQ ID NO: 18) to connect the single-chain antibody fragment with the heavy chain of the anti-HER2 monoclonal antibody, thereby obtaining the heavy chain of the bispecific antibody molecule anti-HER2 / PD1 biclonal antibody a (SEQ ID NO: 20 ), the light chain of HER2 monoclonal antibody (SEQ ID NO: 21) remains unchanged. In order to improve the expression efficiency of antibody molecules in CHO cell...
Embodiment 2
[0118] Example 2. Expression and purification of double antibodies
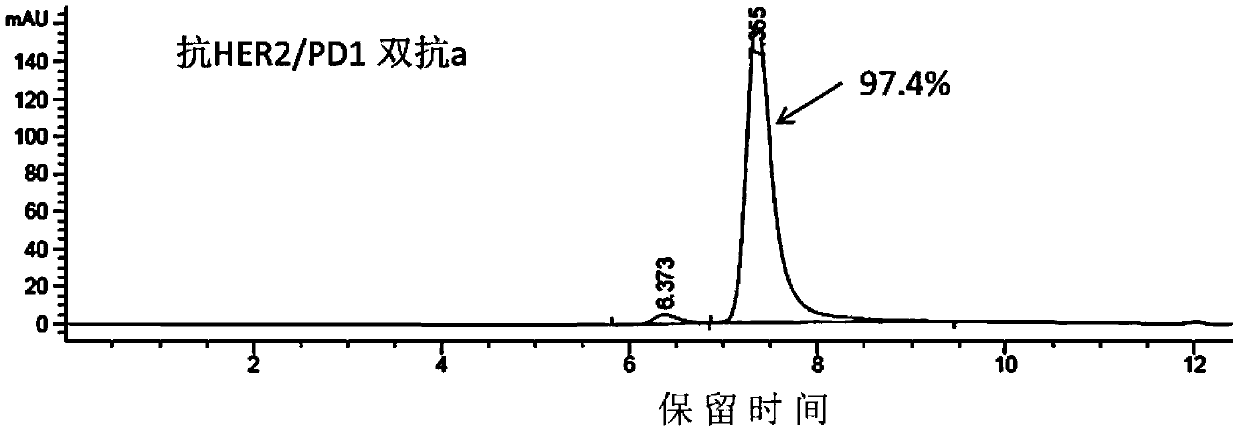
[0119] The heavy chain and light chain DNA fragments of the double antibody were subcloned into the pTT5 vector, and the recombinant plasmids were extracted and co-transfected into CHO cells and / or 293E cells. After the cells have been cultured for 5-7 days, the culture medium is subjected to high-speed centrifugation and vacuum filtration through a microporous membrane, then loaded onto a HiTrap MabSelectSuRe column, and the protein is eluted in one step with an eluent containing 100mM citric acid, pH 3.5, and the target is recovered Samples were dialyzed into PBS pH 7.4. The purified protein was detected by HPLC, and the HPLC detection patterns of anti-HER2 / PD1 double antibody a and b were as follows: Figure 2A , 2B As shown, the molecular state of the antibody is uniform, and the purity of the monomer reaches more than 97%. Add the purified anti-HER2 / PD1 double antibodies a and b to the non-reducing el...
Embodiment 3
[0120] Example 3. Enzyme-linked immunosorbent assay (ELISA) to measure the affinity of the double antibody to the antigen
[0121] In order to detect the affinity of anti-HER2 / PD1 double antibody a and b to HER2 antigen respectively, dilute HER2-ECD-His protein (manufactured by Sunshine Guojian) to 250 ng / ml with pH 7.4 PBS buffer, and then 100 μl / Add the wells to the ELISA plate; incubate overnight at 4°C; wash the plate twice with PBST the next day; add PBST+1% BSA to each well for blocking, block at 37°C for 1 hour; wash the plate twice with PBST; then add PBS+1% The antibody to be tested was serially diluted in BSA, and the HER2 monoclonal antibody was used as a positive control. The initial concentration was 100 nM, and 8 gradients were gradually diluted by 4 times. Incubate at 37°C for 1 hour; wash the plate twice with PBST, add HRP-labeled mouse anti-human Fab antibody, and incubate at 37°C for another 40 minutes; wash the plate three times with PBST and pat dry, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com