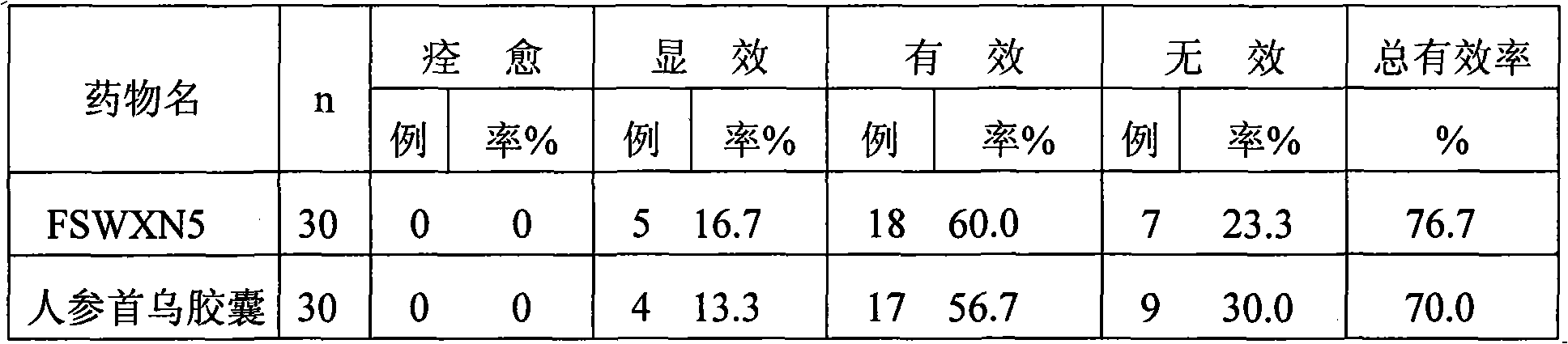
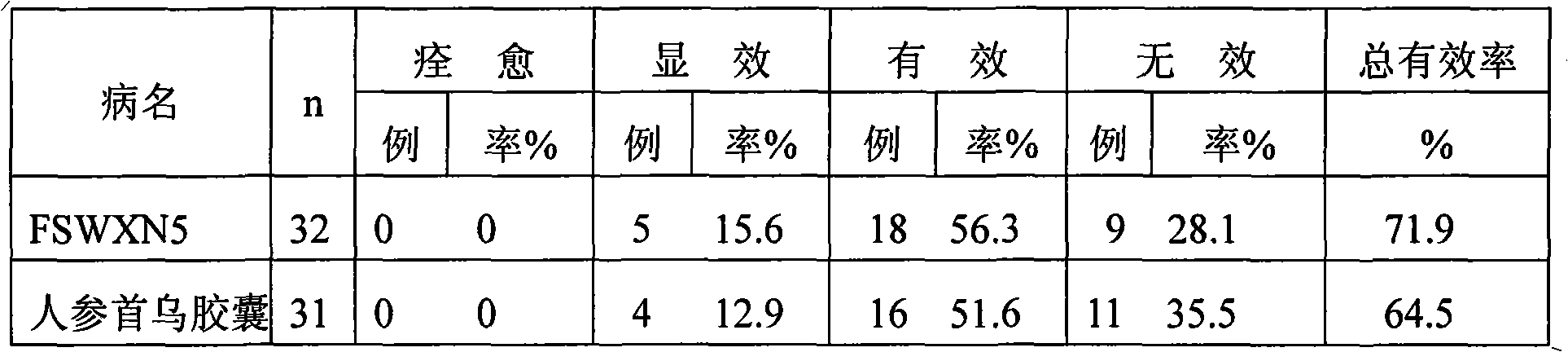
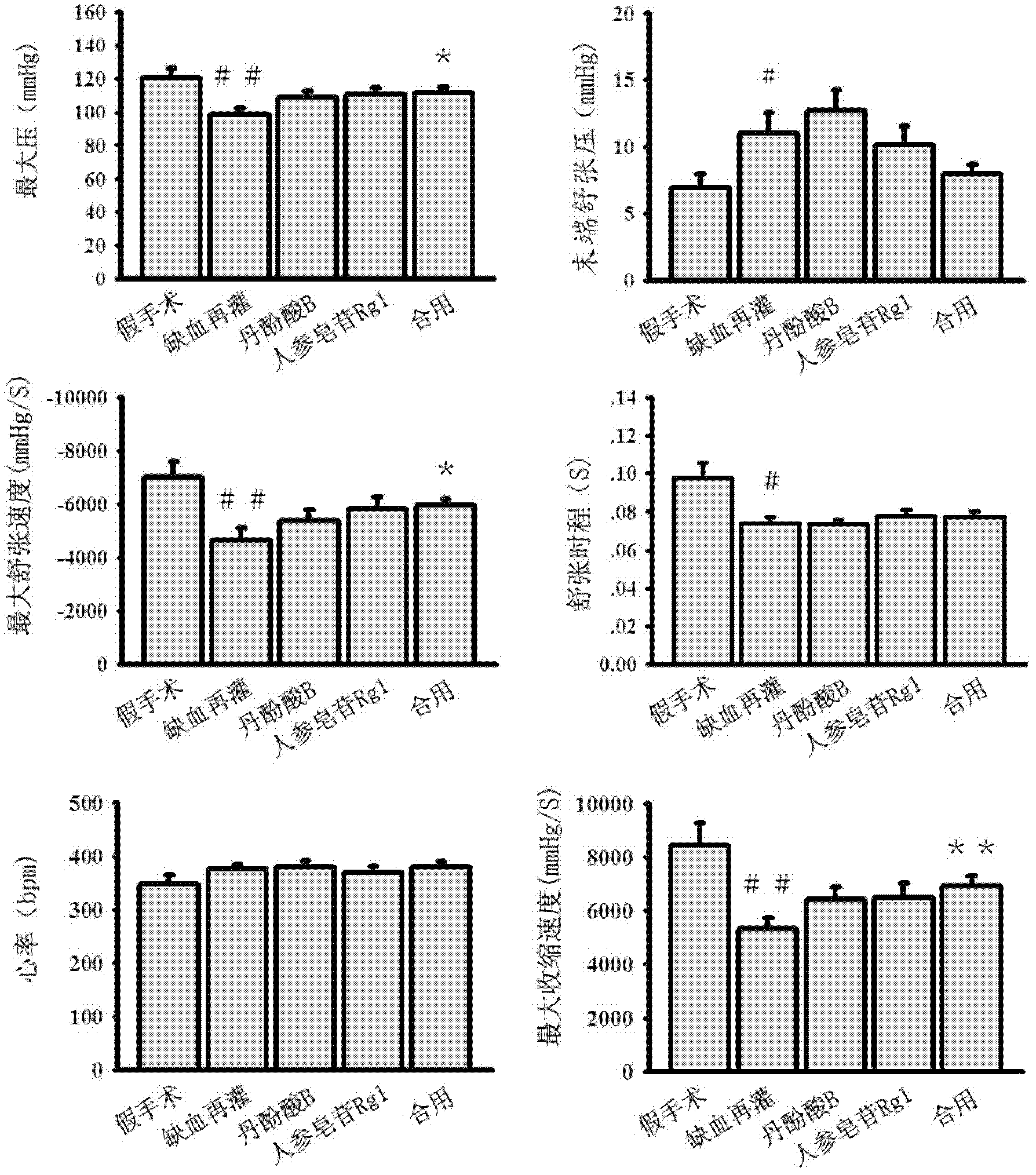
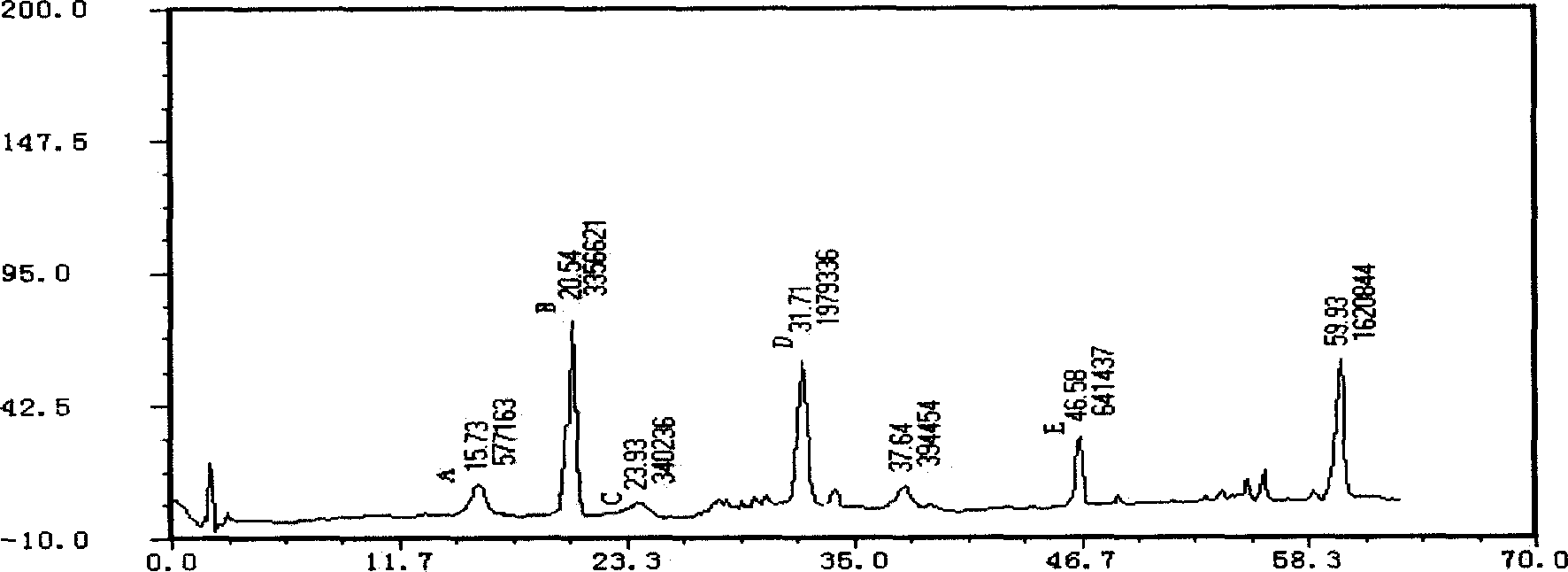
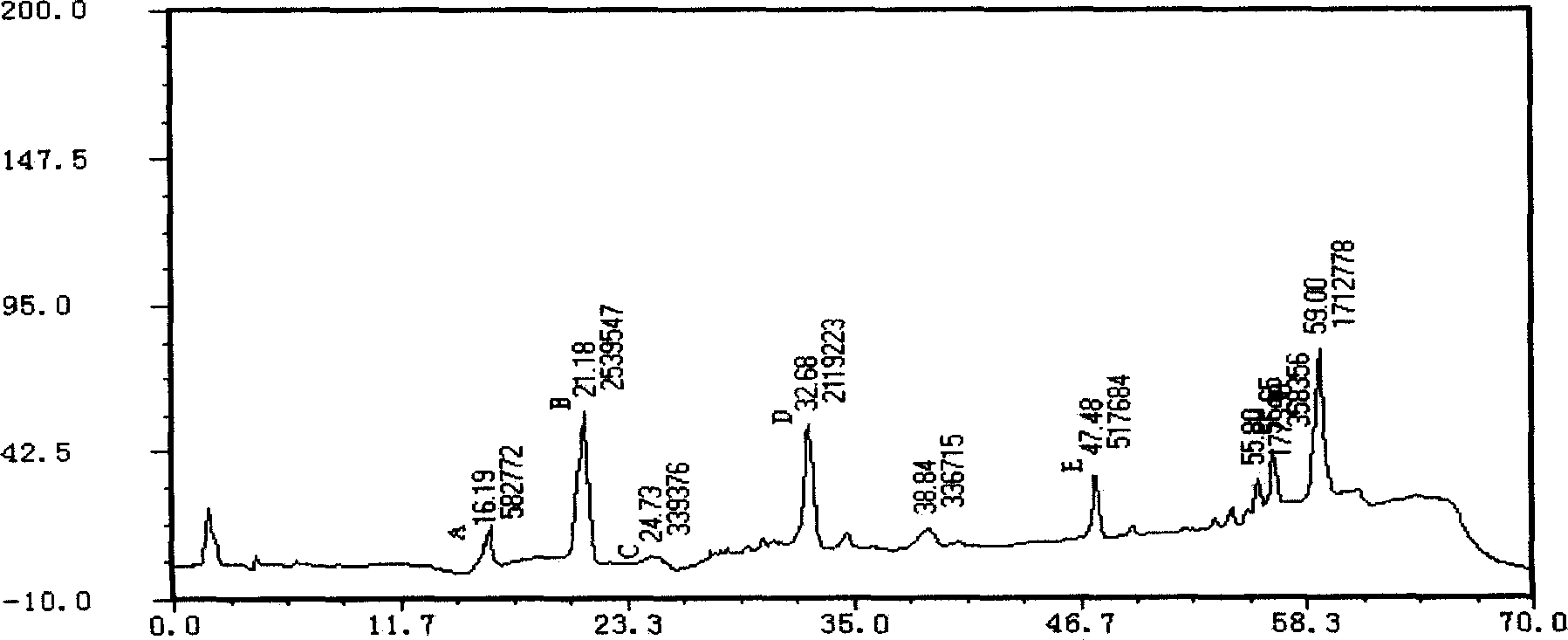
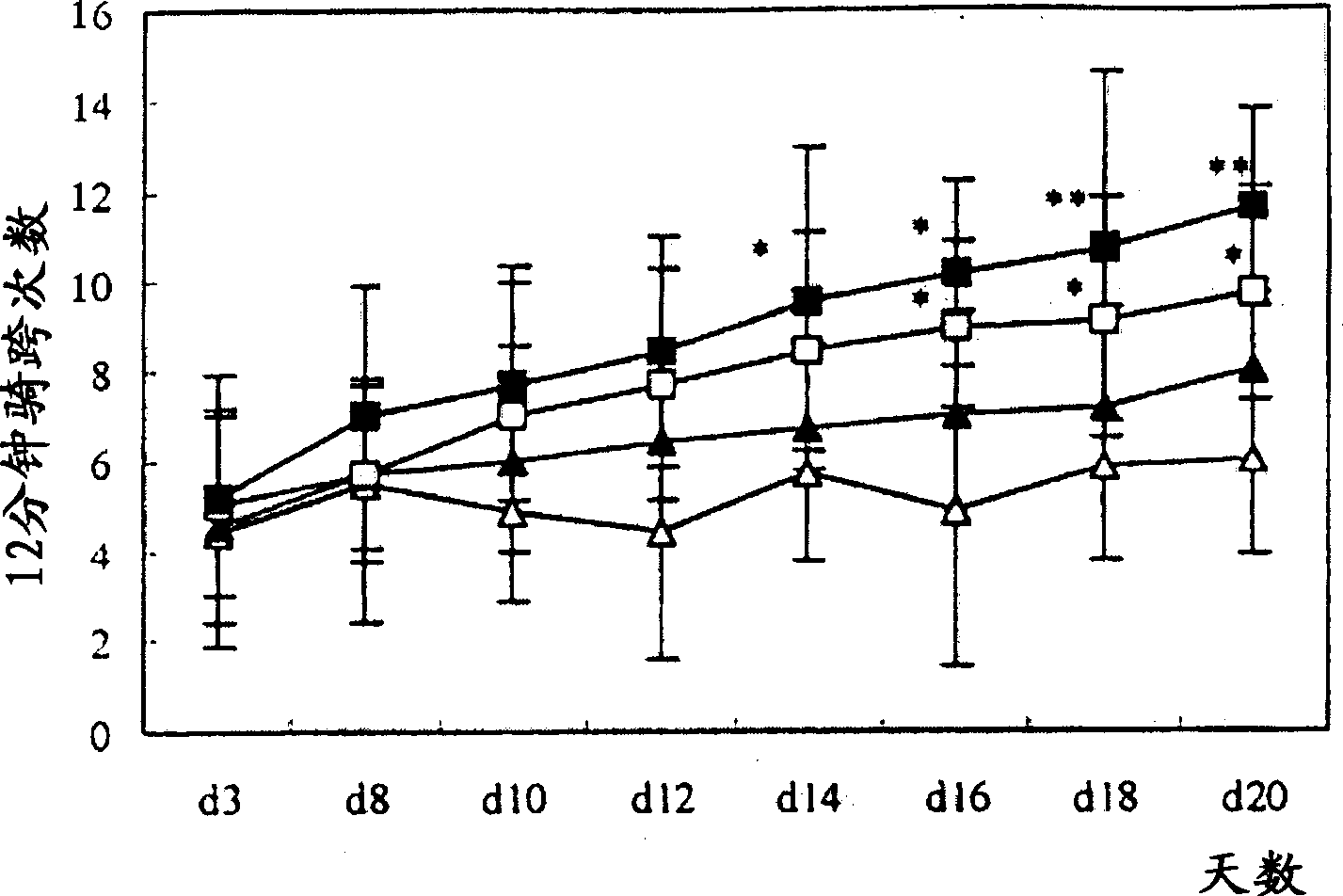
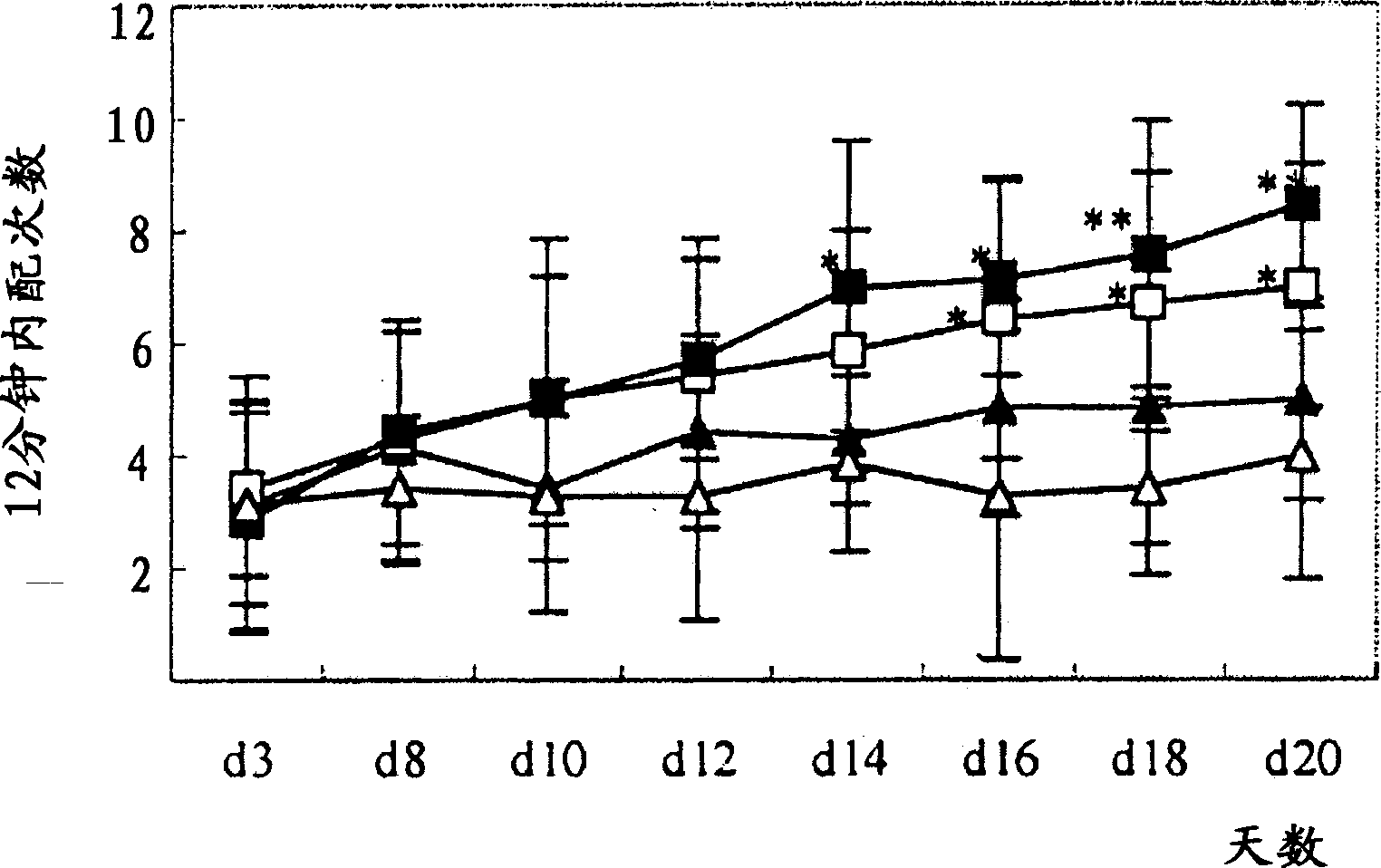
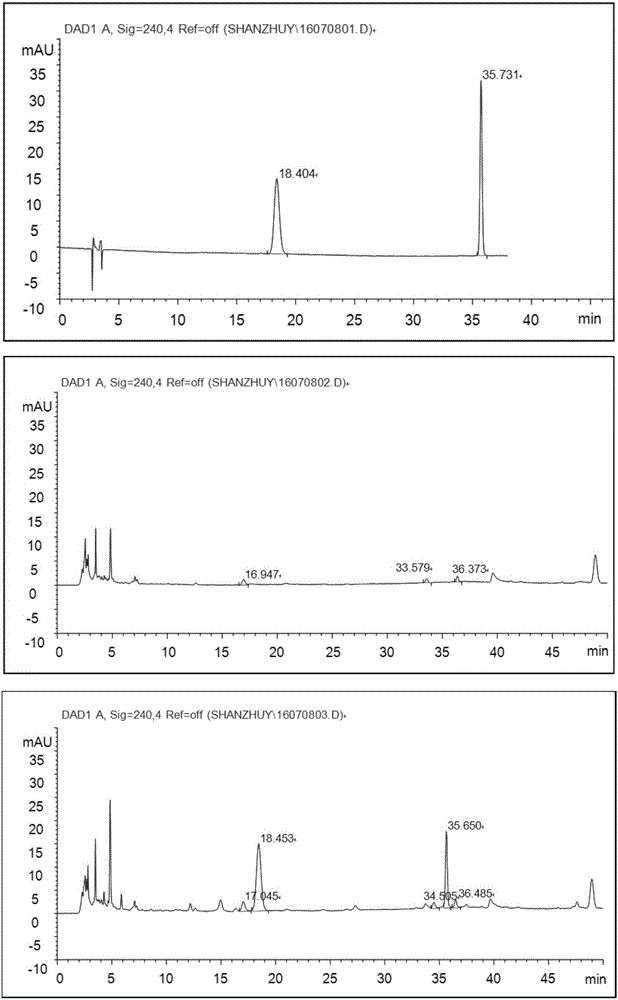
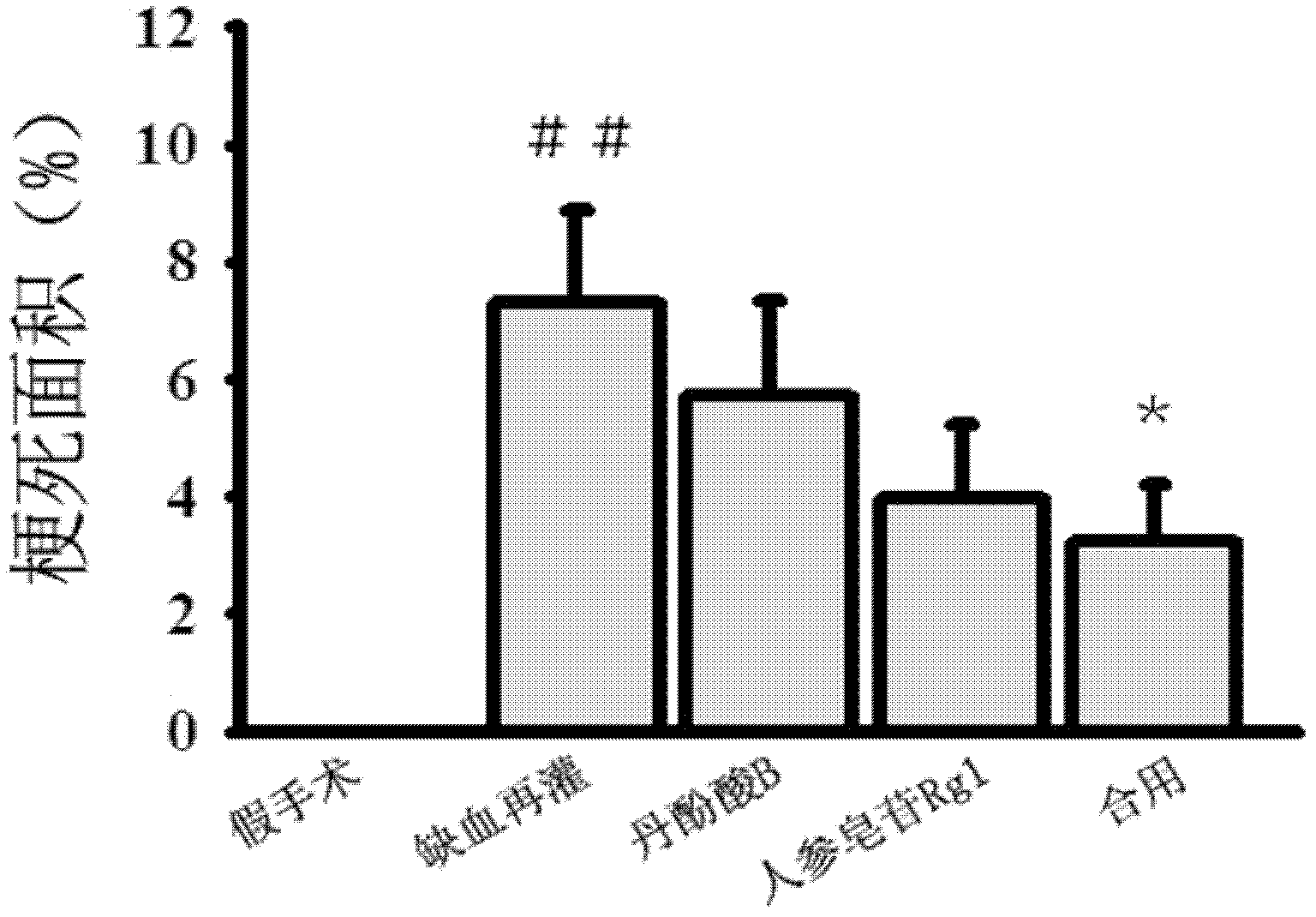Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
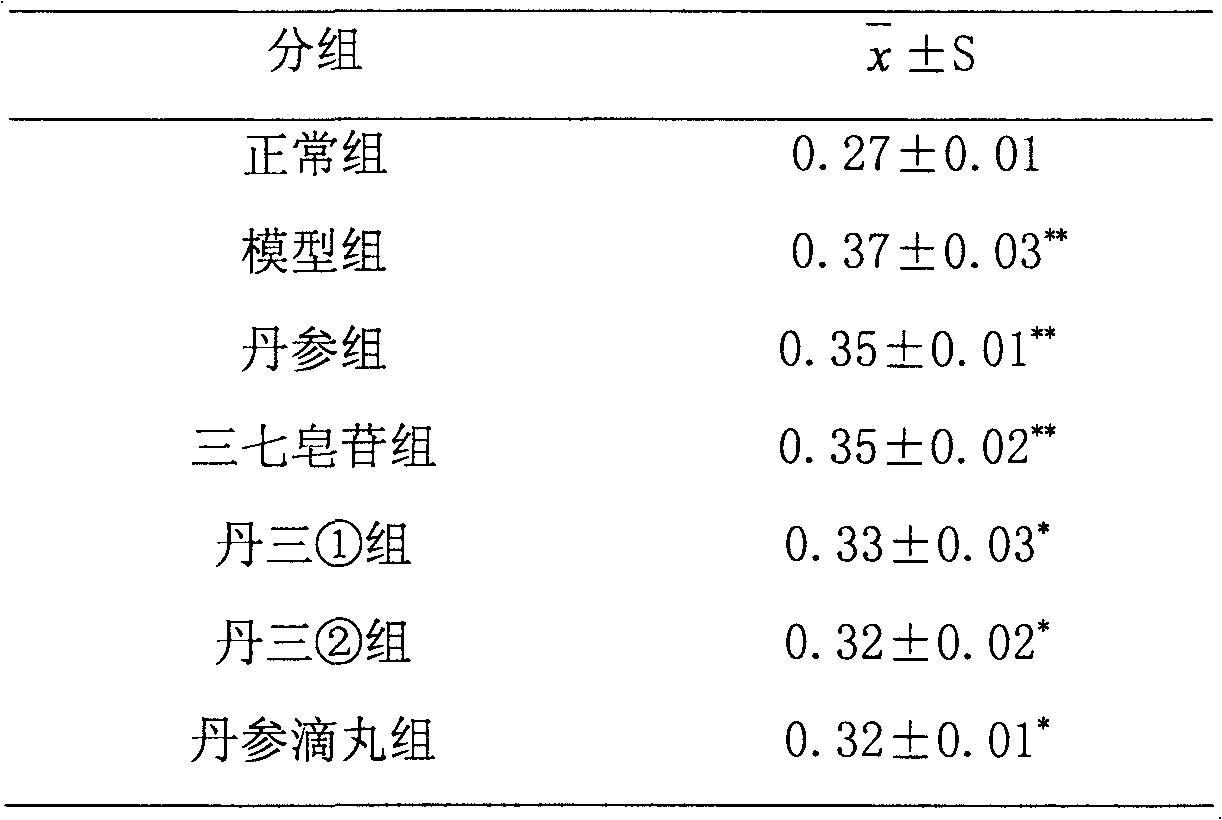
389 results about "Ginsenoside Rg1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Personal care compositions and methods for the beautification of mammalian skin and hair
Personal care composition comprising from about 0.05% to about 5% of at least one aquaporin-stimulating compound selected from the group consisting of xanthine, caffeine; 2-amino-6-methyl-mercaptopurine; 1-methyl xanthine; 2-aminopurine; theophylline; theobromine; adenine; adenosine; kinetin; p-chlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid; indole-3-butyric acid; indole-3-acetic acid methyl ester; beta-naphthoxyacetic acid; 2,3,5-triiodobenzoic acid; adenine hemisulfate; n-benzyl-9-(2-tetrahydropyranyl)adenine; 1,3-diphenylurea; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)urea; zeatin; indole-3-acetic acid; 6-benzylaminopurine; alpha-napthaleneacetic acid; 6-2-furoylaminopurine; green tea extract; white tea extract; menthol; tea tree oil; ginsenoside-RB1; ginsenoside-RB3; ginsenoside-RC; ginsenoside-RD; ginsenoside-RE; ginsenoside-RG1; ginseng root extract; ginseng flower extract; pomegranate extract, extracts from Ajuga turkestanica; extracts from viola tricolor and combinations thereof; an additional ingredient selected from the group consisting of niacinamide, glycerin and mixtures thereof, and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
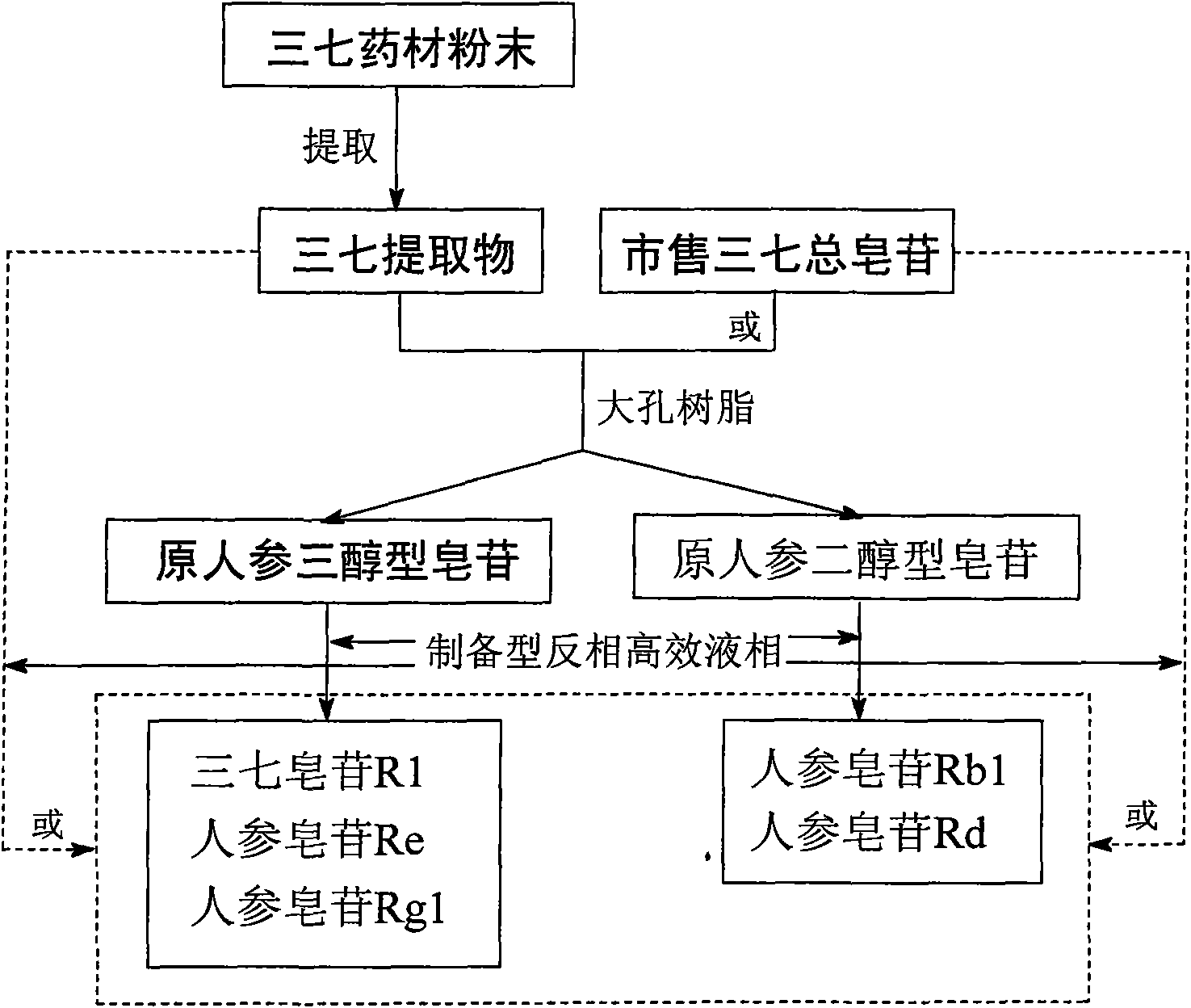
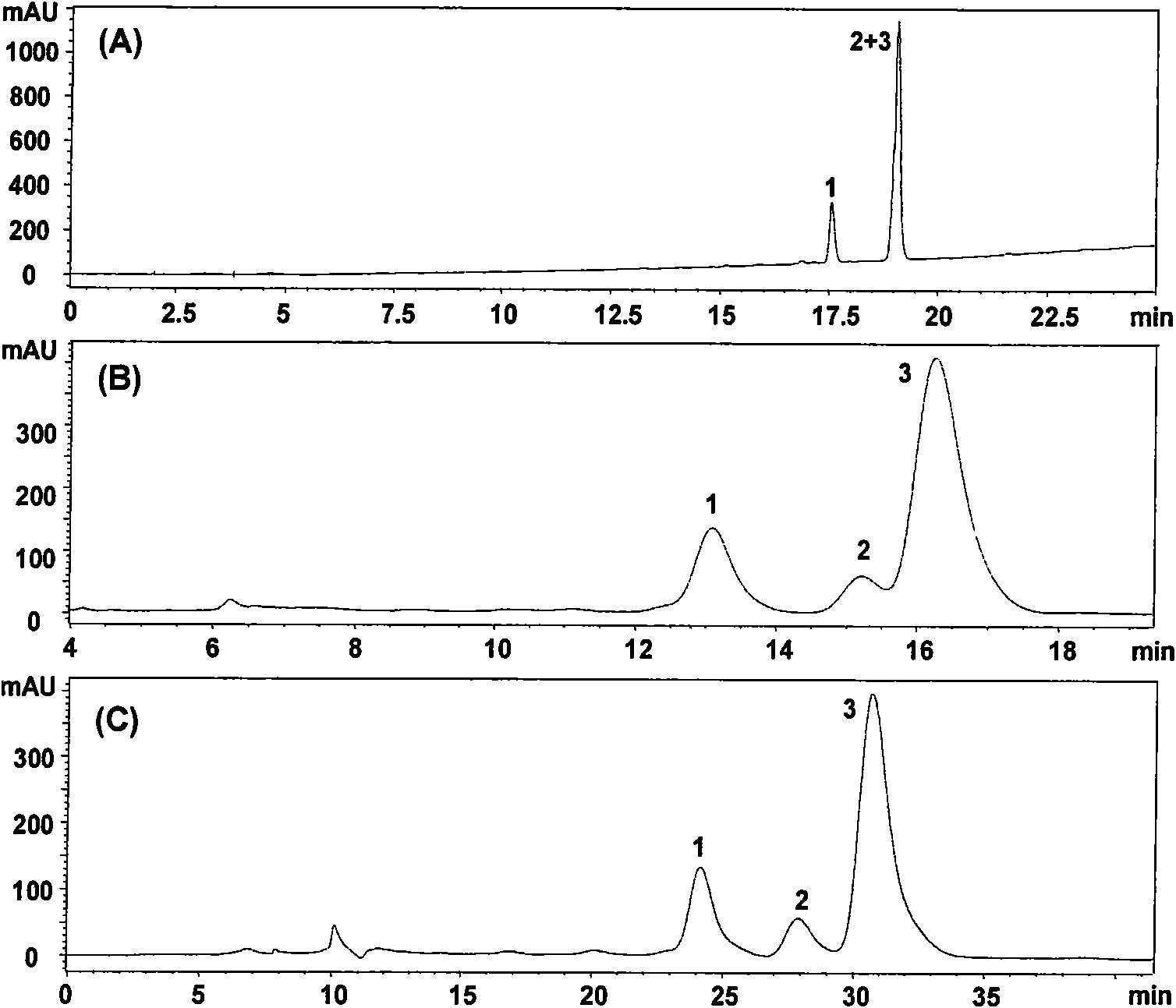
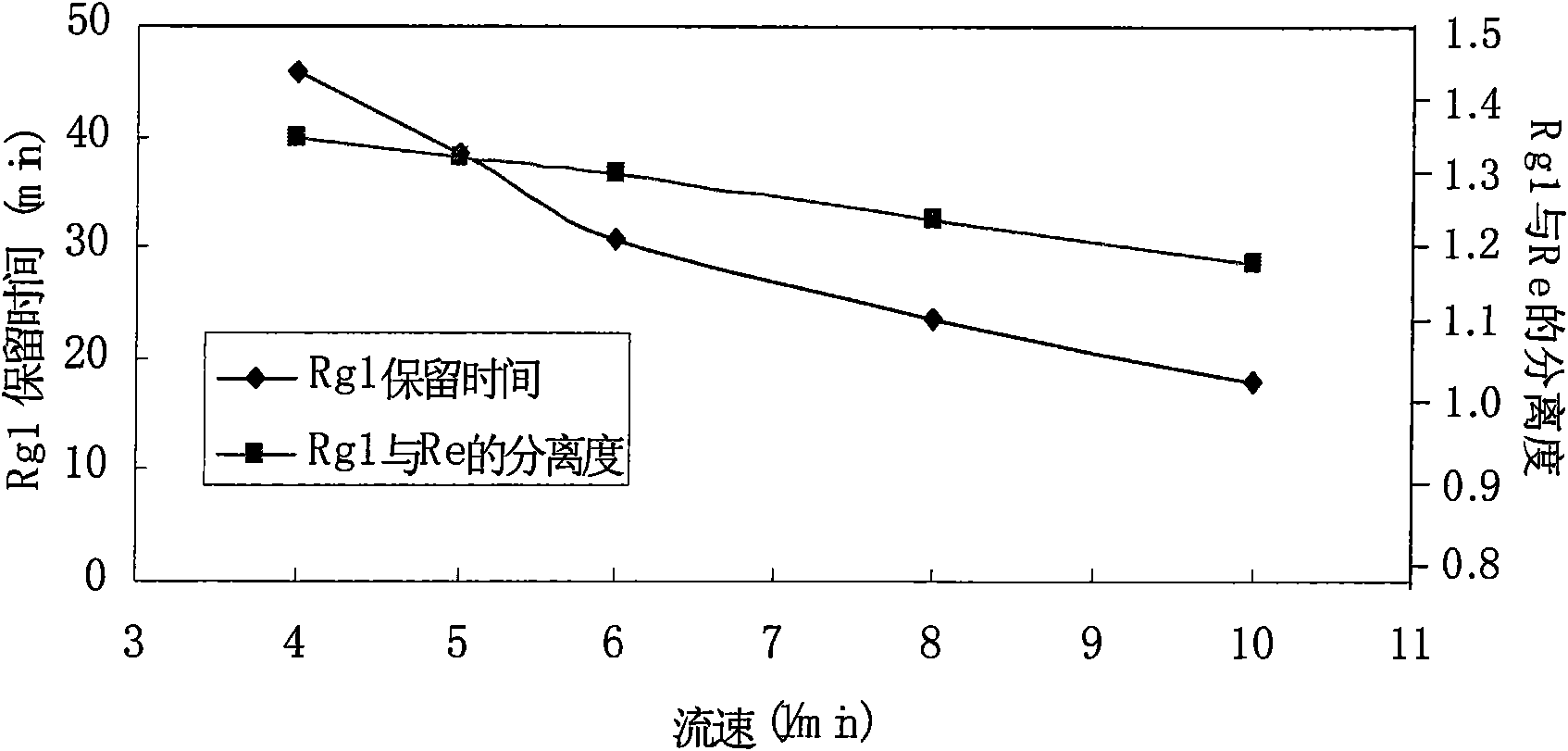
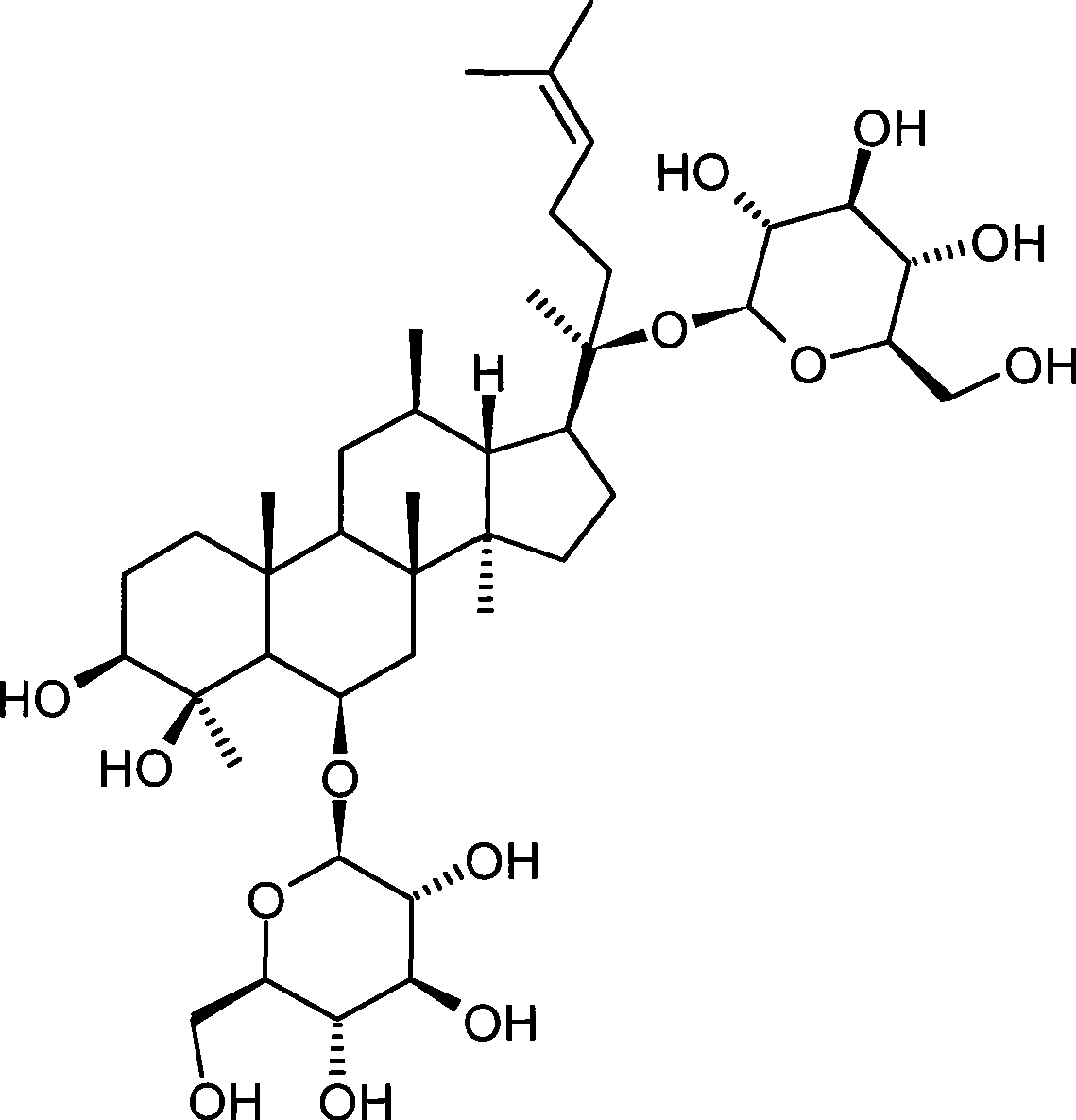
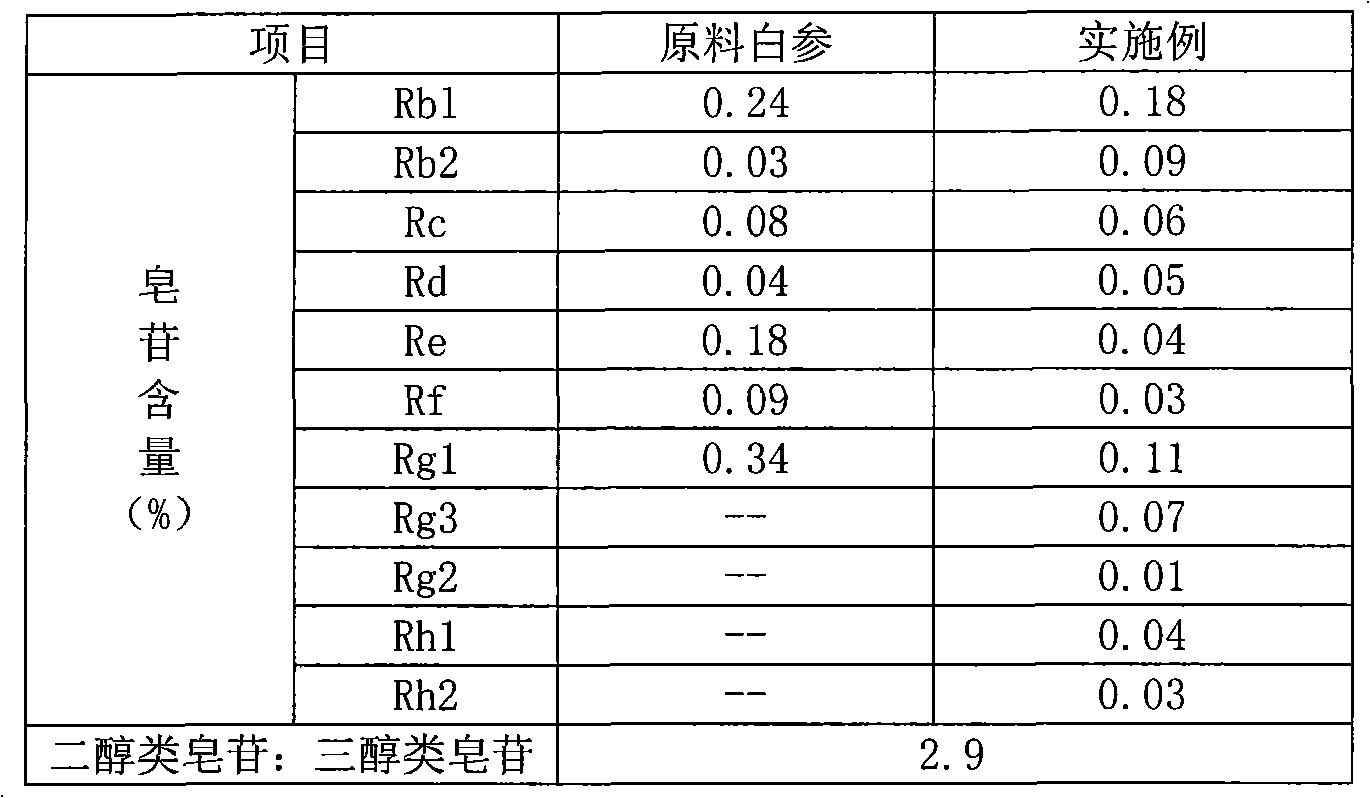
Method for preparing notoginsenoside R1 and ginsenoside Rg1, Re, Rb1 and Rd
InactiveCN101575357ASimple processShorten the production cycleSteroidsIsocratic elutionPanax notoginseng extract
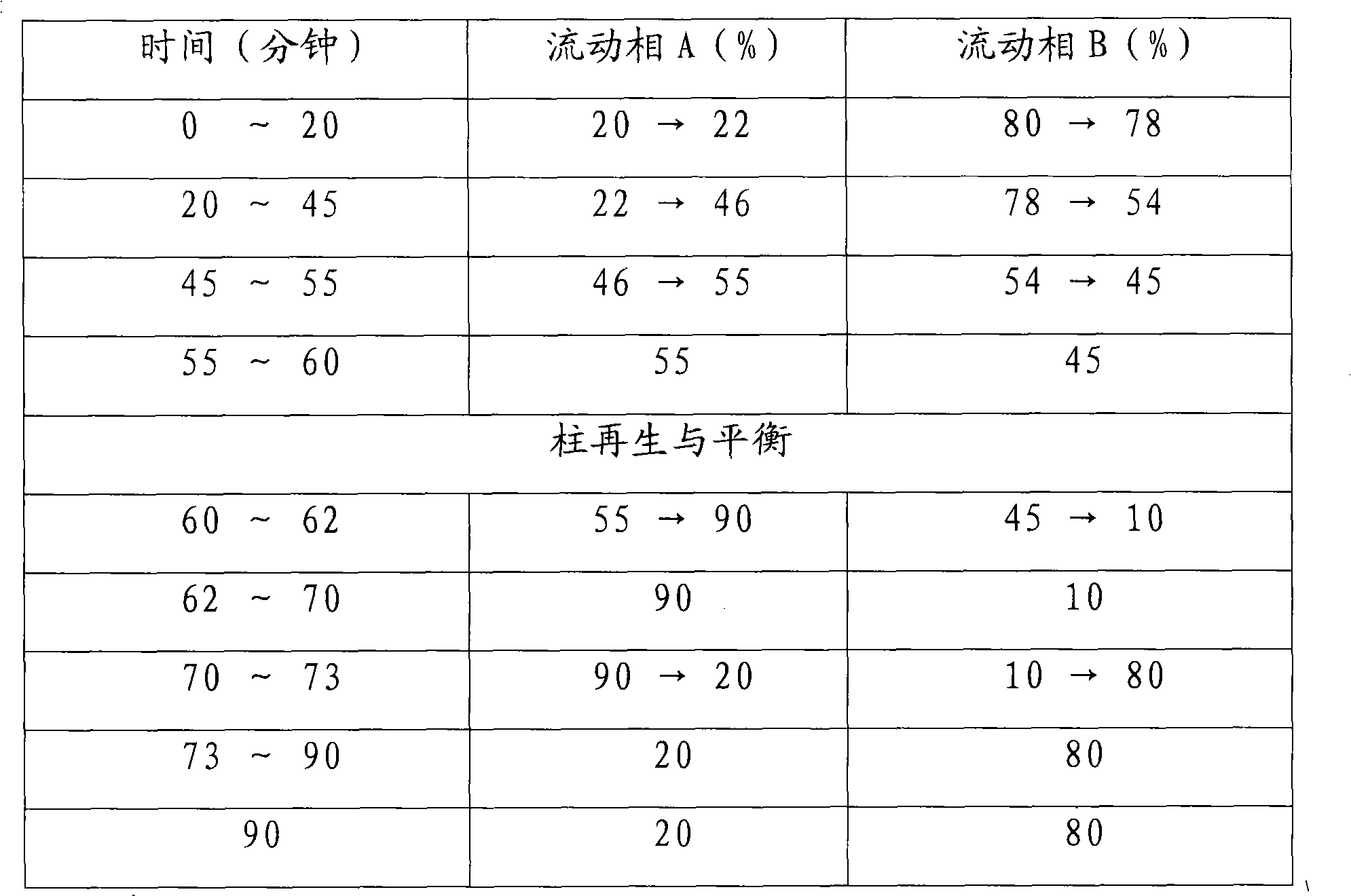
The invention provides a method for simply, conveniently and quickly preparing notoginsenoside R1 and ginsenoside Rg1, Re, Rb1 and / or Rd, which can meet the requirement of industrial production and is environment-friendly. In the method, notoginseng extract and arasaponin or notoginsenoside intermediates are used as original materials, a preparative scale reversed phase high-performance liquid chromatography is adopted, and an ethanol-water system is used as a flow phase to carry out isocratic elution or gradient elution, wherein the ethanol-water system is an ethanol-water solution of 30 percent to 80 percent (V / V). The method has the advantages of simple process, no pollution, low cost and high purity, wherein the purity of products produced by the method is more than 97 percent.
Owner:UNIVERSITY OF MACAU
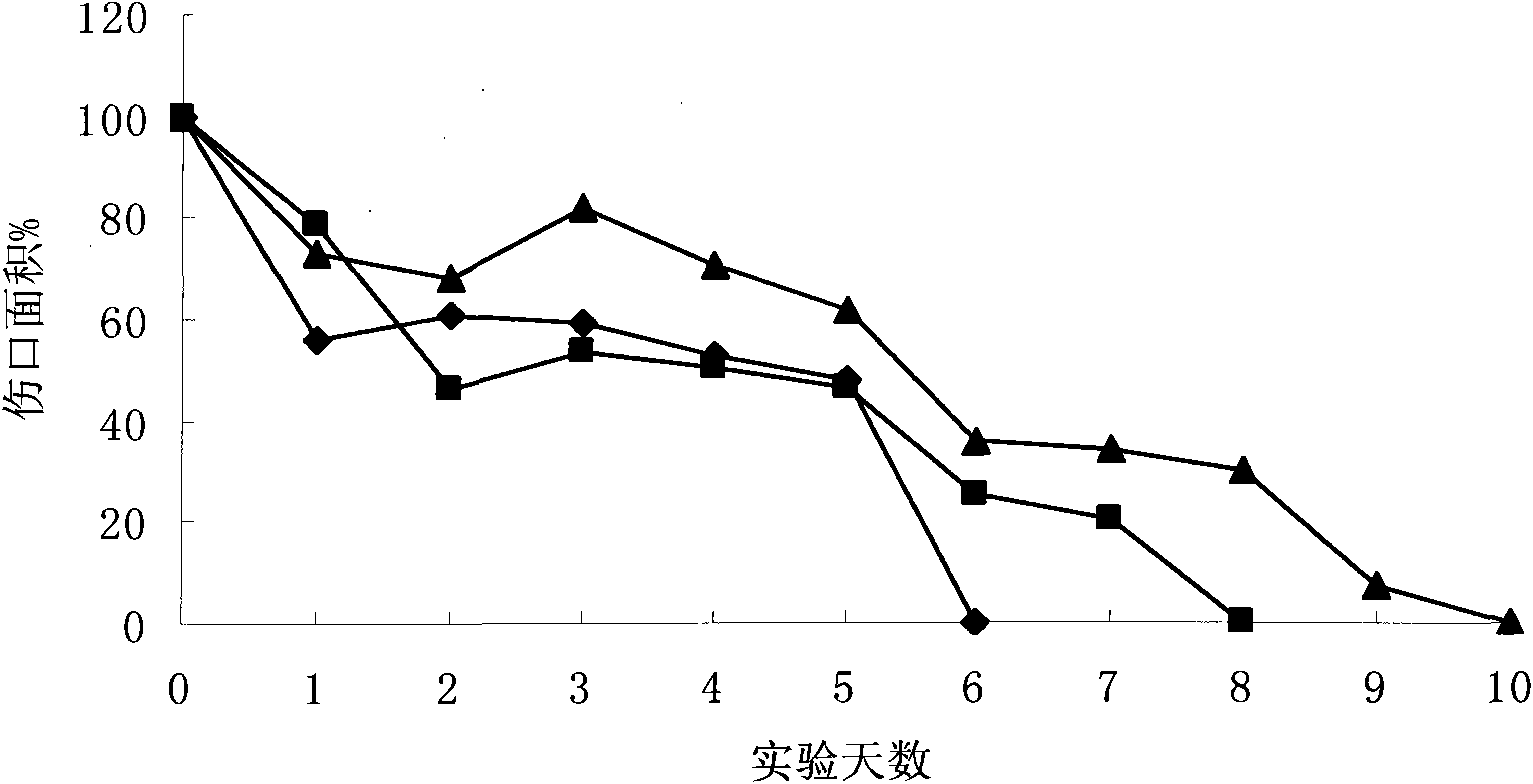
Dressing composition used for inhibiting scars and accelerating wound healing and application thereof
InactiveCN101897990APromote healingShorten healing timeAbsorbent padsBandagesCarboxymethyl-chitosanSodium hyaluronate
The invention discloses a dressing composition used for inhibiting scars and accelerating wound healing and application thereof. The dressing composition consists of chitosan, acidic polysaccharide and panax notoginseng saponins in a weight proportion of 100:10-50:10-100, wherein the chitosan is selected from chitosan, chitosan lactate, acetate and hydrochloride; the acidic polysaccharide is selected from sodium carboxymethyl chitosan, sodium hyaluronate, zinc hyaluronate, sodium alginate and sodium carboxymethyl cellulose; and the panax notoginseng saponins accounts for no less than 40.0 percent of the total weight of ginsenosides Rg1 and Rb1. The formulations of the dressing composition are xerogel powder, capsules, gel membranes, hydrogels and fiber coatings. The dressing composition is used for wounds, burns, scalds, crush injuries, skin ulcers, gastroenteric ulcers and no healing of the wounds, has the advantages of rapid hemostasis, exudate reduction, moisturizing, air permeability, bacteriostasis, inflammation diminishing, itch relieving, no adhesion, effective inhibition of scarring, remarkable reduction of wound healing time and no need of replacing dressings, so that thedressing composition basically meets requirements of perfect functional external dressings.
Owner:DALIAN UNIV OF TECH
Composition of traditional Chinese medicine effective constituent for preventing and treating diseased associated with cerebral ischemia injury
ActiveCN101357136AAvoid gatheringPrevent thrombosisOrganic active ingredientsCardiovascular disorderSequelaAdditive ingredient
The invention relates to a composite of active ingredients of Chinese herb medicine used for preventing the damage of cerebral ischemia and relevant diseases, in particular to a preparation which is prepared by the active ingredients of the Chinese herb medicine; the preparation is prepared by the active ingredients of the Chinese herb medicine and the conventional preparation technique which is used by carriers permitted by pharmacy, and comprises the following components with the parts by weight: 2 to 10 parts of ginsenoside Rb, 2 to 10 parts of ginsenoside Rg1, 1 to 5 parts of ginsenoside Rd, 1 to 5 parts of ginsenoside Re, 2 to 10 parts of stilbene glycoside, 1 to 5 parts of ginkgolide and 1 to 5 parts of flavonoid mixture which consists of kaempferol and quercetin with the weight rate to be 1:1. The invention has the advantages that the defects that in the traditional compounded Chinese medicine, the composition is complex, the product quality is not controlled effectively and the curative effect is not stable are overcome, has clear ingredients, definite effect, stable quality and obvious control effect on ischemic stroke, sequela, cerebral arteriosclerosis and vascular dementia, can improve the functions of motor nerve, and learning and memory of the patients, has low toxicity and simple preparation method without obvious side effect and is suitable for industrial mass production.
Owner:GUANGDONG PHARMA UNIV
Ginseng saponin Rg1 and Rb1 in pseudo-ginseng and preparation of total saponin thereof
The invention belongs to the medicine technical filed, in particular to a preparation method of monomeric compound ginsenoside Rg1, ginsenoside Rb1 and total arasaponin and the application in the medicine field thereof. The fresh medicinal material, the dried medicinal material and the medicinal material on the market of Panax notoginseng are taken as the raw materials; according to the polarity and the solubility property of a compound, separation and purification are carried out by adopting the solvent extraction method, the crystallization process and the chromatography and total arasaponin powder is prepared by combining the common drying means, such as decompression concentration drying, freeze drying, vacuum drying and the like; by carrying out one or more methods of recrystal, normal phase, opposite phase silica gel column chromatography, daiamid column chromatography and sephadex chromatography and the like on the powder, the ginsenoside Rg1 and the ginsenoside Rb1 monomers are prepared. The medicines which take the ginsenoside Rg1 and the ginsenoside Rb1 monomers or the total arasaponin as the active ingredients can be used for preventing and / or curing the senile dementia, the neurodegenerative diseases, the cerebrovascular disorder, various dysmnesia, the central lesion and other diseases.
Owner:YUNNAN JECUI BIOTECH
Radix notoginseng extract and preparation thereof
ActiveCN101732378AHigh purity of ingredientsIncrease concentrationCardiovascular disorderPlant ingredientsGinsenoside RcPanax notoginseng extract
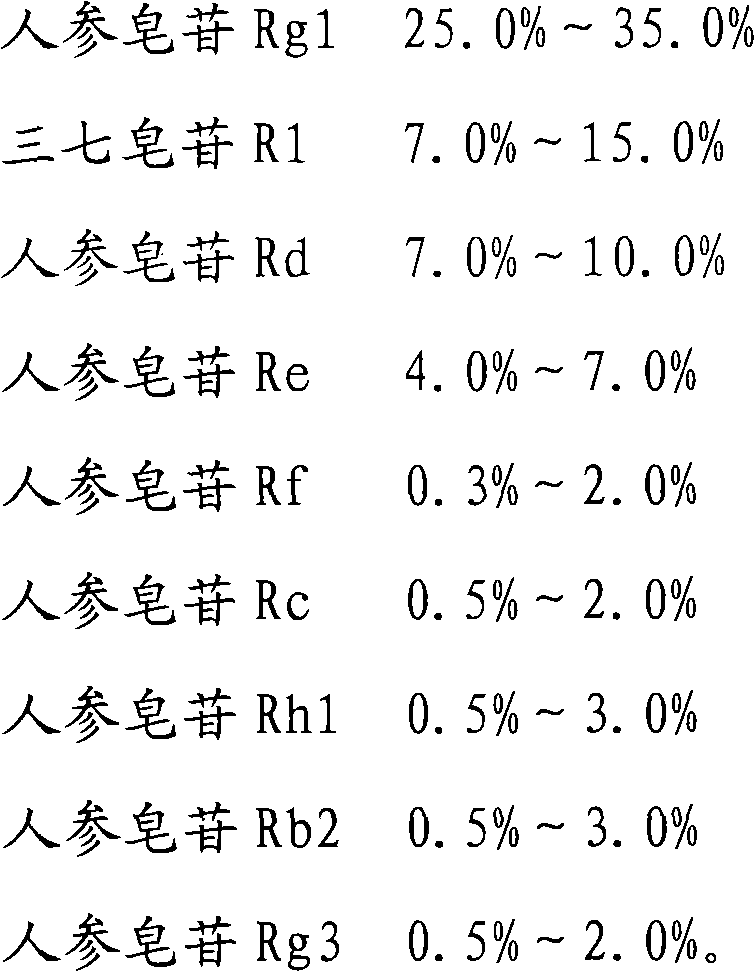
The invention provides a radix notoginseng extract which contains 5-10% of notoginsenoside R1, 25-36% of ginsenoside Rg1, 2.5-5% of ginsenoside Re, 30-39% of ginsenoside Rb1, 5-10% of ginsenoside Rd and at least 2% of ginsenoside Rf, ginsenoside Rh1, ginsenoside Rc, ginsenoside Rb2 and ginsenoside Rg3, wherein the ginsenoside R1, the ginsenoside Rg1, the ginsenoside Re, the ginsenoside Rb1 and the ginsenoside Rd account for 75-95% of the total weight. The invention also provides a preparation method of the radix notoginseng extract. The radix notoginseng extract prepared by the method has little impurities, the purity of the component of total saponin is higher, and especially, the components of the ginsenoside Rf, the ginsenoside Rh1, the ginsenoside Rc, the ginsenoside Rb2, the ginsenoside Rg3 and the like with very low content are purified. The medicinal preparation prepared by the radix notoginseng extract has better curative effect and higher safety.
Owner:HARBIN ZHENBAO PHARMA
Panax processing technique
InactiveCN101332214AQuality improvementReduce drynessPlant ingredientsSocial benefitsAdditive ingredient
A technique for processing ginseng has the steps that gardenia is taken, cleaned and selected; the part of the gardenia which is damaged by worm and rotted is removed; the processed gardenia is ground into powder with 20-100 meshes and is added with 20-30 times of water to be soaked for 1-24 hours in a sealing way, and then gardenia honey mixing suspension is obtained by adding 30% of honey . Fresh ginseng pieces with the thicknesses of 1-3mm are soaked into 0.5 time of gardenia honey mixing suspension for 6-24 hours, stewed for 1-3 hours and taken out; and then the ginseng pieces are placed into a heating oven to be dried at the temperature of 40-50 DEG C until the color of the ginseng pieces burn and ginseng pieces are not sticky, finally, the obtained ginseng pieces are spread out to be dried. The processed ginseng products which are obtained by adopting the method is added with gardenoside having anti-inflammation and heat reducing ingredients, and the content of the active ingredients, which are ginsenosides Rg1, Re and Rb1 is changed, thus achieving the purpose of reducing characteristics of warmth and dryness. The new processed products not only have the efficacy of traditional ginseng, but also reduce the characteristics of warmth and dryness of ginseng; thus the newly processed products can well play the role of health care such as fatigue resistance, anti-aging and intelligence development, and the like, and have good stability and controllable quality, so as to be creative innovation for solving the problem of drug property of ginseng and have certain economic and social benefits.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Medicinal composition and application thereof
ActiveCN102908355AOrganic active ingredientsCardiovascular disorderVascular diseaseSalvianolic acid B
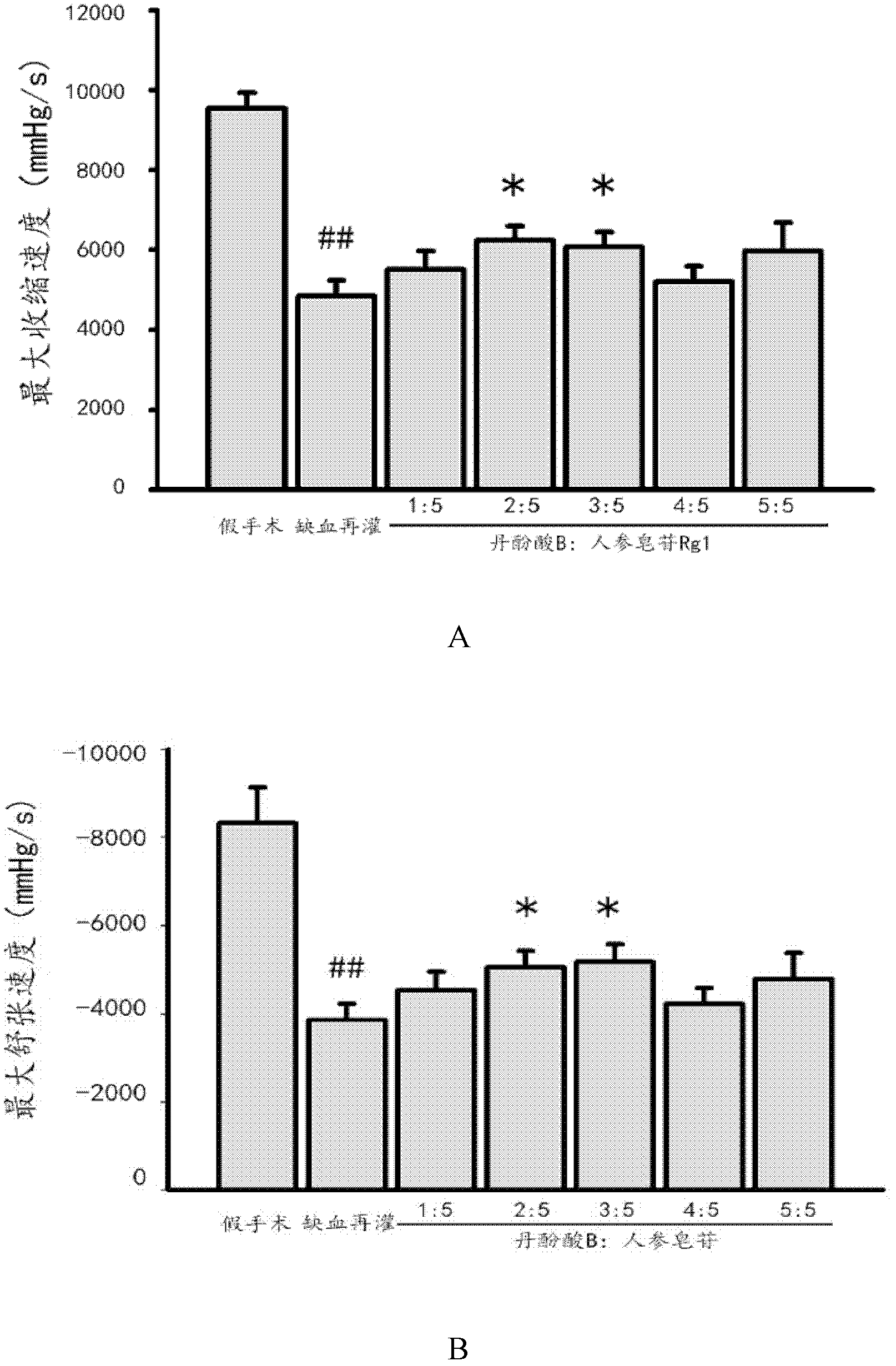
The invention discloses a medicinal composition, which is mainly prepared from compound salvianolic acid B and ginsenoside Rg1 according to a certain weight part ratio. The medicinal composition has a protection effect on cardiac muscle in myocardial ischemia and reperfusion and can be used for treating cardiovascular diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Quality detection method for compound prepn of red sage and notoginseng
InactiveCN1772041ASimple methodGood precisionComponent separationBlood disorderHplc dadSalvianolic acid B
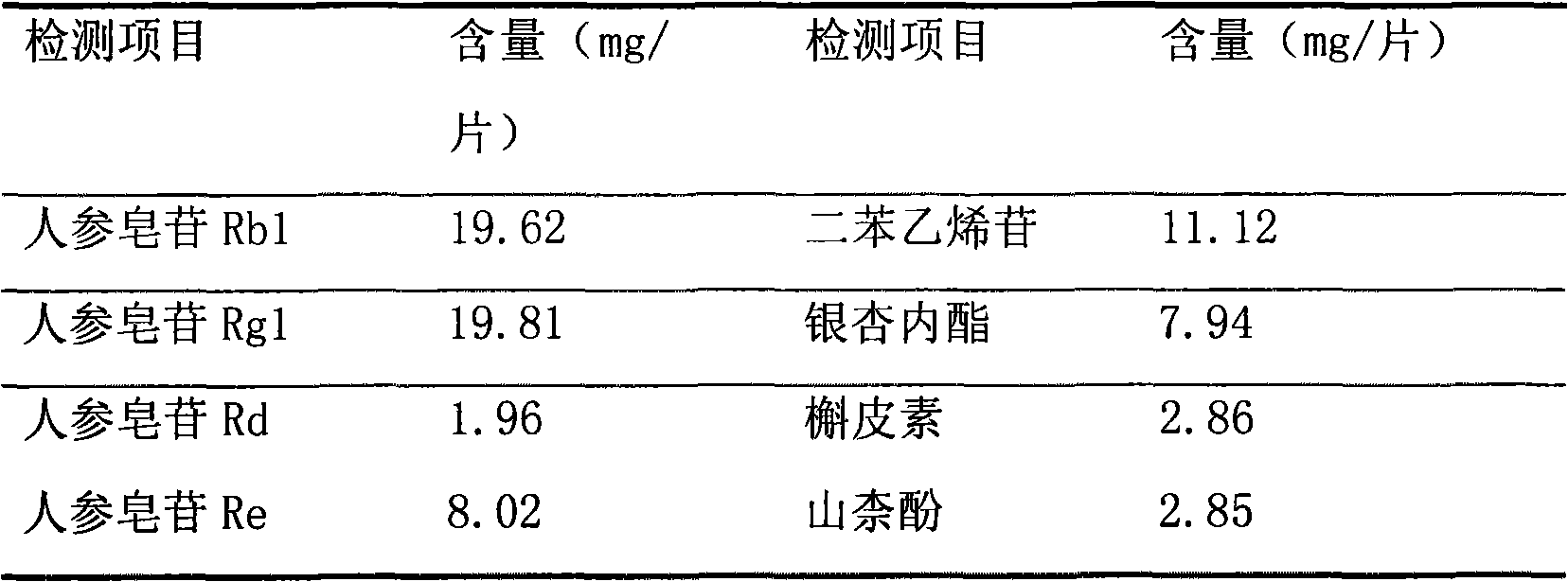
The present invention relates to Chinese medicine quality detecting technology, and is especially the quality detection method for compound preparation of red sage and notoginseng. HPLC-DAD process is adopted to measure the contents of protocatechuic aldehyde, salvianolic acid B, cryptotanshinone, neotanshinone IIA, arasaponin R1, ginsenoside Rg1 and ginsenoside Rb1 in compound red sage preparation simultaneously. The process is simple, precise, repeatable and reliable, and may be used in the quality control of compound red sage preparation.
Owner:CHINA PHARM UNIV
Notoginseng medicine composition for treating cardiac and cerebral vascular diseases
The present invention relates to a kind of total arasaponin composition, which has the active component of total arasaponin comprising arasaponin R1, ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 not less than 55.0 wt% and ginsenoside Rd not more than 2.5 wt%. The total arasaponin composition is prepared into injection, powder for injection, enteric coated tablet, bolus, medicine powder and other preparation forms. The medicine of the present invention has the functions of promoting blood circulation to disperse blood clots and activating collateral flow, and is used in treating blood stasis to block collateral channels, apoplexy, hemiplegia and other cardiac and cerebral vascular diseases.
Owner:GUANGXI WUZHOU PHARMA GRP
Medicinal composition for preventing and treating cardiovascular diseases and application thereof
InactiveCN102526186ADrop in trustQuality is easy to controlOrganic active ingredientsCardiovascular disorderVascular diseaseCoronary heart disease
The invention discloses a medicinal composition for preventing and treating cardiovascular diseases, belonging to the technical field of Chinese medicines. The medicinal composition is prepared from salvianolic acids, total tanshinone and a notoginsenoside mixture in a certain weight ratio, wherein the notoginsenoside mixture is prepared by mixing ginsenoside Rb1, ginsenoside Rg1 and notoginsenoside R1; and the notoginsenoside mixture can be replaced by an equivalent amount of arasaponin. The medicinal composition has remarkable treatment effects on a coronary heart disease, angina and myocardial infarction.
Owner:GUANGANMEN HOSPITAL CHINA ACAD OF CHINESE MEDICAL SCI
Composition for treating cardiovascular disease and preparation thereof
The invention discloses a composition for treating cardiovascular diseases and a preparation method thereof. The composition comprises (by weight part) Radix Ginseng Rubra saponin 2-20, Ophiopogon japonicus saponin 0.2-3, and Ophiopogon japonicus flavone 0.02-3; wherein the Radix Ginseng Rubra saponin extract contains (by dry weight) ginsenoside Rg1 not less than 9.0%, ginsenoside Re not less than 3.0%, ginsenoside Rb1 not less than 12.0%, and Panax ginseng total saponins not less than 80%; the Ophiopogon japonicus saponin extract contains (by dry weight) ophiopogonin D not less than 6.0%, ophiopogonin D' not less than 6.0%, and Ophiopogon japonicus total saponins not less than 80%; and the Ophiopogon japonicus flavone extract contains (by dry weight) methylophiopogonone A and methylophiopogonone B in total not less than 80%. Animal experiments showed that the composition has good therapeutic action on myocardial ischemia and has better anti-shock effect.
Owner:巩洪刚 +1
Rg2 group and Rh1 group of red ginseng saponin and preparation method as well as applications in preparing cosmetics preventing skin aging
ActiveCN102302420ASynthesis function improvedSimple processing methodCosmetic preparationsToilet preparationsBiotechnologyCollagenan
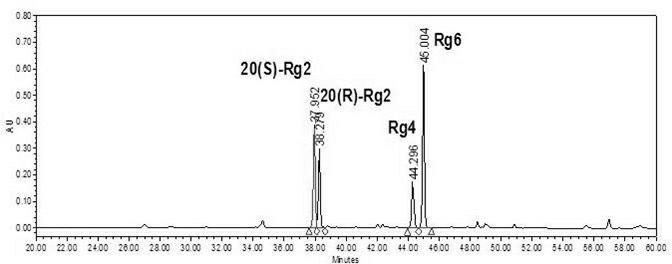
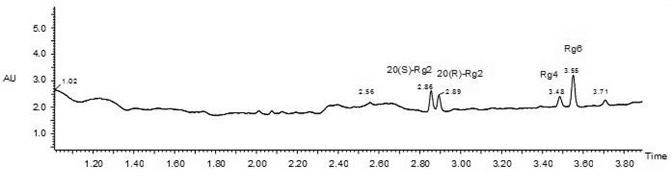
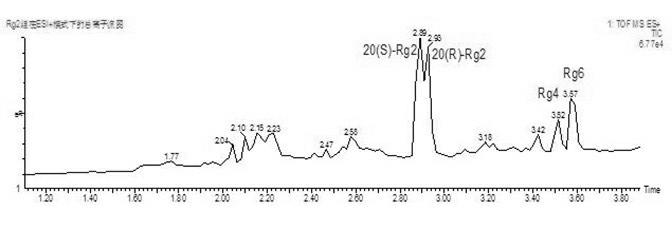
The invention discloses an Rg2 group and an Rh1 group of red ginseng saponin and a preparation method as well as applications in preparing cosmetics preventing skin aging. Extracts which are extracted from ginseng roots, do not contain saponin, and contain saponin enzyme of ginseng and other water-soluble matters react with ginsenoside Re to obtain red ginseng mixed saponin Rg2 group consisting of red ginseng rare saponin 20(S)-Rg2, 20(R)-Rg2, Rg4 and Rg6, and the extracts react with ginsenoside Rg1 to obtain the red ginseng mixed saponin Rh1 group consisting of red ginseng rare saponin 20(S)-Rh1, 20(R)-Rh1, Rh4 and Rk3. Compared with a saponin Rg2 monomer and a saponin Rh1 monomer, the function of the Rg2 group and Rh1 group of red ginseng mixed saponin for promoting skin fibroblast collagen synthesis is improved obviously, the Rg2 group and Rh1 group of red ginseng mixed saponin are more safe to skin and human bodies, and have less side effects.
Owner:SUNFLOWER PHARM GRP (TIANJIN) DRUG RES INST CO LTD
Medicinal preparation for treating nerve-root cervical spondylopathy, and preparation method and quality detection method thereof
InactiveCN104887771AMeet needsVarious dosage formsNervous disorderComponent separationClinical efficacyCervical spondylopathy
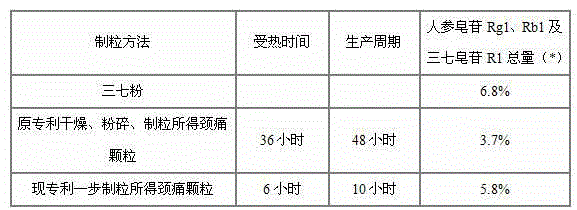
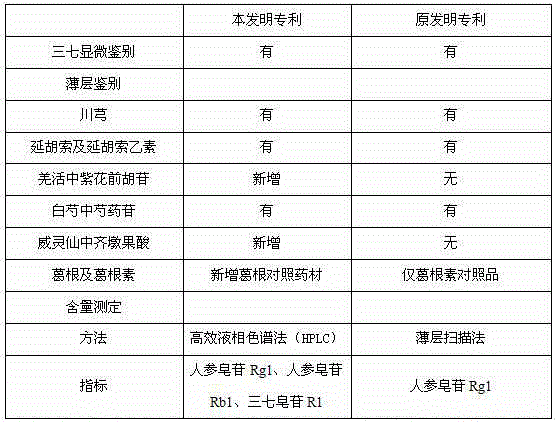
The invention relates to a medicinal preparation for treating nerve-root cervical spondylopathy, and a preparation method and a quality detection method thereof. The invention is an extension of an original invention. The medicinal preparation comprises a granule, a tablet and a capsule, the preparation method is an optimized and screened production technology based on the original invention, a modern new device, a new process and a new technique are adopted to realize industrial production; and quality standard researches are completed and improved on the basis of original standards, HPLC is adopted to simultaneously determine the content of ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 in a finished product, and thin layer discrimination of all medicines is carried out to comprehensively control the quality, so the clinic curative effects are guaranteed.
Owner:SHANDONG MINGREN FURUIDA PHARMA
Medicine composition of Panax notoginseng saponins
InactiveCN101390887AImprove protectionInhibit aggregationOrganic active ingredientsMetabolism disorderPANAX NOTOGINSENG ROOTTreatment effect
The invention relates to panax notoginseng saponins medicine combination and the application thereof, which are characterized in that the active ingredient is composed of panax notoginseng saponins which contains notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Re, ginsenoside Rd, ginsenoside Rb3 and ginsenoside Rb2; the combination with different contents and ingredients of saponins are prepared; the combinations have different treatment effects.
Owner:HEILONGJIANG ZBD PHARMA
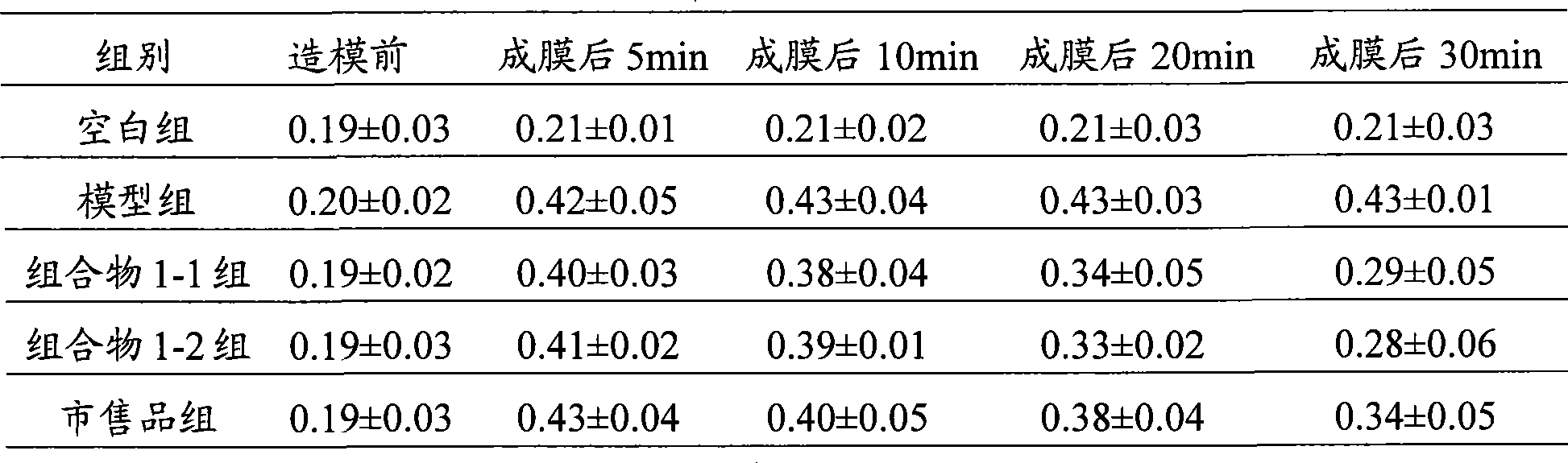
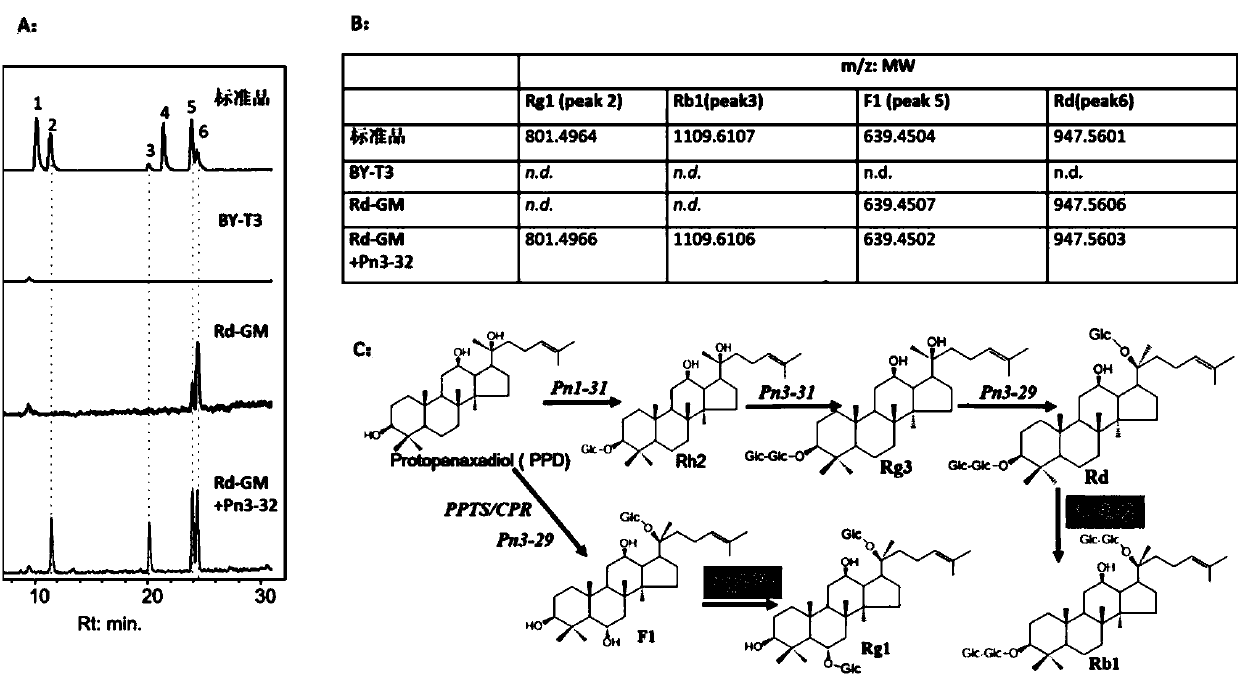
Applications of glycosyltransferase and related materials thereof in construction of engineering bacteria for producing ginsenoside Rb1 and Rg1
The invention discloses applications of glycosyltransferase and related materials thereof in the construction of engineering bacteria for producing ginsenoside Rb1 and Rg1. A glycosyltransferase genePn3-32 which can catalyze ginsenoside Rd to generate ginsenoside Rb1 can be successfully identified through a synthetic biological method; and the gene can simultaneously catalyze ginsenoside F1 to generate ginsenoside Rg1 and construct recombinant yeast producing the ginsenoside Rb1 and the ginsenoside Rg1. Through experiments, the constructed recombinant yeast producing the ginsenoside Rb1 and the ginsenoside Rg1 can simultaneously generate the ginsenoside Rb1 and the ginsenoside Rg1. Pn1-31, Pn-3-29, Pn3-31 and Pn3-32 glycosyltransferase genes in medicinal plant radix notoginseng are firstly utilized to continuously catalyze protopanaxadiol and protopanaxatriol to synthetize the ginsenoside Rb1, the ginsenoside Rg1 and corresponding intermediates, so that novel cases can be provided formicrobial strains to produce natural products.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Medicinal composition and preparation method thereof
ActiveCN102028700AIncrease the maximum tolerated doseRaise the median lethal doseOrganic active ingredientsPeptide/protein ingredientsHemolysisAcute toxicity testing
The invention provides a medicinal composition, which comprises the following components: ginsenoside Rb1, ginsenoside Rg1, notoginsenoside R1, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rc, ginsenoside Rh1, ginsenoside Rb2 and ginsenoside Rg3. The invention also provides a preparation method for the medicinal composition. The medicinal composition has definite components; compared with the total notoginsenoside in the prior art, the medicinal composition has stable quality and good controllability; and results of experiments of acute toxicity, undue toxicity, hemolysis and the like show that the medicinal composition has higher safety and wide clinical application prospect.
Owner:KPC PHARM INC
Cooked pseudo-ginseng and preparation method thereof
ActiveCN106138141AEnhance immune functionImprove anti-tumor effectOrganic active ingredientsImmunological disordersLower gradeLow graded
The invention discloses cooked pseudo-ginseng. By weight, the total saponin content is 6.0-12.0%, and the sum of the content of the low-grade saponin in the transformation accounts for 40-90% of the total saponins of the cooked pseudo-ginseng. Inherent saponins in pseudo-ginseng contain ginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1, the low-grade saponins generated by transformation contains ginsenoside 20 (S)-Rh1, ginsenoside 20 (R)-Rh1, ginsenoside Rd, ginsenoside Rk3, ginsenoside Rh4, ginsenoside 20 (S)-Rg3, ginsenoside 20(R)-Rg3, ginsenoside Rk1, and ginsenoside Rg5. The invention also discloses a preparation method of the cooked pseudo-ginseng. By precisely controlling the water addition, processing temperature and processing time, the cooked pseudo-ginseng having a certain saponin and the proportion thereof is obtained. The cooked pseudo-ginseng is rich in low-grade saponins that rarely exists in the dried pseudo-ginseng and can improve the immunity and the anti-tumor effect.
Owner:四川仟源中药饮片有限公司 +1
Detection method of compound danshen dripping pills
ActiveCN102119961AQuality improvementImprove product qualityHydroxy compound active ingredientsComponent separationSalvia miltiorrhizaChromatographic fingerprint
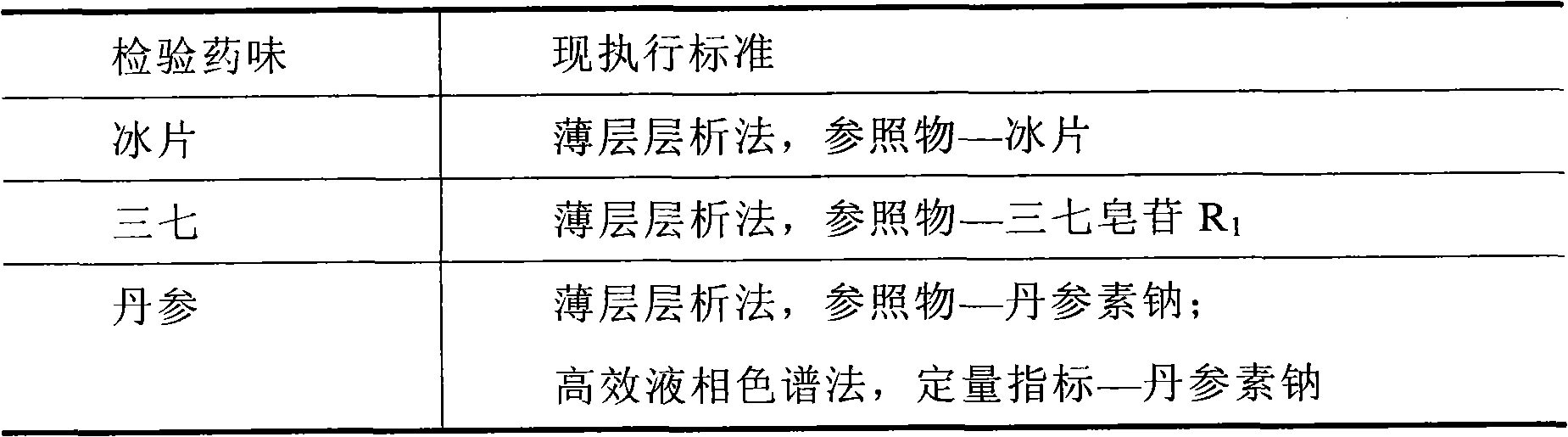
The invention relates to a detection method of compound danshen dripping pills, comprising the following contents of: observation of characters, discrimination of contents, inspection of contents, comparison of finger prints and assaying of contained components. The detection method provided by the invention comprises a discriminating method of the medicinal component panax notoginseng, the discriminating method adopts thin-layer chromatography, the reference materials adopt a panax notoginseng reference medicinal material, a ginsenoside Rg1 reference substance, a ginsenoside Re reference substance, a ginsenoside Rb1 reference substance and a panax notoginseng saponin R1 reference substance. The detection method provided by the invention also comprises a discriminating method and an assaying method of the medicinal component danshen, wherein the discriminating method adopts a sub-2 mu m liquid chromatography technology to perform chromatographic fingerprint discrimination, and adopts sodium danshensu as a reference material; and the assaying method adopts a sub-2 mu m liquid chromatography technology to perform assaying on the components such as danshensu, panax notoginseng saponin R1, ginsenoside Rg1 and ginsenoside Rb1 in the danshen.
Owner:TIANJIN TASLY PHARMA CO LTD
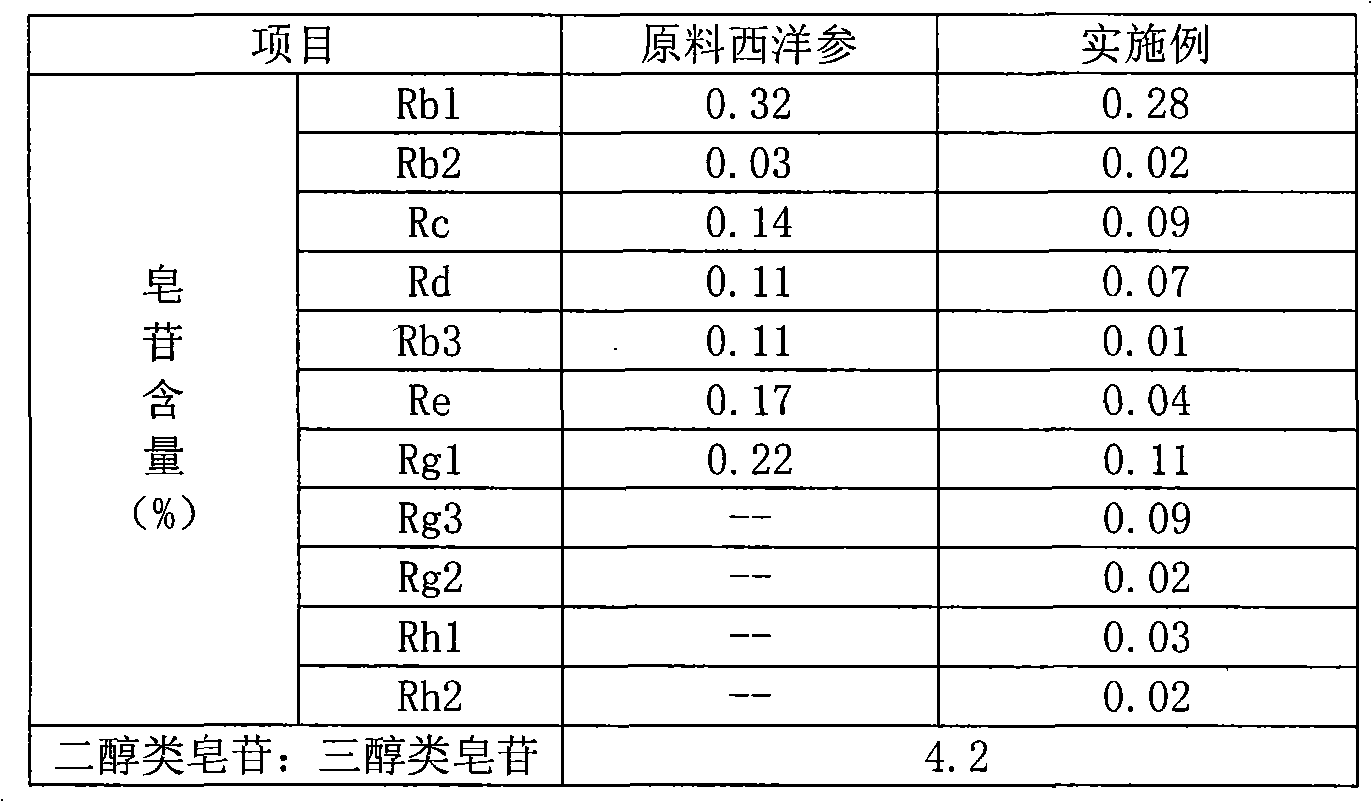
Method for extracting and separating total saponins of panax ginseng from american ginseng
ActiveCN102772462AHigh purityHigh yieldNervous disorderAntinoxious agentsAMERICAN GINSENG ROOTGinsenoside Rc
The invention discloses a method for extracting and separating total saponins of panax ginseng from american ginseng. The method employs a series of high-efficiency extraction, separation and purification technical measures of alcohol-backflow extracting, water precipitating, purifying by a macroporous adsorption resin, decolorizing with an ion exchange resin, etc. The obtained total saponins of panax ginseng are white powder and comprise ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, ginsenoside Rc and ginsenoside Rd, wherein the total purity of ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 is more than 74.0%. The production process is simple and practical, has no pollution, and is suitable for large-scale production.
Owner:HEBEI YILING MEDICINE INST
Production method of flavor panax ginseng
The invention relates to a preparation method of flavor ginseng; after ginseng is added with nature fruit juice or organic acid for reaction, the content of the precious ginsenoside in products is enhanced, the efficacies of ginseng is improved, while the specific flavor of ginseng is maintained. The main quality characteristics is that: the flavor ginseng includes at least one of the following ingredients: ginsenoside Rg3, ginsenoside Rh1 and ginsenoside 20R-Rh2; wherein, the ratio of the total amount of precious ginsenoside group, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rd and ginsenoside Rc to the total amount of ginsenoside Re, ginsenoside Rg1 and ginsenoside Rf is more than 2.5; the preparation method can convert part of the component causing internal heat, namely, panaxatriol ginsenoside, into precious ginsenoside.
Owner:JILIN HONGJIU BIO TECH
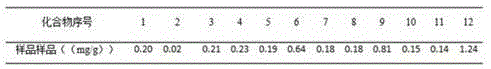
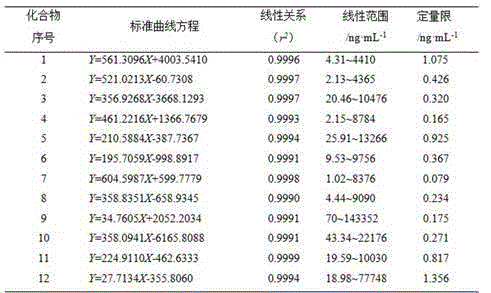
Determining method for contents of twelve components in traditional Chinese medicine composition preparation
ActiveCN104914199AQuality improvementGood repeatabilityComponent separationBiotechnologyAstragaloside
The present invention discloses a UPLC-MS quantitative method for the contents of twelve components in a traditional Chinese medicine composition preparation, specifically determination of the contents of calycosin-7-O-beta-D-glucoside (1), isoquercitrin (2), narirutin (3), hesperidin (4), ginsenoside Re (5), ginsenoside Rg1(6), periplocoside (7), ginsenoside Rf (8), ginsenoside Rb1 (9), astragaloside (10), ginsenoside Rd (11) and periplocin H1 (12) in the traditional Chinese medicine composition, and belongs to the field of traditional Chinese medicine composition preparation component detection. The determining method of the present invention has characteristics of short period, good reproducibility and high sensitivity.
Owner:HEBEI YILING MEDICINE INST
Extract of panax notoginseng saponins and preparation method thereof
InactiveCN101829170AIncrease cerebral blood flowHigh trafficBlood disorderCardiovascular disorderPANAX NOTOGINSENG ROOTAdjuvant
The invention relates to an extract of panax notoginseng saponins, a preparation method thereof, a preparation prepared from the total saponins and a method for preparing the preparation. The extract comprises panaxtriol saponins (PTS) and panaxadiol saponins (PDS), wherein the PTS mainly comprises notoginsenoside R1 and ginsenoside Rg1; the PDS mainly comprises the ginsenoside Rb1 and the ginsenoside Rd; the extract is characterized in that the weight ratio of the PTS to the PDS is 1:0.5-2; and the total content of the notoginsenoside R1, the ginsenoside Rg1, the ginsenoside Rb1 and the ginsenoside Rd accounts for over 80 weight percent of the extract of the panax notoginseng saponins. The preparation of the extract of the panax notoginseng saponins comprises the therapeutically effective amount of the panax notoginseng saponins and pharmaceutically acceptable adjuvant.
Owner:北京中海康医药科技发展有限公司
Quality detection method for weinai'an tablet
ActiveCN104165962AHigh precisionQuantitatively accurateComponent separationBULK ACTIVE INGREDIENTContent determination
The invention discloses a quality detection method for a weinai'an tablet. The quality detection method adopts one or more of the following identification and content determination items: thin-layer identification of radix astragali; thin-layer identification of radix astragali, red ginseng and pseudo-ginseng; thin-layer identification of atificial cow-bezoar; high performance liquid chromatography qualitative and quantitative identification method of weinai'an tablet; and high performance liquid chromatography quantitative identification method of Astragaloside IV. The method provided by the invention improves and revises the original weinai'an capsule quality standards, revises and enlarges thin-layer identification of radix astragali's four flavonoid components and Astragaloside IV, simultaneously revises and enlarges thin-layer identification of red ginseng, and identifies the index components Astragaloside IV, ginsenoside Rg1, Rb1 and notoginsenoside R1 at the same time. The method employs an HPLC-PDA-ELSD combined technology to establish a main active ingredient content determination and characteristic spectrum to realize effective control of the weinai'an tablet quality. At the same time, the method has the advantages of high efficiency, accurate quantification, good stability, high precision and excellent repeatability.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill detection method
InactiveCN104483315AStrong process controllabilityImprove quality controlComponent separationMaterial analysis by optical meansControllabilityHigh-performance liquid chromatography
The invention discloses a traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill detection method, according to the method, rehmannia glutinosa, yam, tuckahoe, schisandra, chrysanthemum and astragalus in a lumbus-strengthening kidney-tonifying pill can be microscopically identified, a radix angelicae sinensis referenee crude herb, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, dipsacus asper saponin VI, astragaloside and deoxyschizandrin are used as reference substances, whether the lumbus-strengthening kidney-tonifying pill contains the radix angelicae sinensis ,ginseng, dipsacus asper, astragalus and schisandra can be identified by thin-layer chromatography; the dipsacus asper saponin VI content in the lumbus-strengthening kidney-tonifying pill preparation can be detected by HPLC, according to the detection method, the quantitative index is that the dipsacus asper saponin VI (C47H76O18) content in every 1g of the traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill may not be less than 0.60mg, the controllability of quality standards of the traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill can be improved to further ensure the product intrinsic quality and effect, so that the quality standard is more perfect, and the drug quality control level is improved.
Owner:JINGFUKANG PHARMA GRP CHIFENG DANLONG PHARMA CO LTD
Novel use of ginsenoside Rg1
The invention relates to a novel use of ginsenoside Rg1, especially the use in preventing or treating male sexual dysfunction such as ejection dysfunction.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Quality detection method of ginseng basis-consolidating oral solution
PendingCN106370749AEffective quality controlGuaranteed clinical efficacyComponent separationMedicineContent determination
The invention discloses a quality detection method of a ginseng basis-consolidating oral solution with the functions of nourishing yin and qi and consolidating basis. According to the quality detection method, the preparations of ginseng, dogwood, cortex moutan and rhizoma alismatis are distinguished with thin layer chromatography, and meanwhile the contents of ginsenosides Rg1 and Re of the ginseng, the content of paeonol of the cortex moutan, the contents of morroniside and loganin of the dogwood in the preparations are measured by adopting HPLC (high performance liquid chromatography). The quality detection method disclosed by the invention is stable, reliable, high in specificity and good in repeatability; content determination is carried out by selecting monarch drugs such as ginseng and cortex moutan containing volatile constituents in the prescription, so that the quality of the ginseng basis-consolidating oral solution can be comprehensively and effectively controlled, the stabilization for product quality is facilitated, the safety and effectiveness of clinical medication are guaranteed, and requirements of medical treatment and market are preferably met.
Owner:杨本官 +1
Preparation process for ginsenoside Rg1
The invention relates to a preparation process for panaxoside monomer comprising the steps of, (1) dissolving total notoginseng saponin with alcohol, (2) passing through pretreated big hole resin columns, washing panaxatriol saponin with alcohol, rinsing the resin columns, (3) reclaiming alcohol through decompression, spray drying, obtaining panaxadiol saponin, (4) adsorbing on chromatographic silica gel, drying, (5) proceeding low pressure chromatography, (6) reclaiming solvent, vacuum drying to obtain the panaxoside Rg1 crude product, (7) proceeding step 4, 5 and 6 to obtain refined Rg1.
Owner:云南特安呐制药股份有限公司
Pharmaceutical composition and application thereof
InactiveCN102125572AFunction increaseEasy to synthesizeOrganic active ingredientsNervous disorderDiseaseMedicine
The invention relates to a pharmaceutical composition and a preparation method thereof. The pharmaceutical composition contains ginsenoside Rg1, ginsenoside Rb1 and ginsenoside Re, wherein the weight ratio of ginsenoside Rg1: ginsenoside Rb1: ginsenoside Re is 1:0.7:1.2. The composition provided by the invention has notable effects and high safety, can alleviate apoplexy symptoms, obviously shortens the course of disease, improves depression of patients with apoplexy sequelae, and the like.
Owner:HEILONGJIANG ZBD PHARMA
Pharmaceutical composition for invigorating qi and strengthening spleen
ActiveCN103191358AEasy extractionImprove the extraction effectDigestive systemPlant ingredientsTreatment effectSallow complexion
The invention provides a pharmaceutical composition for invigorating qi and strengthening spleen. The pharmaceutical composition is a ginseng spleen-strengthening tablet which is prepared from the following materials such as 20-30g of ginseng, 45-55g of white atractylodes rhizome, 20-30g of liquorice, 70-80g of Chinese yam, 45-55g of lotus seed, 45-55g of white hyacinth bean, 15-25g of elecampane, 20-30g of alpinia katsumadai, 45-55g of pericarpium citri reticulatae, 45-55g of pericarpium citri reticulatae viride, 45-55g of maticated leaven, 45-55g of rice sprout, 45-55g of hawthorn, 45-55g of gorgon fruit, 95-105g of semen coicis, 45-55g of angelica sinensis, and 20-30g of fructus aurantii. The ginseng spleen-strengthening tablet is high in content of effective ingredients; especially, the content of saponin ingredients such as ginsenosides Rg1 and Re is higher than that in the prior art; and the pharmaceutical composition is better in treatment effect on the symptoms such as eating less and loose stool, vomiting or diarrhea, abdominal fullness and distention, limb weakness and sallow complexion caused by dampness stagnancy due to spleen deficiency.
Owner:ZHEJIANG WECOME MEDICINE IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com