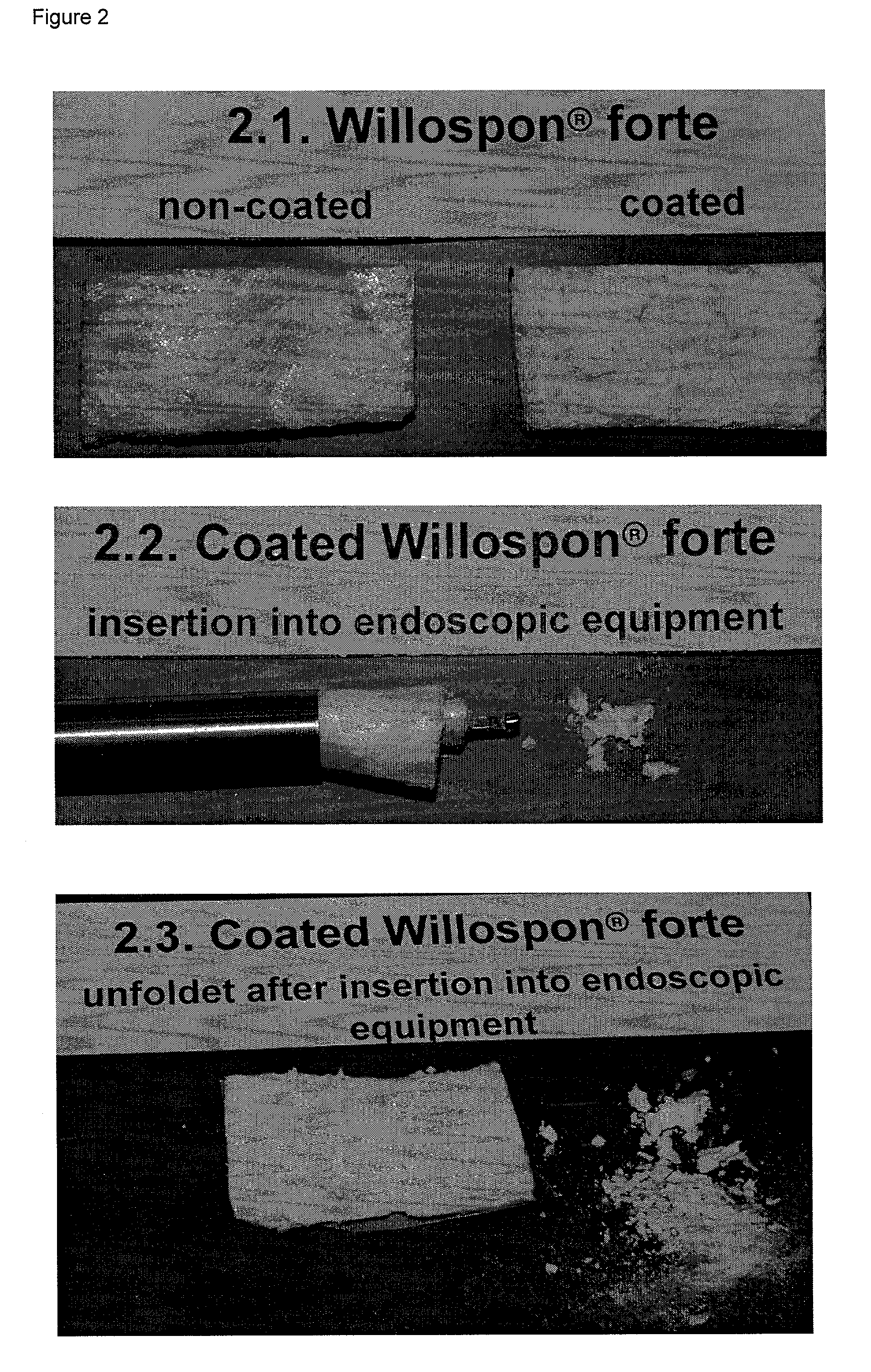
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
85 results about "Collagen ADP" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
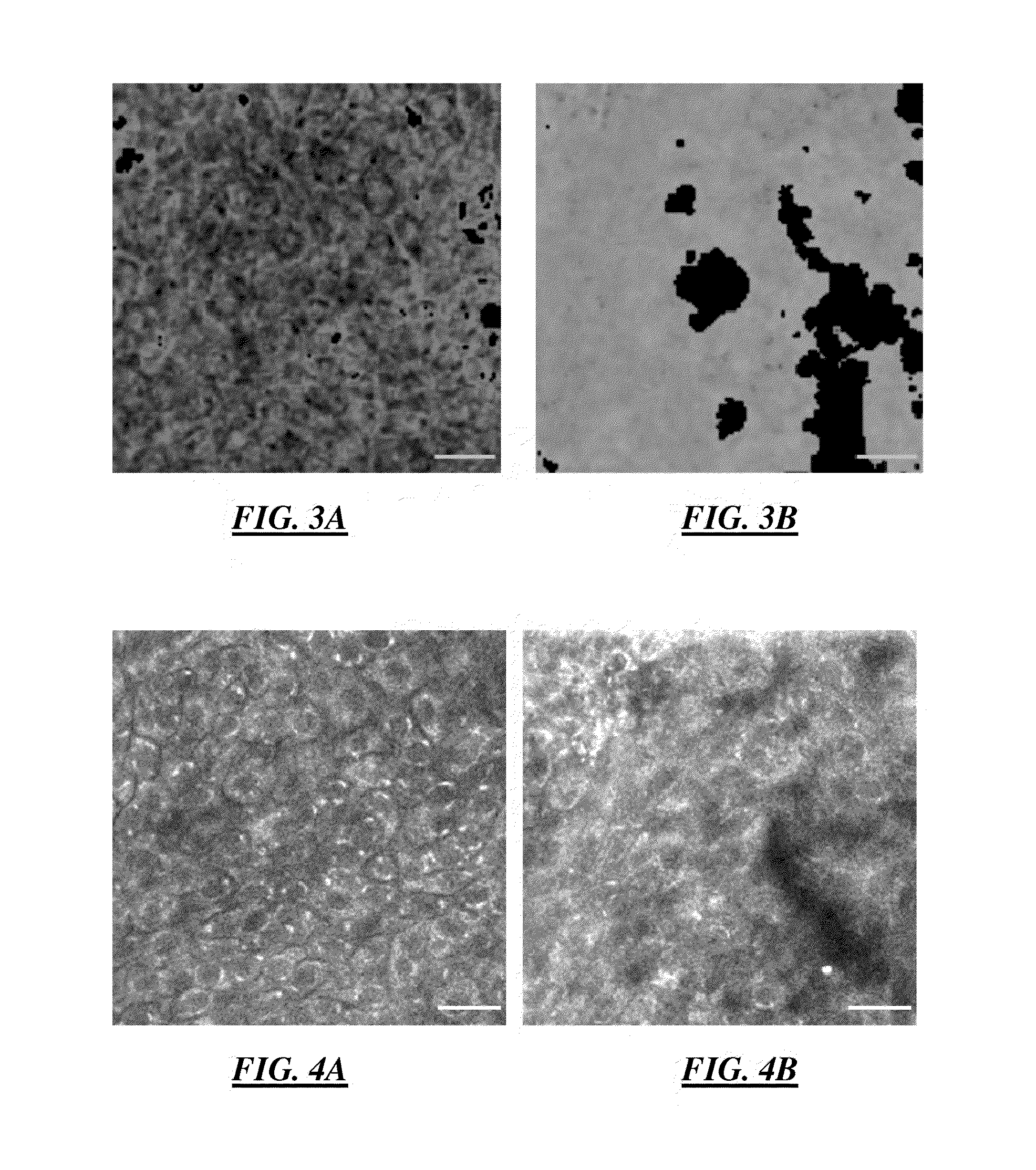
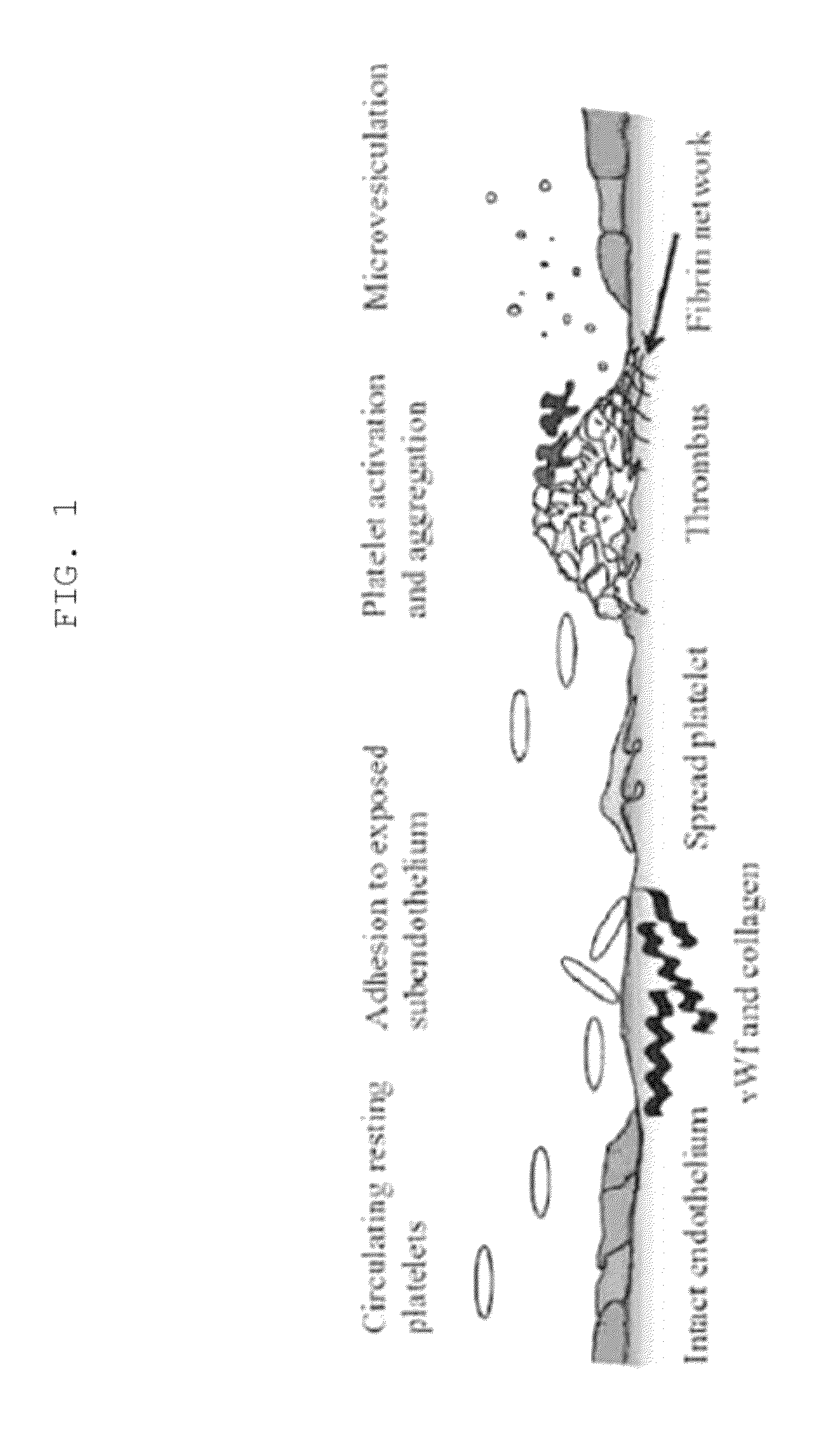
The cartridge membrane is coated with collagen, and with one of two platelet agonists (epinephrine or ADP). The platelets adhere to the collagen and aggregate in response to the collagen and epinephrine (or ADP). The response measured is known as the Closure Time (CT) and is reported in seconds.
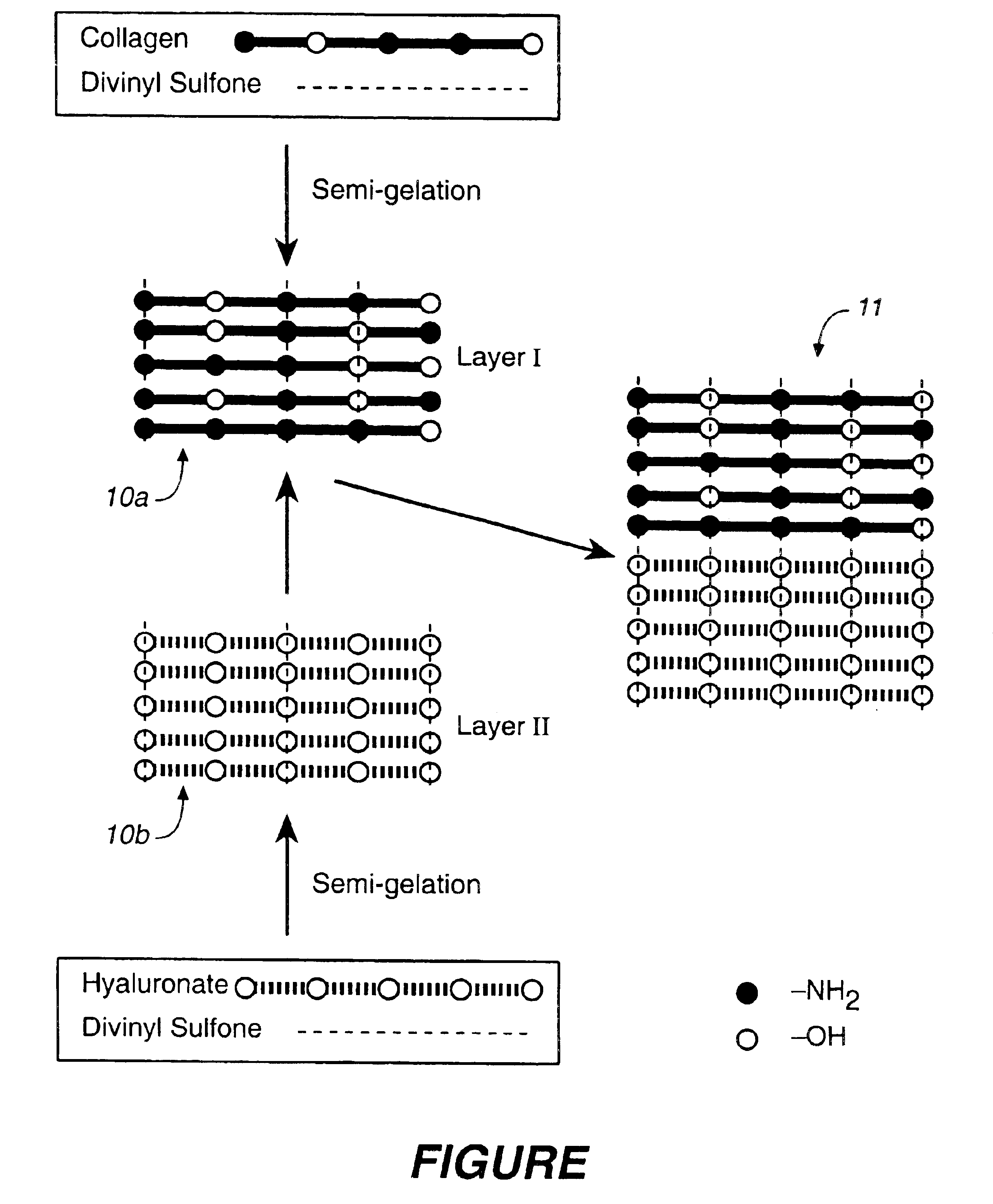
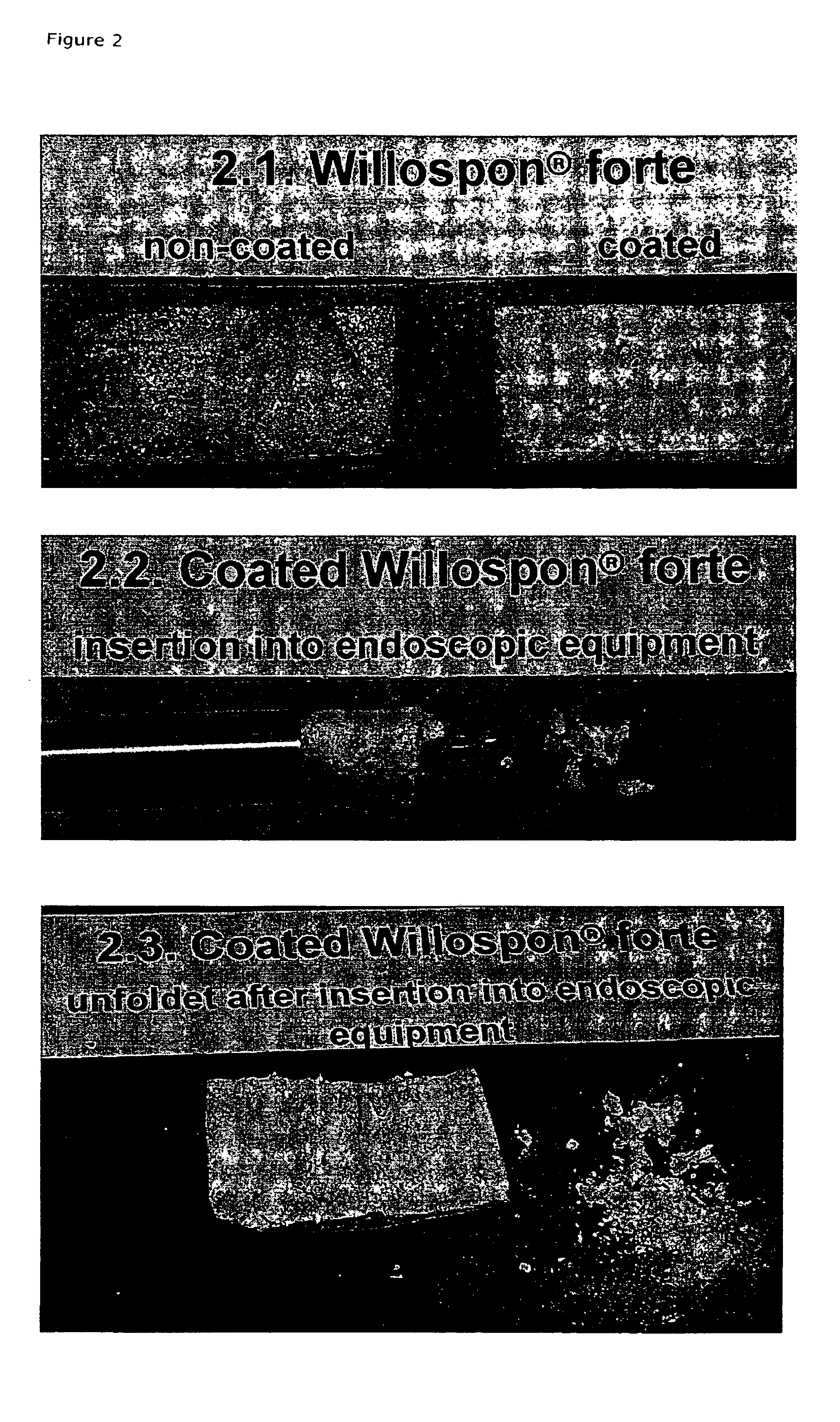
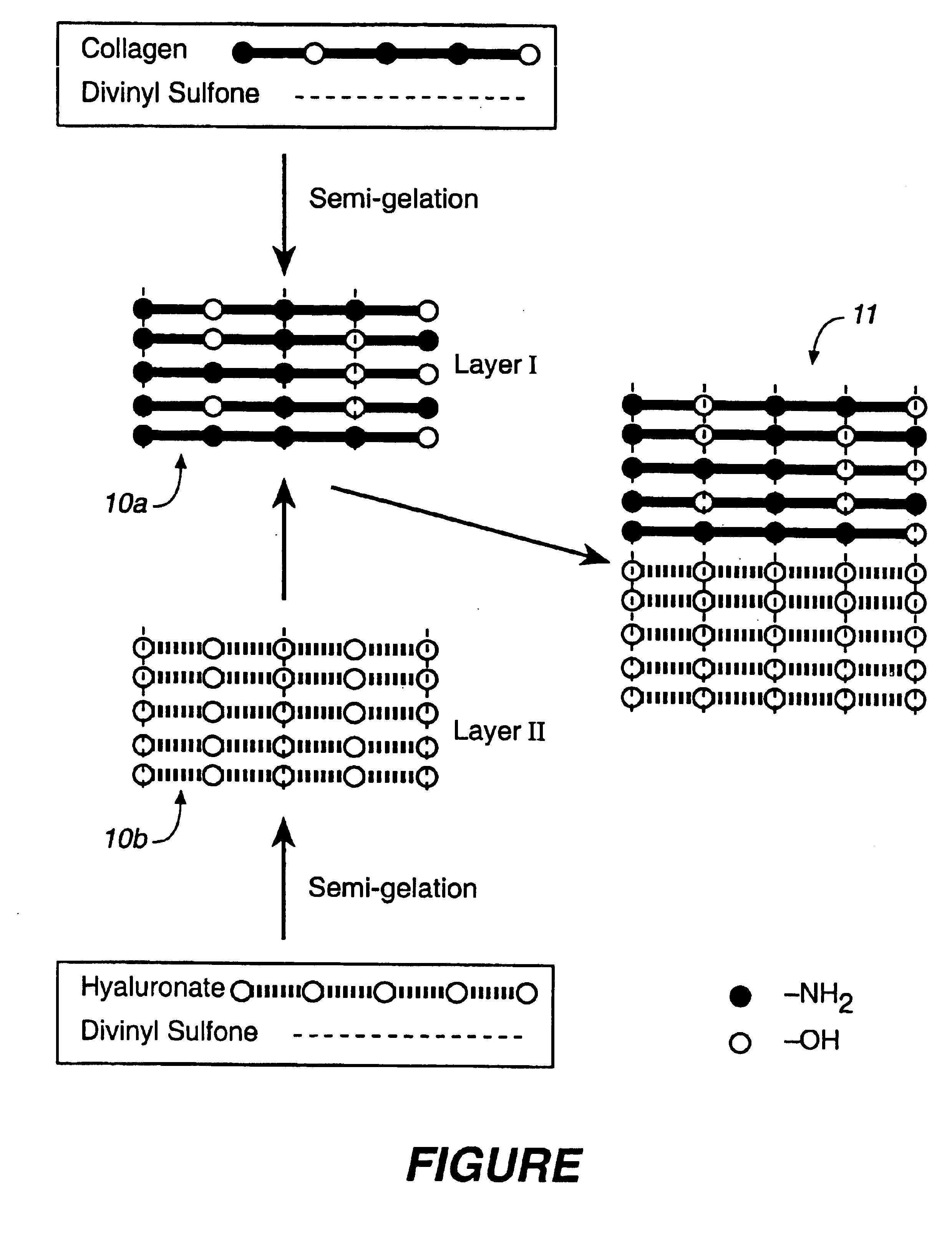
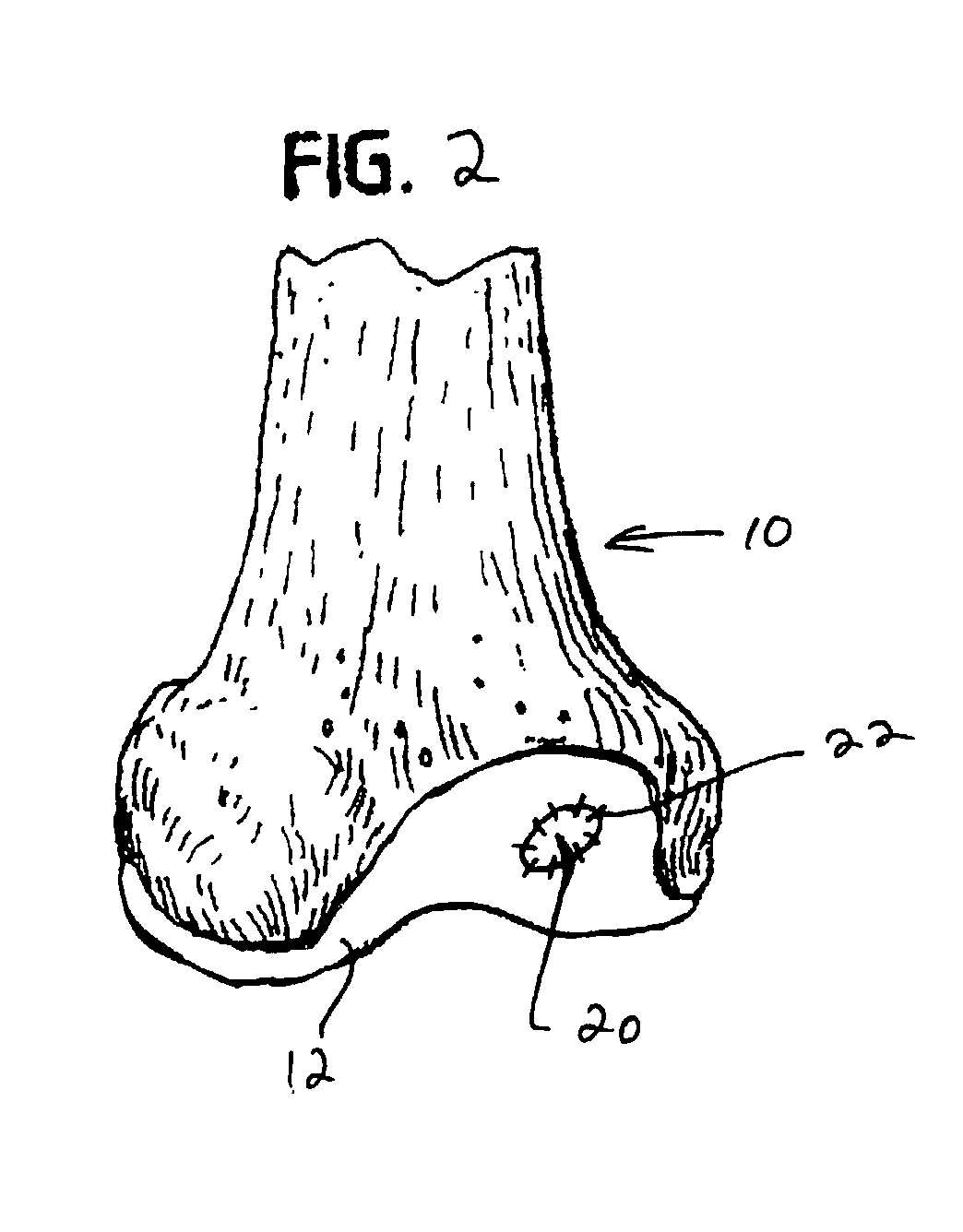
Collagen/polysaccharide bilayer matrix
Disclosed are bilayer matrices of a polysaccharide such as collagen (COL) and another polysaccharide such as hyaluronic acid (HA) with various COL / HA ratios. Each layer has a porous structure. These materials are useful for tissue regeneration, particularly when used with orthopedic implants and drug delivery.
Owner:DEPUY SPINE INC (US)
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm<3>, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsilon-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Intra-serum and intra-gel for modeling human skin tissue
InactiveUS6475800B1Diagnostics using spectroscopyScattering properties measurementsConfocalCrosslinking reagent
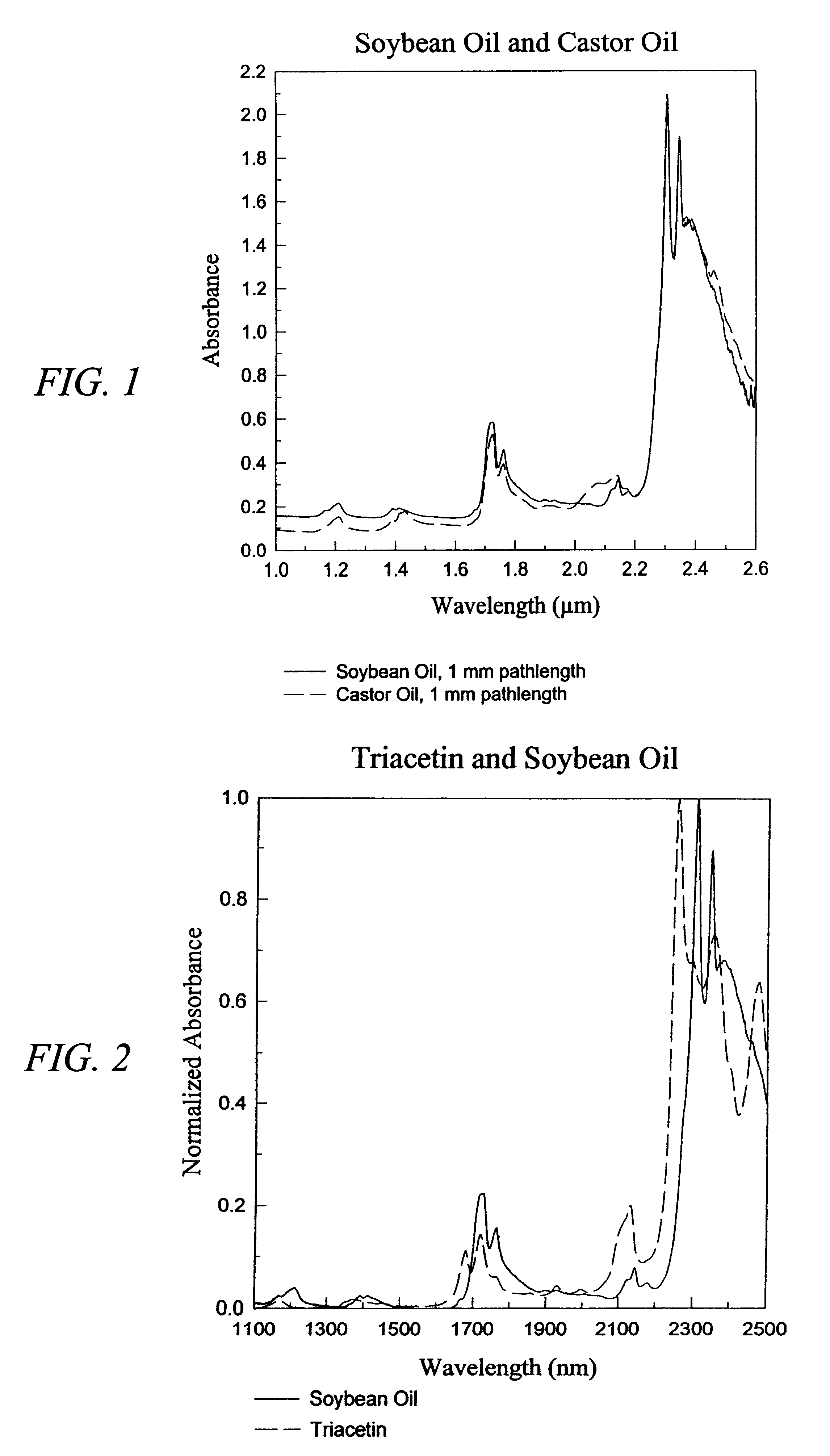
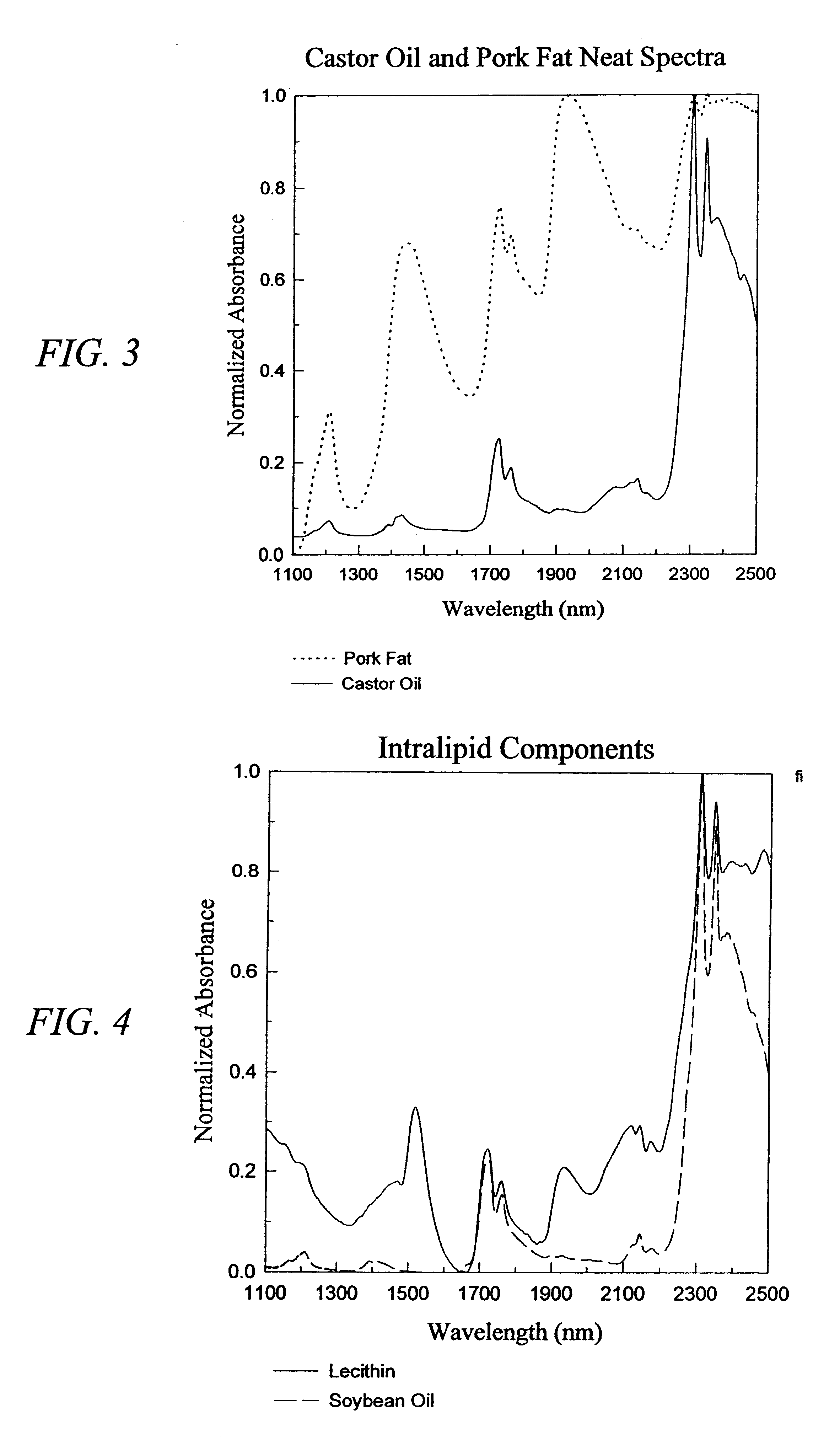
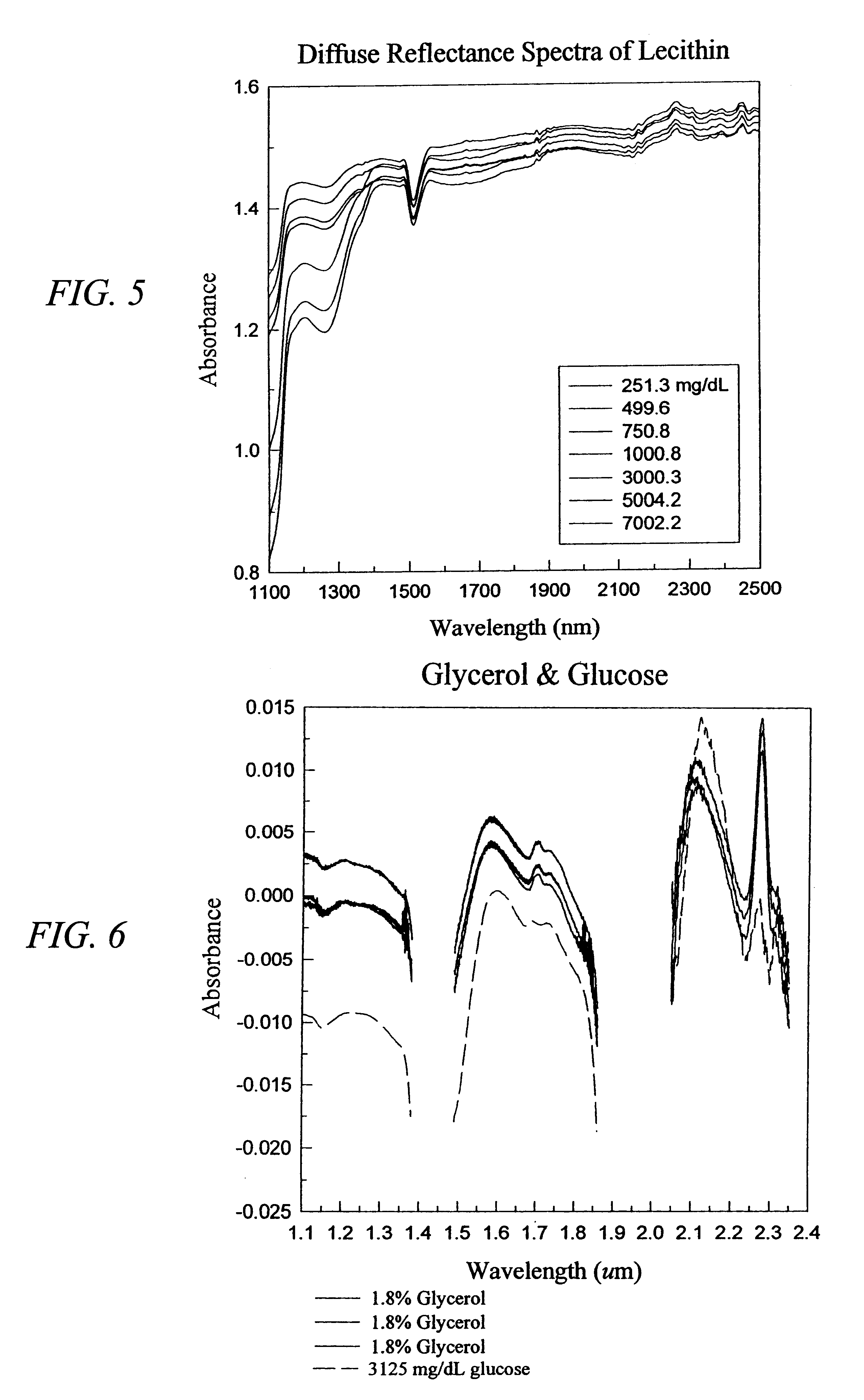
The invention provides a class of samples that model the human body. This family of samples is based upon emulsions of oil in water with lecithin acting as the emulsifier. These solutions that have varying particle sizes may be spiked with basis set components (albumin, urea and glucose) to simulate skin tissues further. The family of samples is such that other organic compounds such as collagen, elastin, globulin and bilirubin may be added, as can salts such as Na+, K+ and Cl-. Layers of varying thickness with known index of refraction and particle size distributions may be generated using simple crosslinking reagents, such as collagen (gelatin). The resulting samples are flexible in each analyte's concentration and match the skin layers of the body in terms of the samples reduced scattering and absorption coefficients, mums and muma. This family of samples is provided for use in the medical field where lasers and spectroscopy based analyzers are used in treatment of the body. In particular, knowledge may be gained on net analyte signal, photon depth of penetration, photon radial diffusion, photon interaction between tissue layers, photon density (all as a function of frequency) and on instrument parameter specifications such as resolution and required dynamic range (A / D bits required). In particular, applications to delineate such parameters have been developed for the application of noninvasive glucose determination in the near-IR region from 700 to 2500 nm with an emphasis on the region 1000 to 2500 nm (10,000 to 4,000 cm-1).
Owner:GLT ACQUISITION
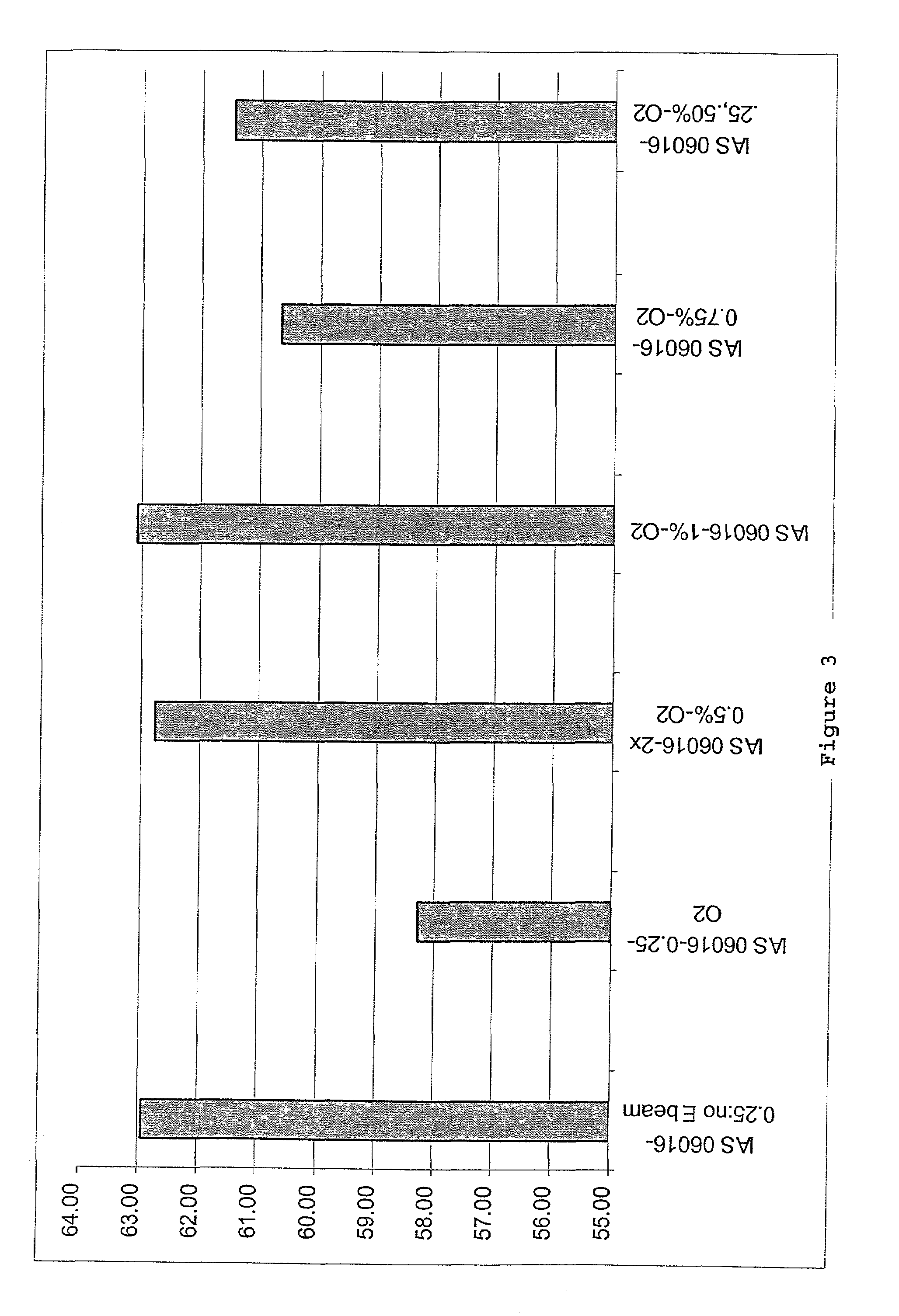
Collagen/glycosaminoglycan matrix stable to sterilizing by electron beam radiation
InactiveUS6969523B1Facilitate cross-linkingImprove performanceBiocideMechanical working/deformationCross-linkGlycosaminoglycan
Compositions of cross-linked collagen and a glycosaminoglycan are provided which retain characteristics rendering them useful as tissue engineering matrices or scaffolds following terminal sterilization. Also provided are methods for producing these compositions and terminally sterilized matrices or scaffolds from these compositions as well as methods of using these matrices or scaffolds as tissue engineering devices.
Owner:INTEGRA LIFESCI
Carrier with solid fibrinogen and solid thrombin
InactiveUS7052713B2Safety and efficacyShorten hemostasis timePowder deliverySurgical adhesivesNatural sourceFiber
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and hemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin.The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibers. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, ε-aminocaproic acid or α2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
Collagen/polysaccharide bilayer matrix
Disclosed are bilayer matrices of a polysaccharide such as collagen (COL) and another polysaccharide such as hyaluronic acid (HA) with various COL / HA ratios. Each layer has a porous structure. These materials are useful for tissue regeneration, particularly when used with orthopedic implants and drug delivery.
Owner:DEPUY ACROMED INC
Collagen carrier of therapeutic genetic material, and method
InactiveUS20030039695A1Powder deliveryGenetic material ingredientsGenetic MaterialsNucleic acid sequencing
A collagen matrix material is charged with a cell growth-promoting derived nucleic acid sequence. The nucleic acid sequence-charged collagen matrix material may be utilized in a method of promoting regeneration of surface cartilage of a joint. In the method, an area of injury is covered with the nucleic acid sequence-charged collagen matrix material, the collagen matrix material is fixed over the area to be treated, and the area is allowed to heal.
Owner:ED GEISTLICH SOHNE FUR CHEM IND
Prolonged delivery of heparin-binding growth factors from heparin-derivatized collagen
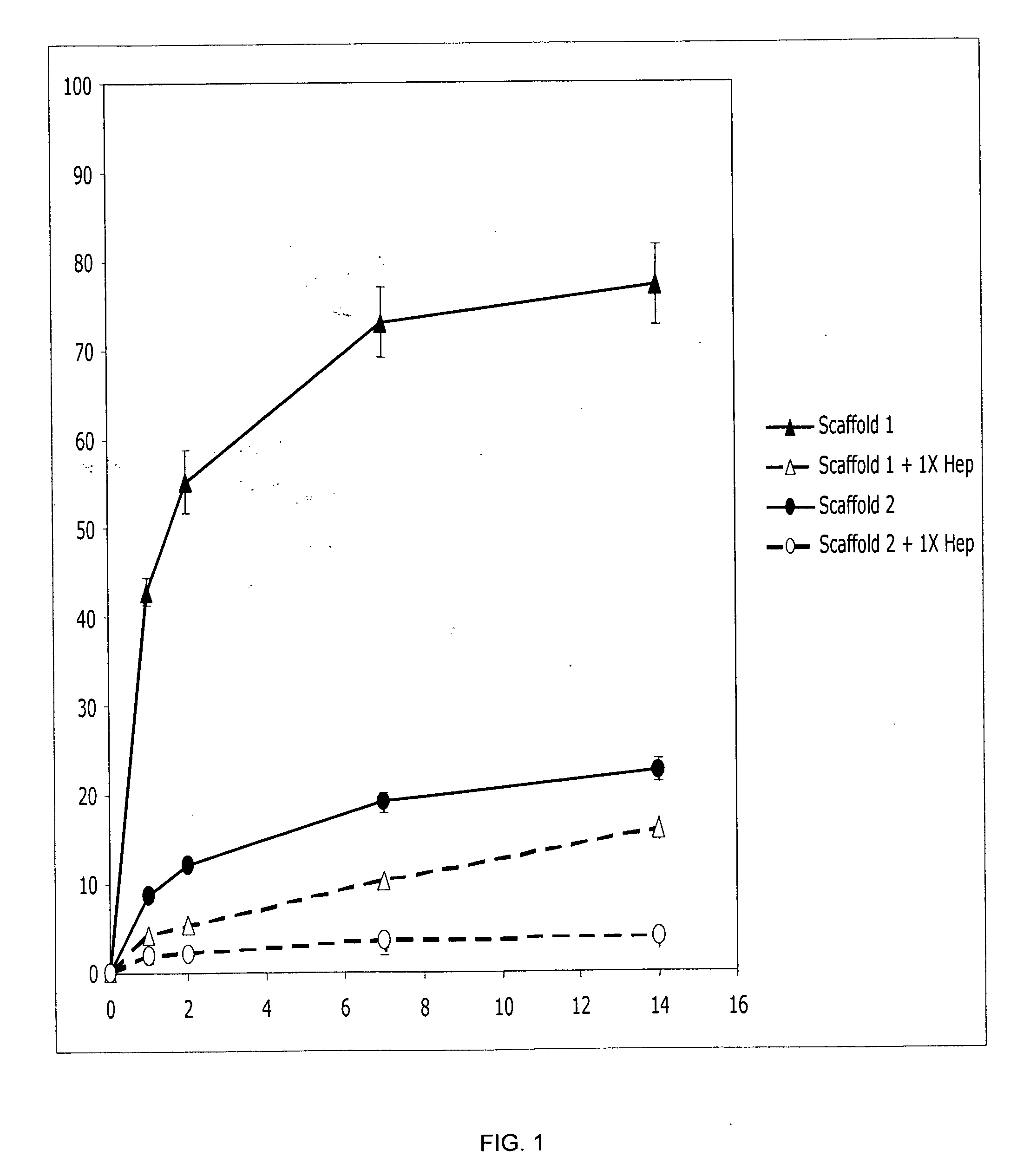
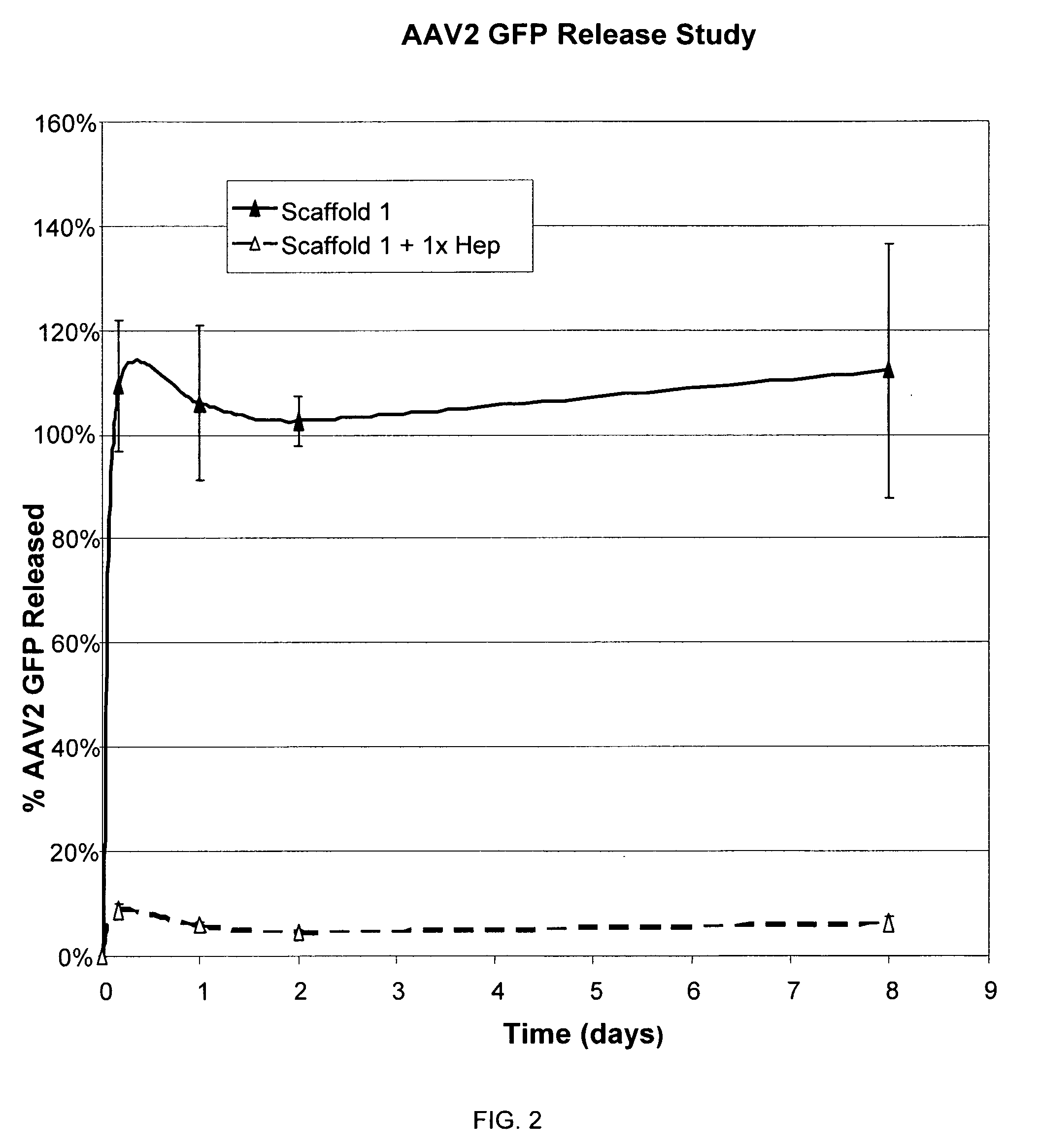
InactiveUS20090192079A1Improve biological activityPeptide/protein ingredientsGenetic therapy composition manufactureMuscle tissueCollagen scaffold
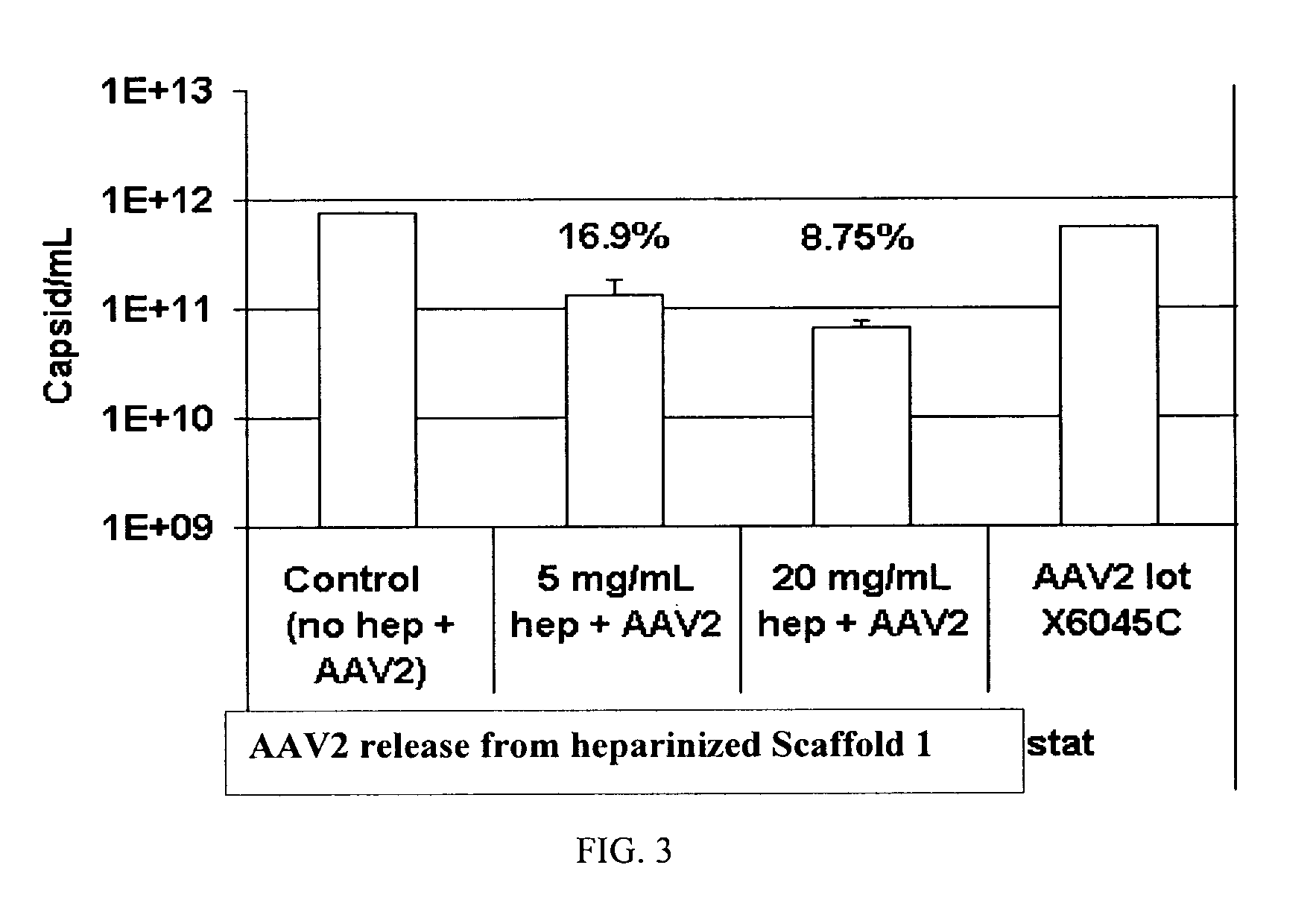
The present invention relates to a heparin-derivatized collagen matrix comprising a fragment of heparin covalently linked to a collagen scaffold, wherein the fragment of heparin has molecular weight of less than about 15 kDa, and at least one heparin-binding growth factor (HBGF) or heparin-binding adeno-associated virus (HB-AAV) or a combination thereof and methods for promoting bone growth, bone repair, cartilage repair, bone development, neo-angiogensis, wound healing, tissue engraftment and muscle tissue regeneration and / or tissue augmentation comprising administering a heparin-derivatized collagen matrix that includes at least one heparin-binding growth factor or heparin-binding adeno-associated virus or a combination thereof.
Owner:GENZYME CORP
Dosage unit formulations of autologous dermal fibroblasts
ActiveUS8529883B2Reduce severityAdditional componentBiocideArtificial cell constructsWrinkle skinNasolabial fold
Dosage units consist of an autologous cell therapy product composed of fibroblasts grown for each individual to be treated. The suspension of autologous fibroblasts, grown from a biopsy of each individual's own skin using current good manufacturing practices (CGMP), and standard tissue culture procedures, is supplied in vials containing cryopreserved fibroblasts or precursors thereof, having a purity of at least 98% fibroblasts and a viability of at least 85%, for administration of from one to six mL, preferably two mL, of cells at a concentration of from 1.0-2.0×107 cells / mL. When injected into the nasolabial fold wrinkles (creases on the sides of the nose that extend to the corners of the mouth), the autologous fibroblasts are thought to increase the synthesis of extracellular matrix components, including collagen, reducing the severity of these wrinkles. Dosage and timing of administration have been demonstrated to be critical to achieving clinically significant outcomes.
Owner:CASTLE CREEK BIOSCIENCES LLC
Method for protecting and restoring skin using selective MMP inhibitors
InactiveUS20020119107A1Type of reductionEliminate the effects ofCosmetic preparationsToilet preparationsUltravioletSolar ultraviolet radiation
The invention is based on selective inhibition of the enzyme (MMP-1), which causes the dermal matrix damage in humans, while sparing the enzyme(s) (MMP-9 and perhaps MMP-2) which not only do not cause the damage (based on extrapolation from our in vitro collagen gel system to real skin) but actually "clear away" the damage produced by MMP-1 to restore normal function to the skin. Matrix metalloproteinase-1 (MMP-1; fibroblast collagenase) is induced by UV radiation from the sun and is naturally elevated in old age. Human fibroblasts exposed to the degradation products of MMP-1 contract collagen, but when this debris is removed from their environment, the fibroblasts behave normally. Inhibiting MMP-1 but sparing enzymes that remove the debris improves human skin after onslaught from solar UV radiation, old age, and acne.
Owner:RGT UNIV OF MICHIGAN
Carrier with solid fibrinogen and solid thrombin
The present invention relates to a solid composition useful for tissue gluing, tissue sealing and haemostasis consisting essentially of a) a carrier which has at least one of the following physical properties: elasticity module in the range of 5-100 N / cm, density of 1-10 mg / cm3, chamber diameter of more than 0.75 mm and less than 4 mm and / or having a chamber diameter average below 3 mm and evenly distributed and fixed upon said carrier, b) solid fibrinogen, and c) solid thrombin. The carrier is a biodegradable polymer such as a polyhyaluronic acid, polyhydroxy acid, e.g. lactic acid, glucolic acid, hydroxybutanoic acid, a cellulose, gelatine or collagen, such as a collagen sponge, e.g. a collagen sponge consisting essentially of collagen type I fibres. The fibrinogen and thrombin are preferably human, purified from a natural source, or transgenic or recombinant human fibrinogen and / or thrombin. In a preferred embodiment the composition does not comprise any antifibronolytic agent such as aprotinin, epsi-aminocaproic acid or alpha2-antiplasmin,
Owner:TOPAZ INVESTMENT AS
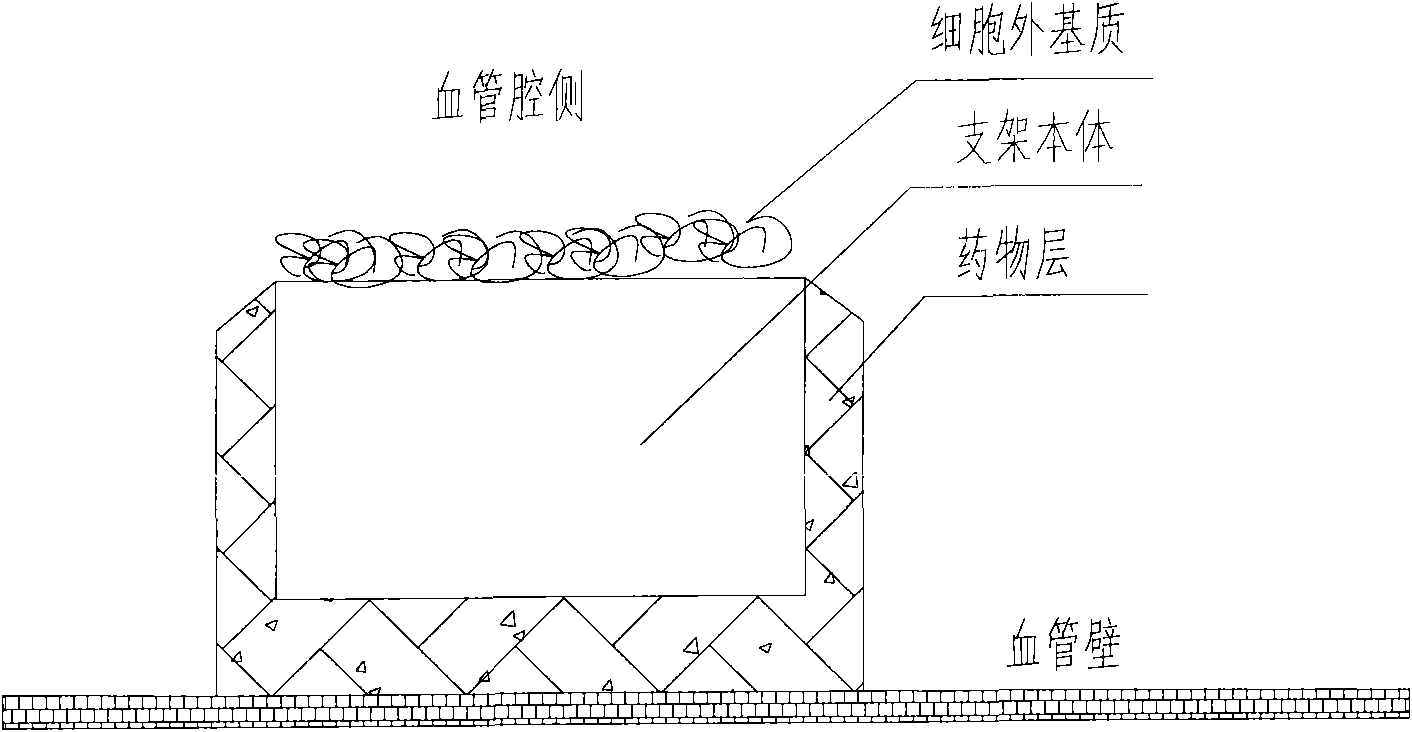
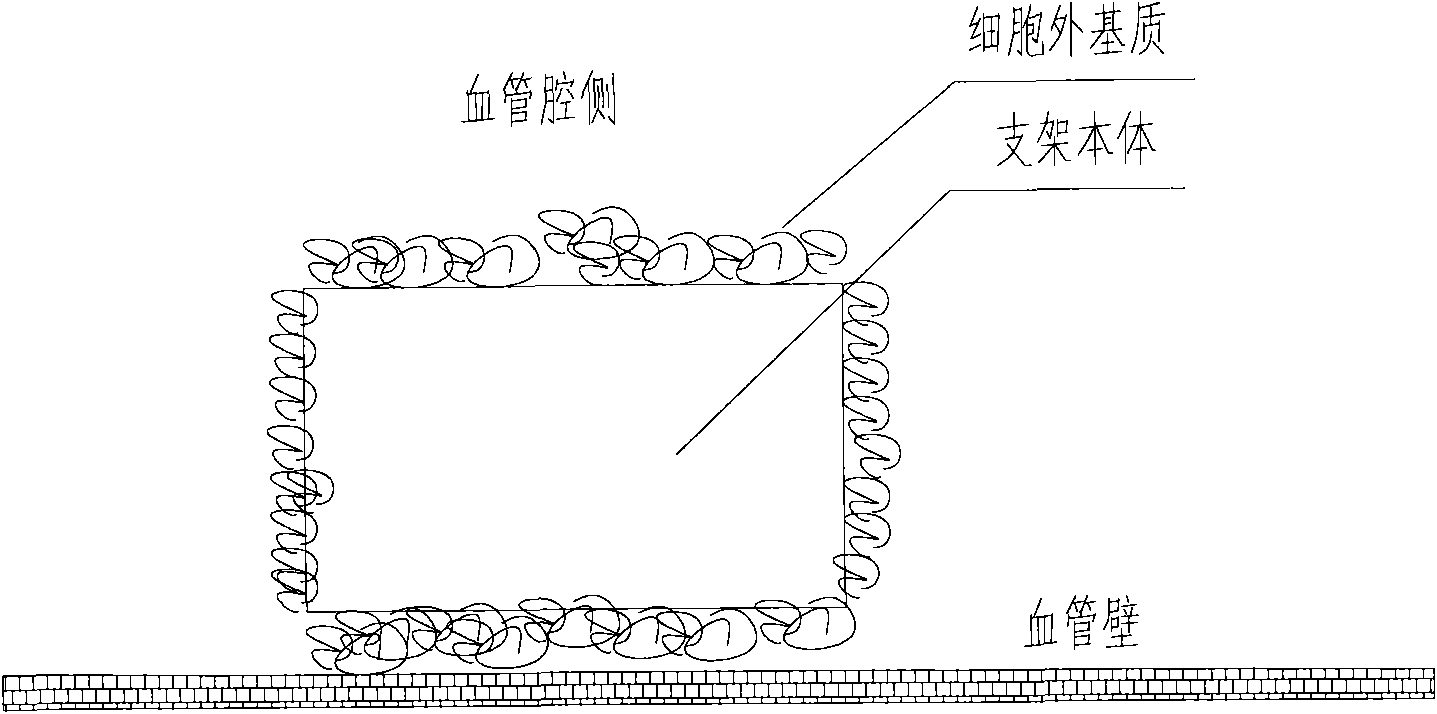
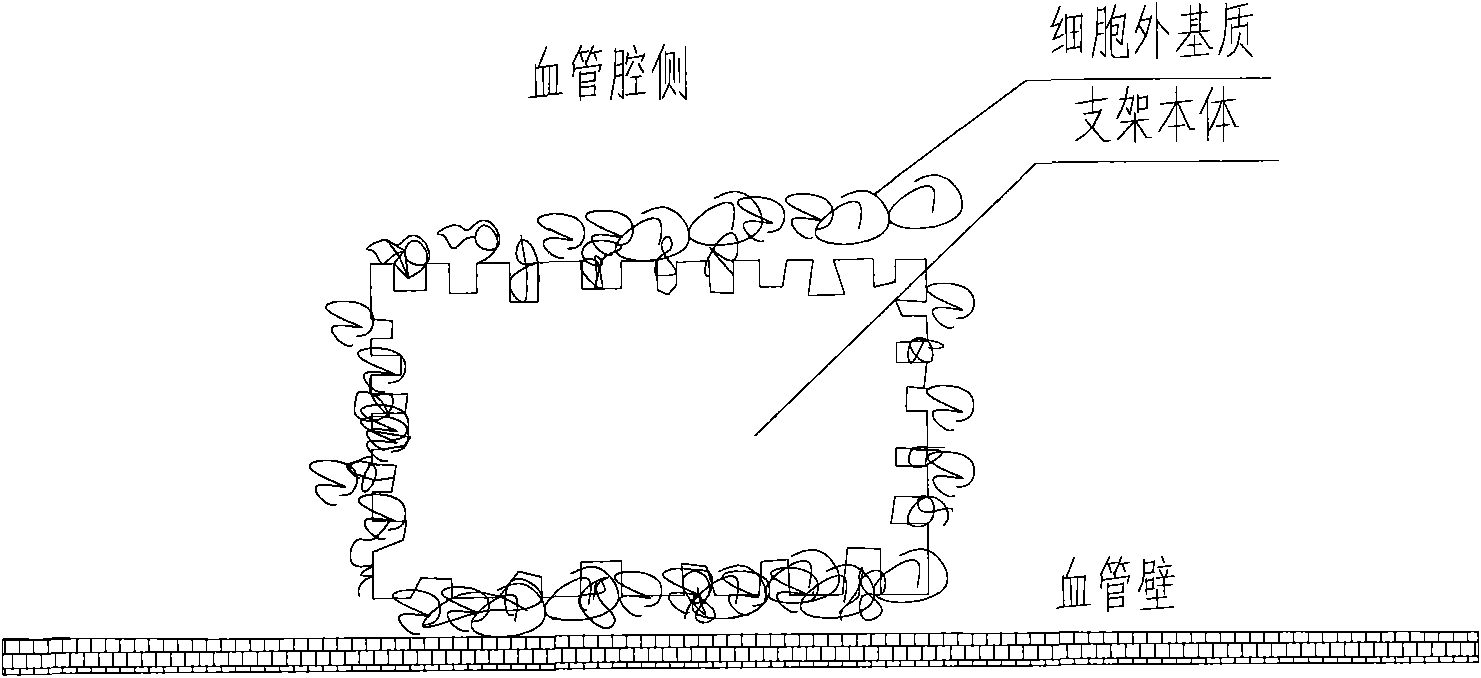
A medical equipment carrying extracellular matrix and its production method
ActiveCN101548916APromote endothelializationReduce restenosis rateStentsHeart valvesSide effectCell-Extracellular Matrix
The invention relates to a kind of medical equipment carrying extracellular matrix and its production method. It includes the medical equipment body and the layer of extracellular matrix coating on the surface of the medical equipment body. The described extracellular matrix is collagen, laminin, non-collagenous glycoprotein, GAG and proteoglycan, elastin, and one or multiple endothelial cell extracellular matrixes obtained through cell treatment from the cultured endothelial cells. For the described medical equipment in the invention, the extracellular matrix on the body surface can withstand the wash of blood and other body fluids and advance the vascular endothelialization through slow release; if working together with the drug, it can not only reduce the restenosis rate, but also speed up the surface endothelialization to improve treatment; the preparation method of the invention is simple and may have no carrier. It uses the electrostatic and / or micropore adsorption principle to coat the extracellular matrix directly on the body surface, thus it can prevent the side effects such as the inflammation brought by the carrier.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
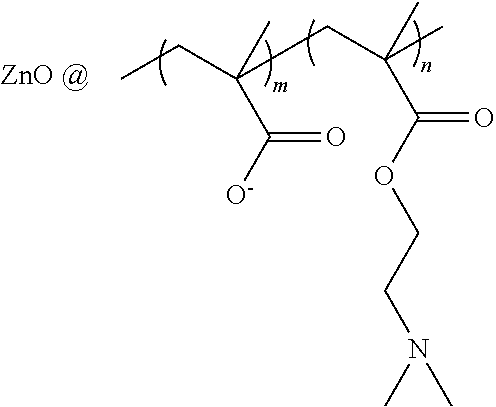
COLLAGEN-BASED HYDROGELS LOADED WITH ZnO QDs/pDNA COMPLEXES AS CORNEAL SUBSTITUES
InactiveUS20120009223A1Good biocompatibilityImprove mechanical propertiesOrganic active ingredientsSenses disorderFluorescenceFreeze-drying
Owner:WENGUANG LIU +3
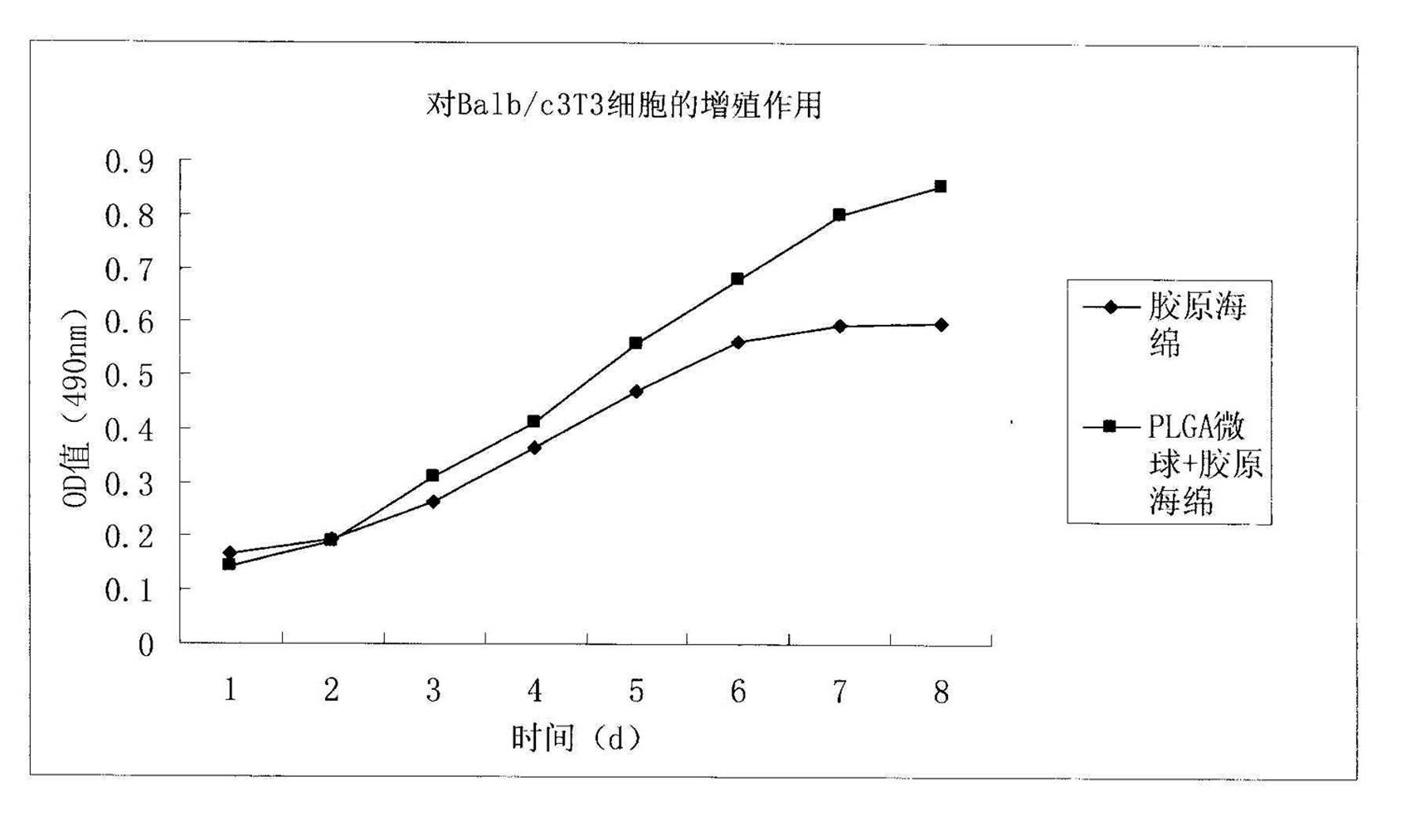
Method for preparing basic fibroblast growth factor sustained-release carrier
InactiveCN102228695AMaintain biological activityGood biocompatibilityPeptide/protein ingredientsPharmaceutical delivery mechanismMicrosphereFreeze-drying
The invention discloses a method for preparing a basic fibroblast growth factor (bFGF) sustained-release carrier, which comprises the following steps of: 1, preparing bFGF-poly(lactide-co-glycolide) (PLGA) sustained-release microspheres encapsulating 0.00001 to 0.001 percent of bFGF; 2, dissolving type I collagen in an acetic acid solution to obtain a collagen solution; 3, mixing and stirring the bFGF-PLGA sustained-release microspheres and the collagen solution, performing freeze drying, solidifying, and performing freeze drying again to obtain a bFGF-PLGA collagen sustained-release carrier; and 4, performing in-vitro release experiments and Balb / c3T3 cell proliferation promotion experiments, wherein experimental results show that the sustained-release carrier has good biocompatibility, can slowly control the release of the bFGF and effectively promote Balb / c3T3 cell growth, and is an ideal bFGF sustained-release carrier.
Owner:舒泰经贸(广州)有限公司
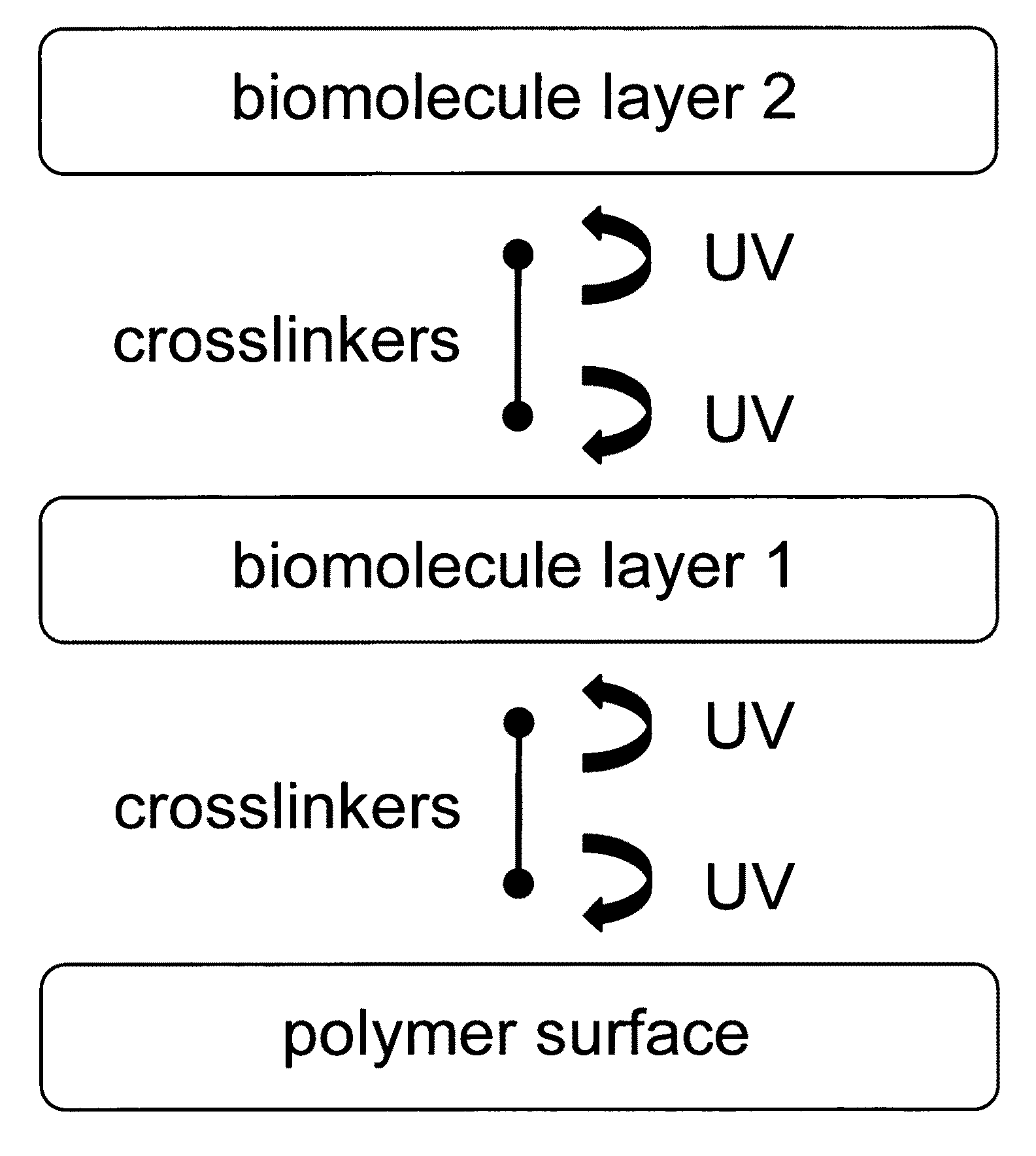
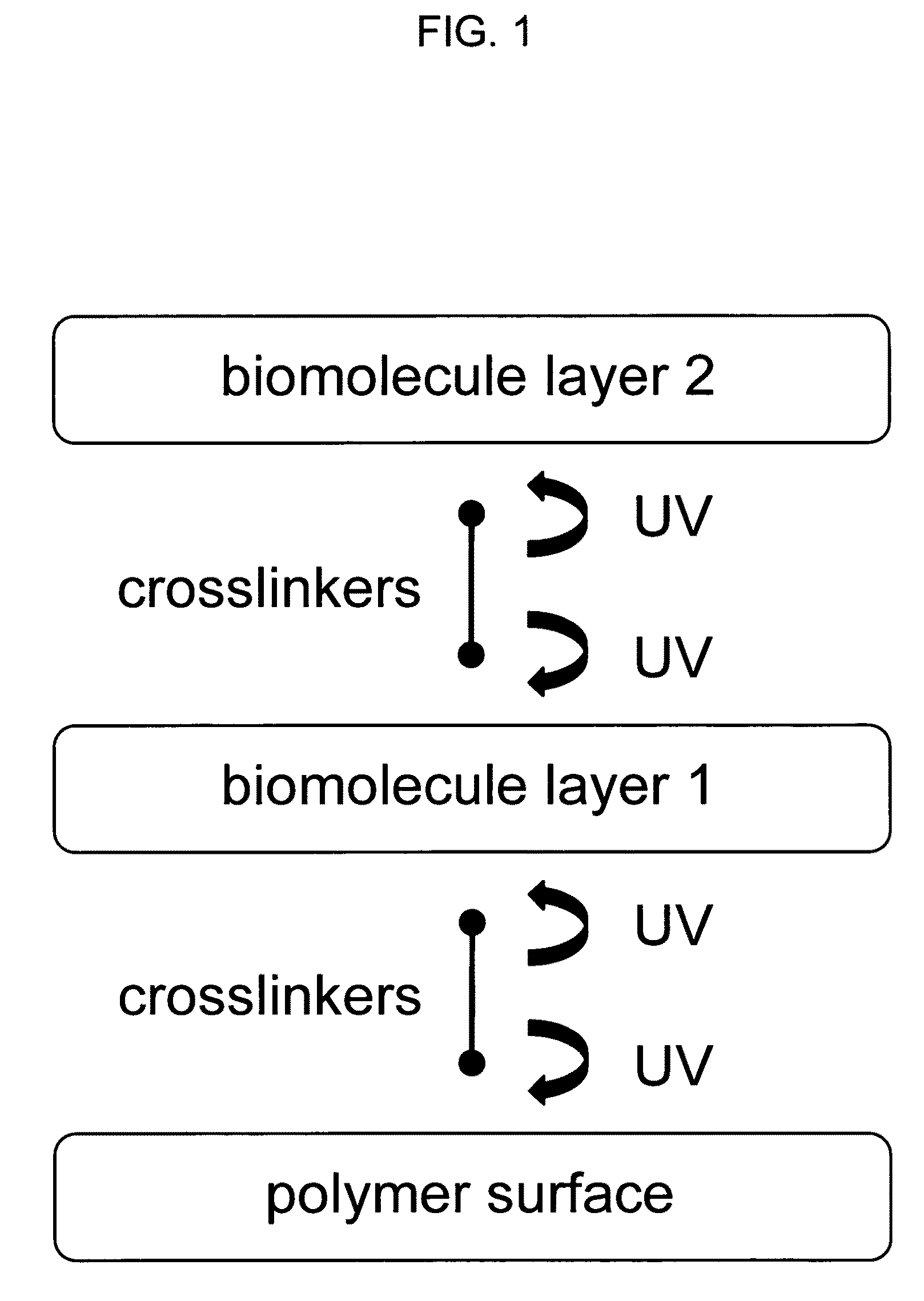
Sequential coupling of biomolecule layers to polymers
InactiveUS20090117166A1Used in fieldPowder deliveryPeptide/protein ingredientsCell-Extracellular MatrixECM Protein
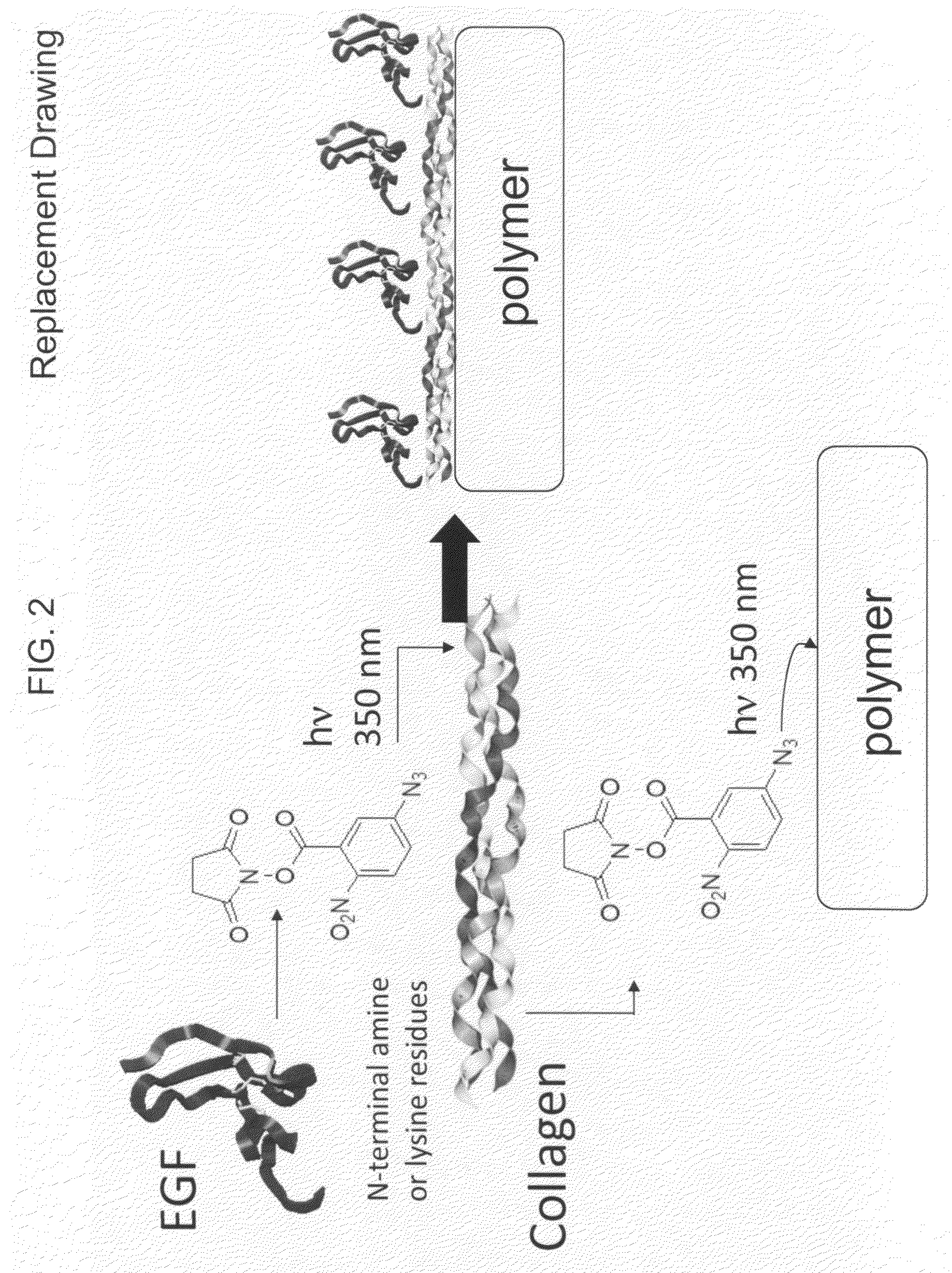
A bio-mimetic or bio-implantable material based on a sequential process of coupling biomolecule layers to a polymer layer is provided. In general, the material could be based on two or more biomolecule layers starting with one of the layers covalently linked to the polymer layer via cross-linkers and the other layers sequentially and covalently linked using cross-linkers to the previously added layer. The polymer layer could be a hydrogel or an interpenetrating polymer network hydrogel. The first layer of biomolecules could be a collagen type, fibronectin, laminin, extracellular matrix protein, or any combinations thereof. The second layer of biomolecules typically is a growth factor, protein or stimulant. The cross-linkers are either water soluble or insoluble bifunctional cross-linkers or azide-active-ester crosslinkers. The material and process as taught in this invention are useful in the field of tissue engineering and wound healing.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
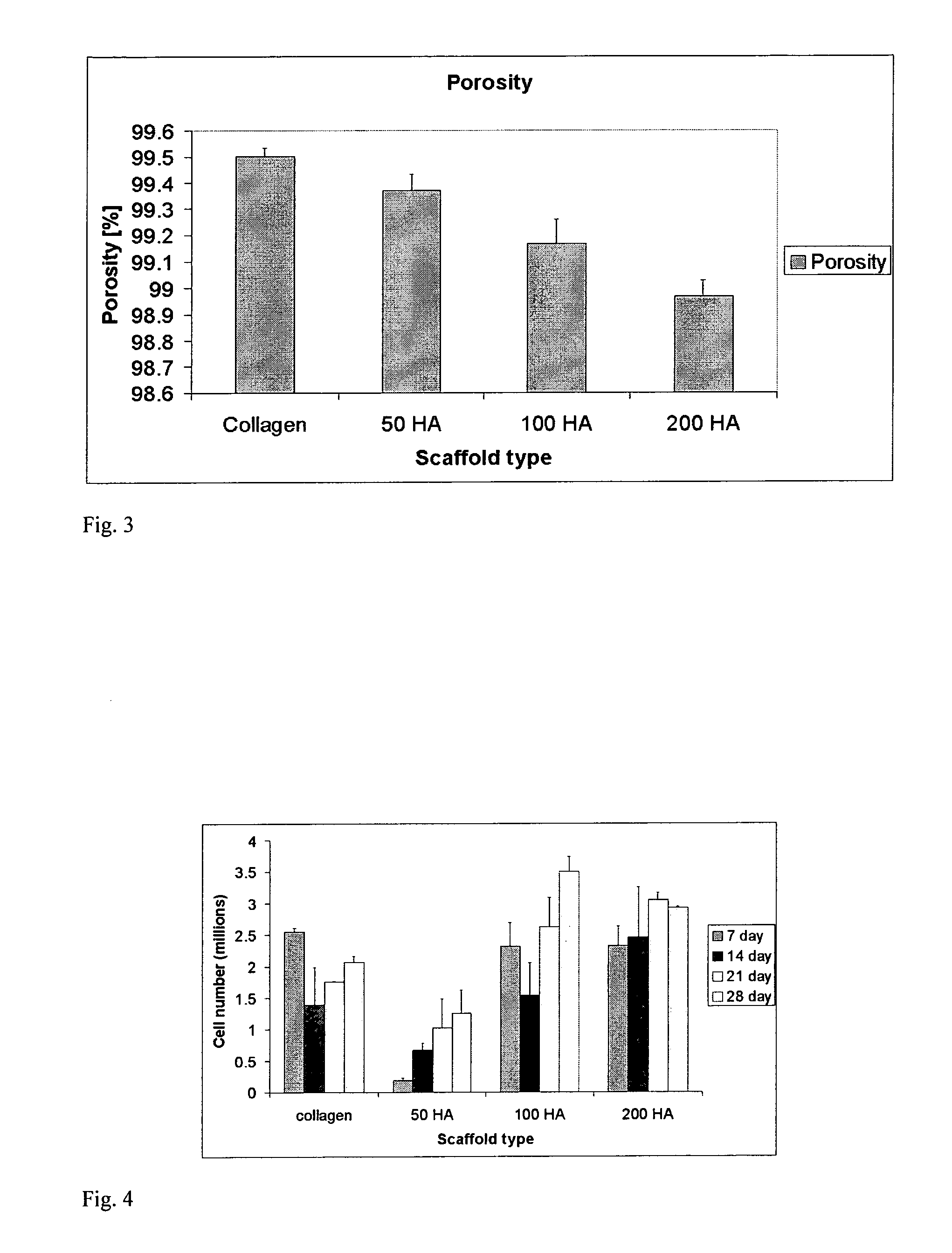
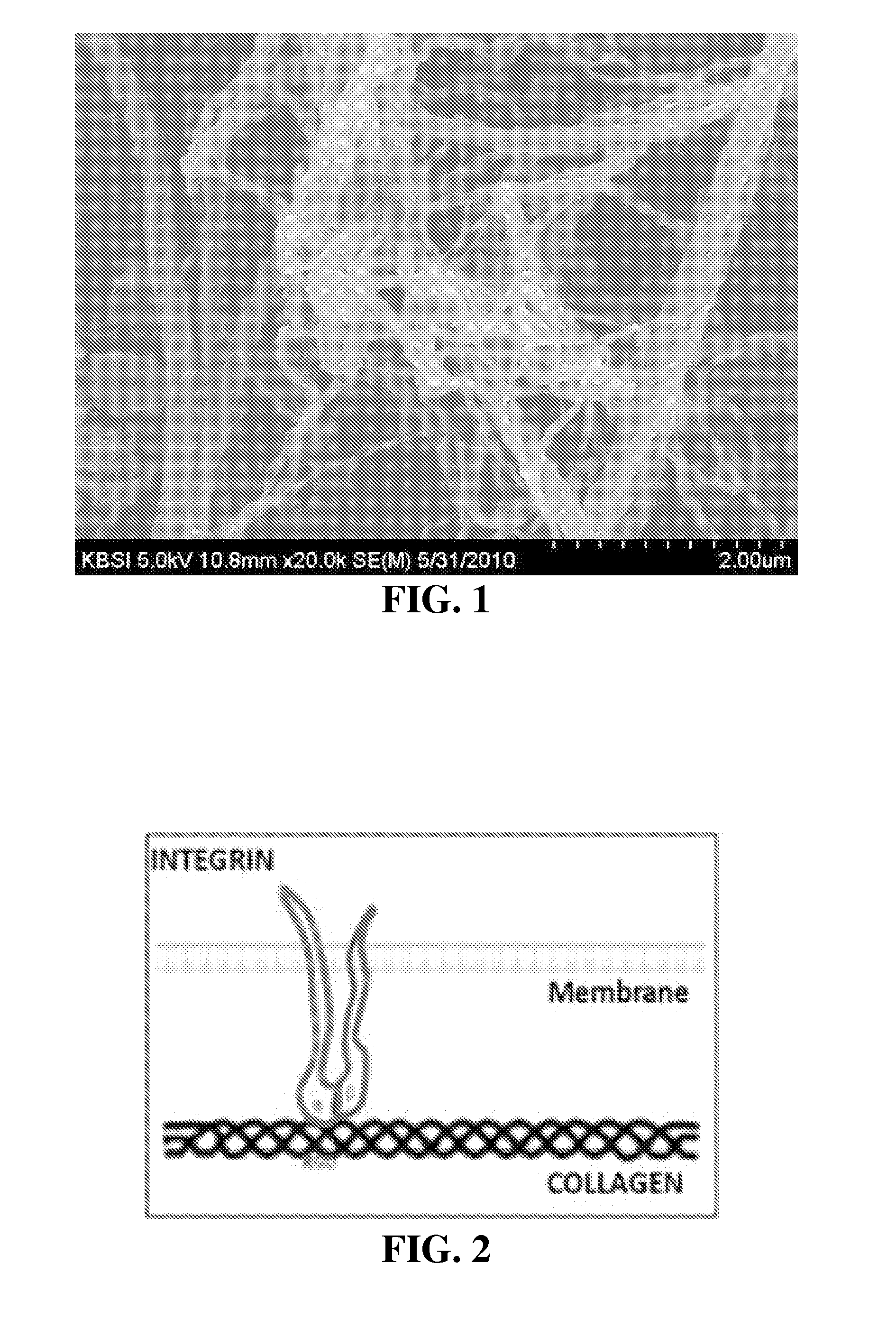
Collagen/hydroxyapatite composite scaffold, and process for the production thereof
ActiveUS20100158976A1Good biocompatibilityPromotes cell adhesionPowder deliveryPeptide/protein ingredientsCompressive stiffnessCross-link
A process for producing a collagen / hydroxyapatite (HA) composite scaffold comprises the steps of forming a homogenous suspension of collagen and HA in an acidic solution, lyophilising the suspension until a desired final freezing temperature is reached to produce the composite scaffold, and optionally cross-linking the composite scaffold, wherein the ratio of HA to collagen is at least 1:10 (w / w). Also provided is a collagen / hydroxyapatite (HA) composite scaffold comprising a homogenous distribution of hydroxyapatite within a porous, crosslinked, collagen matrix, wherein the ratio of HA to collagen is at least 1:10 (w / w). Suitably, the composite scaffold has a porosity of at least 99% (v / v), and a compressive stiffness of at least 0.3 KPa. Composite scaffolds of the invention may be used to provide osteoconductive bone implants and tissue engineering implants.
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
Skin-care, defect-repairing, anti-aging and winkle-removing cosmetic preparation
InactiveCN101884602AAchieve anti-aging effectCosmetic preparationsToilet preparationsWrinkle skinAcidic Fibroblast Growth Factor
The invention discloses a skin-care, defect-repairing, anti-aging and winkle-removing cosmetic preparation. A recombinant human platelet-derived growth factor (rhPDGF) which contains bioactive constituents and has a dermis repairing function, other growth factors such as a transforming growth factor (TGF-beta), an acidic fibroblast growth factor (aFGF), a basic fibroblast growth factor (bFGF) and an epidermal growth factor (EGF), and a hyaluronic acid (HA) compound preparation are introduced into the dermis by a daily skin care or cosmetic introducing method to stimulate the growth and the mitosis of fibroblasts in the dermis, the synthesis and the secretion of collagen and the HA and the growth and repair of collagenous fiber and elastic fiber so as to repair skin tissue defects such as skin depression, deep wrinkle, fracture of dermis fibrous tissue and the like caused by aging or skin damage. Therefore, skin beautifying and anti-aging effects are achieved.
Owner:孙杰
Autologous collagen-containing water needle and preparation method thereof
InactiveCN109044948APromote growthPromote proliferation and differentiationCosmetic preparationsToilet preparationsVitamin CAntioxidant
The embodiment of the invention discloses an autologous collagen-containing water needle and a preparation method thereof. The autologous collagen-containing water needle comprises the following components in parts by weight: 10-20 parts of autologous collagen, 10-20 parts of hyaluronic acid, 5-10 parts of biopolysaccharide glue, 5-10 parts of a sheep placenta extract, 3-8 parts of a levorotatoryC stock solution, 2-3 parts of a grape seed extract, 2-5 parts of fructan, 2-5 parts of beta-glucan, 1-5 parts of vitamin C, 2-5 parts of amino acid, 2-3 parts of an coenzyme, 2-4 parts of an anti-allergic agent, 2-5 parts of an antioxidant, 1-3 parts of a sun-screening agent and 3-8 parts of a moisturizer. The water needle is prepared from the autologous collagen extracted from human venous blood, is autologous, does not generate exclusion, contains a variety of nutrients required for cell repair and cell growth, and has relatively strong efficacies of accelerating wound healing of skin and mucosa, promoting the cell growth, promoting wound healing and strengthening the immune system.
Owner:白晋
Compositions for elastogenesis and connective tissue treatment
ActiveUS7666829B2Increase elasticityGood lookingOrganic active ingredientsBiocideArterial smooth muscle cellsManganese
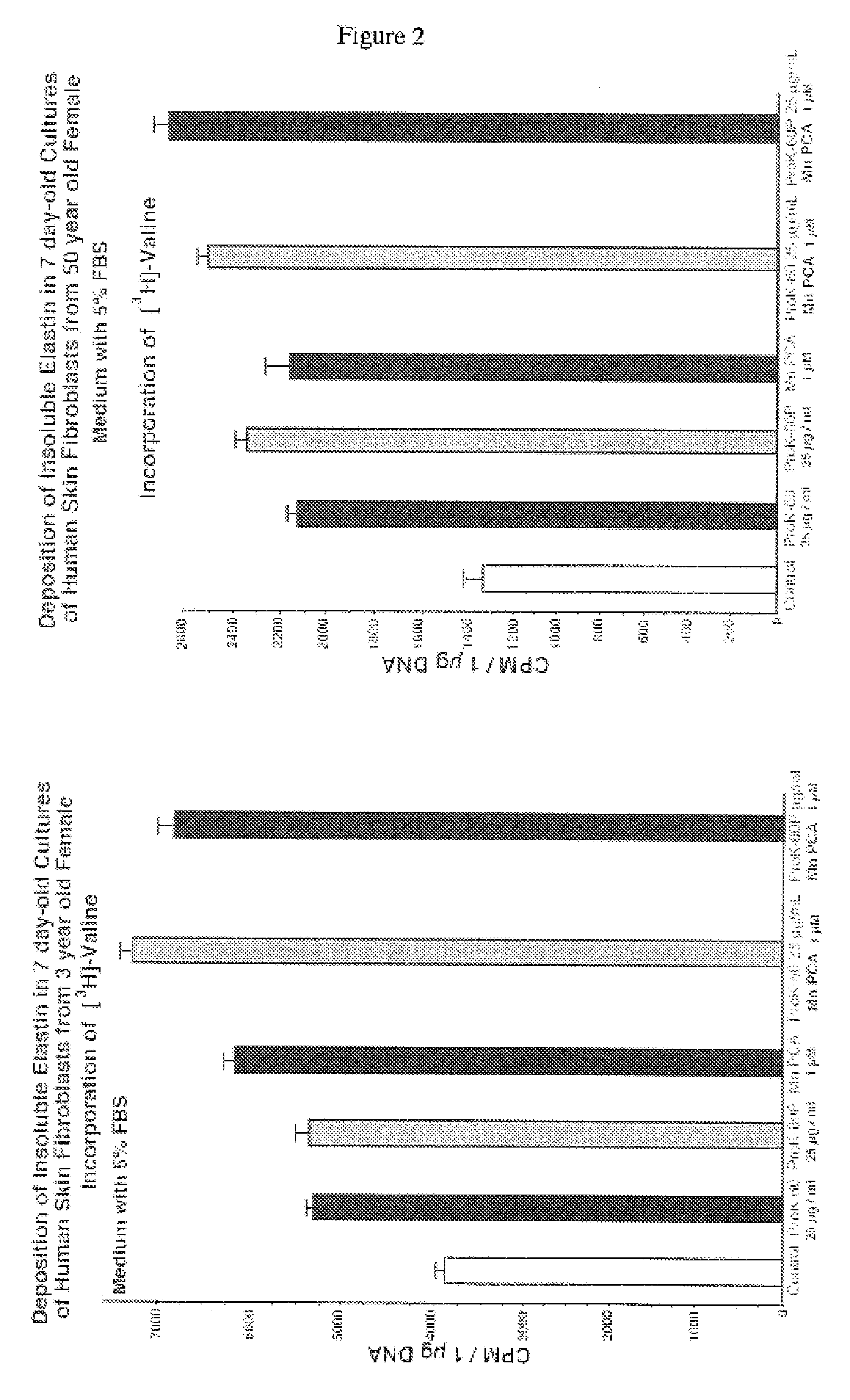
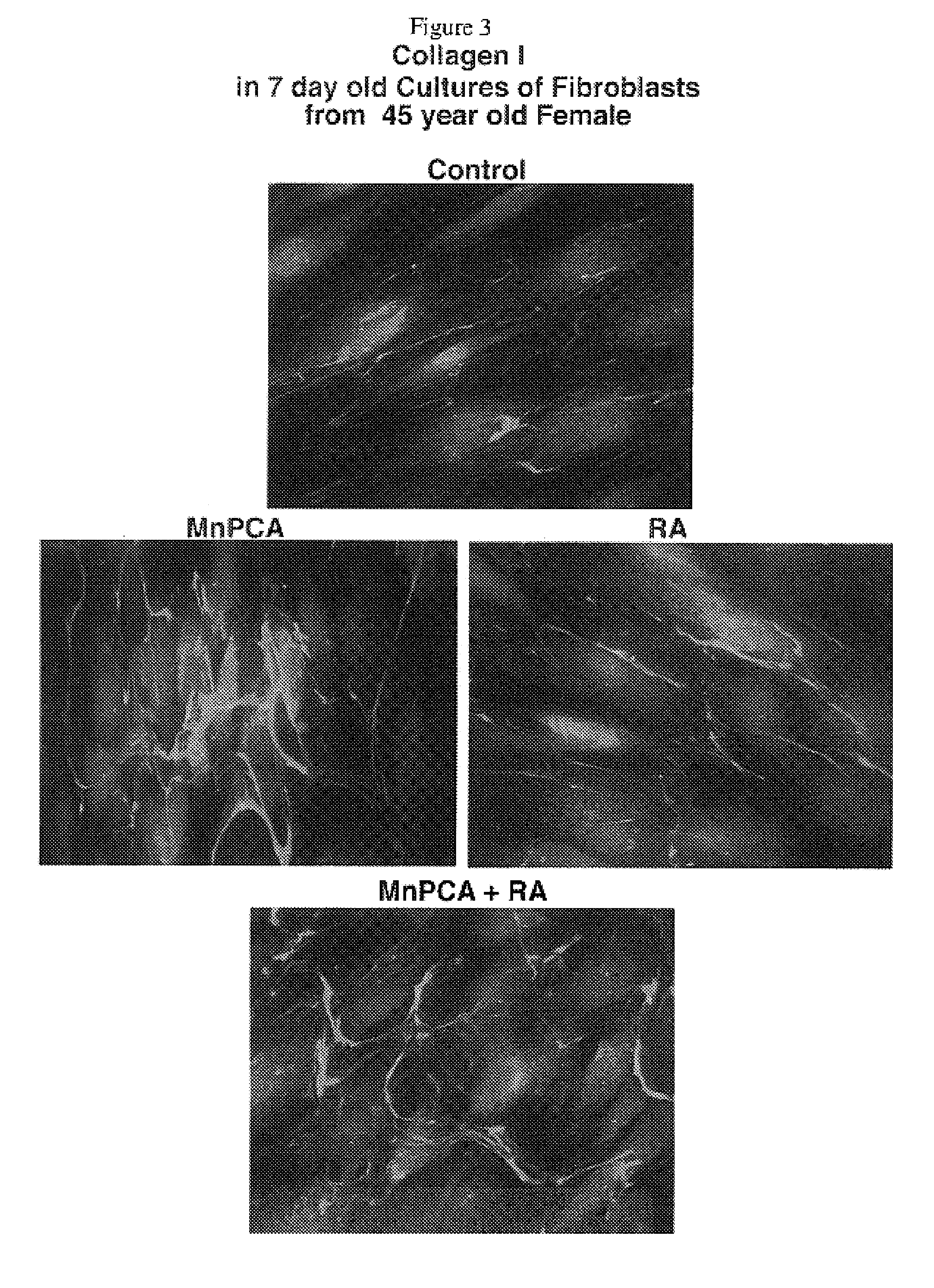
The present invention describes therapeutic compositions comprising one or more minerals, including trivalent iron, divalent manganese and salts thereof, suitable in facilitating synthesis and deposition of connective tissue matrix, particularly rich of elastin and collagen, and mitogenic potential in human dermal fibroblasts. It also describes the phenomenon in which stimulation of elastogenesis by arterial SMC associates with a net decrease in proliferation of these cell types. The present invention also describes methods of treatment of human skin fibroblasts and arterial smooth muscle cells. The therapeutic compositions of the present invention comprise one or more of trivalent iron or divalent manganese or salts thereof and may be combined with an elastic tissue digest.
Owner:HOSPITAL FOR SICK CHILDREN +1
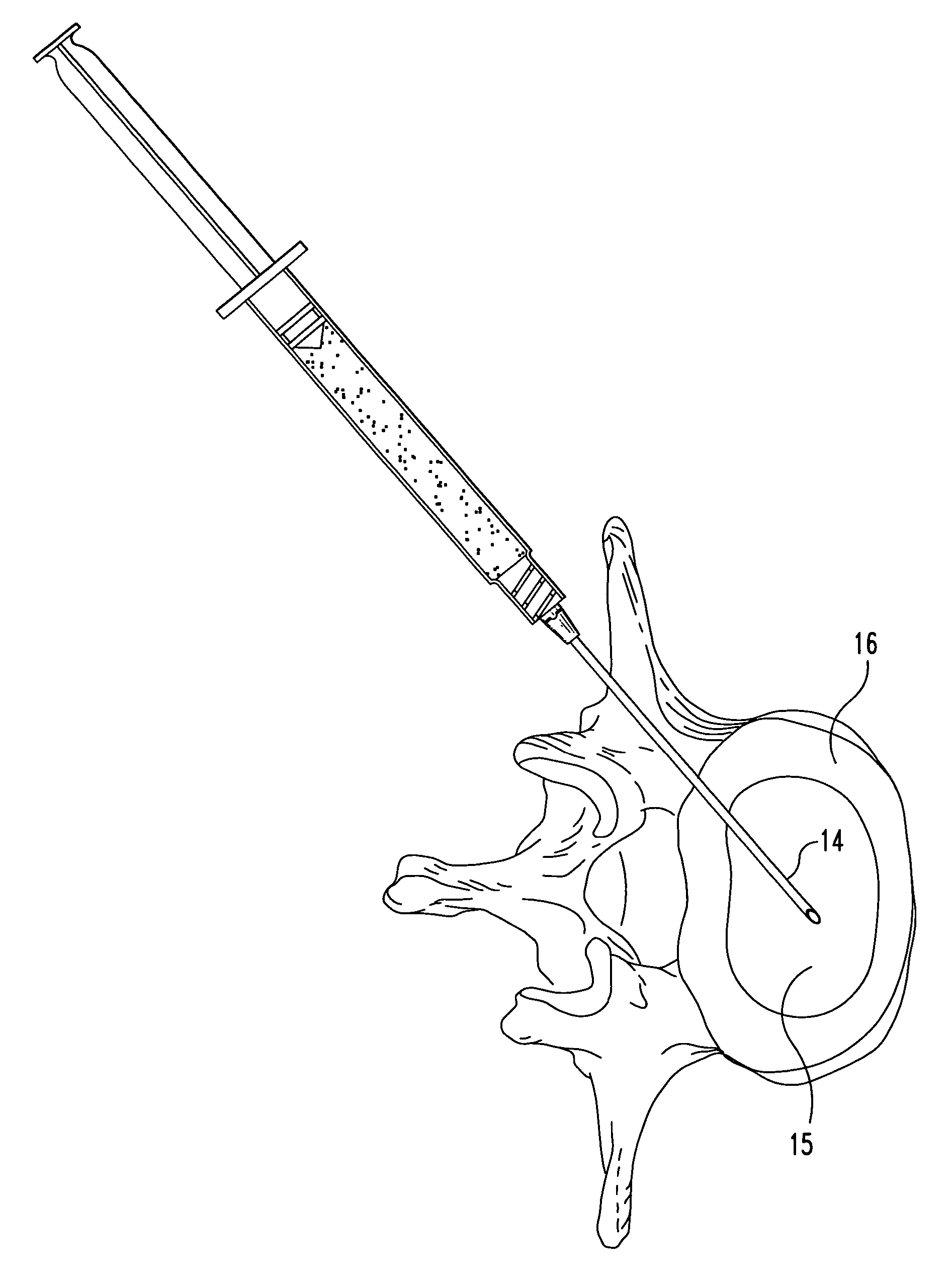
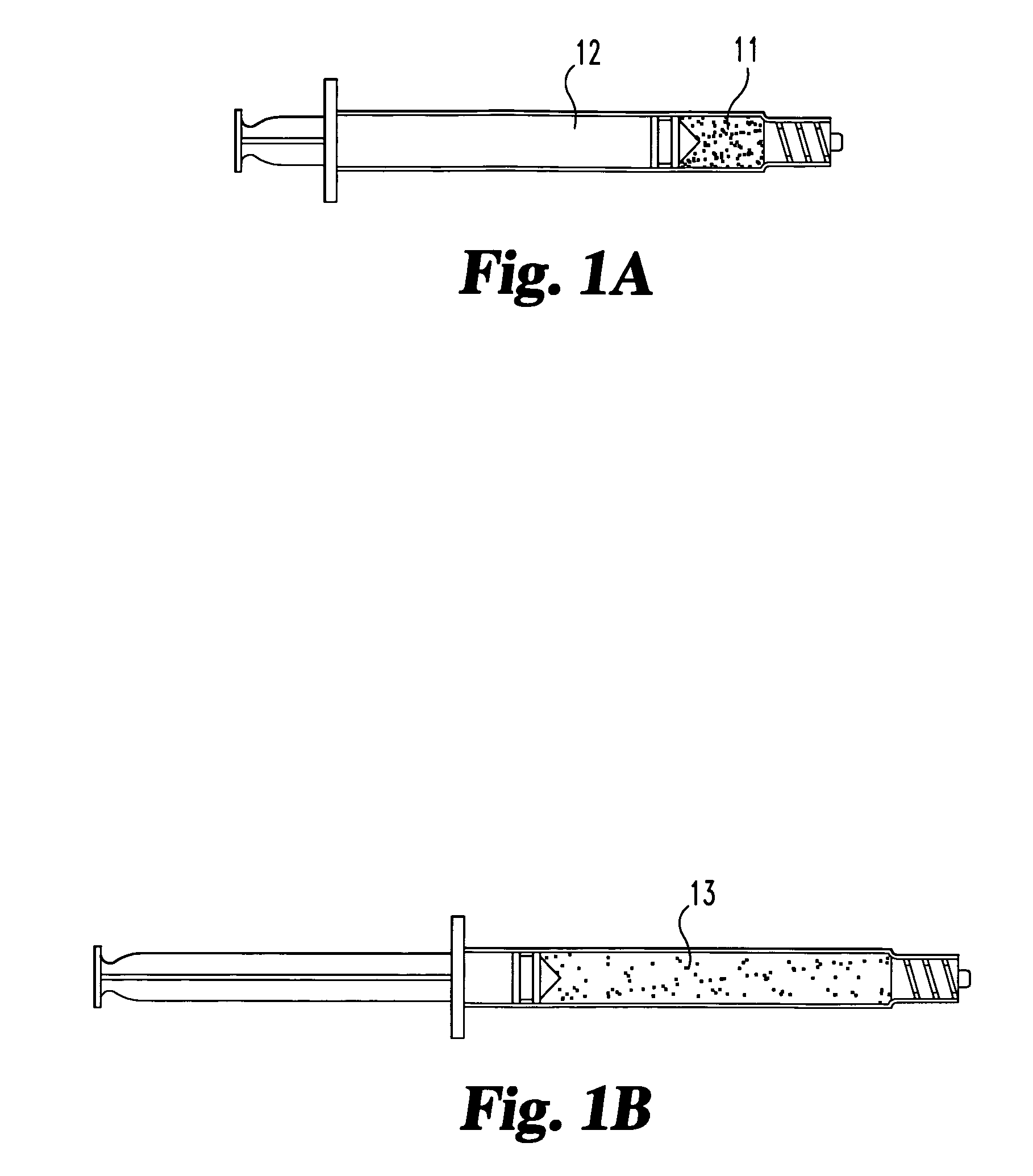
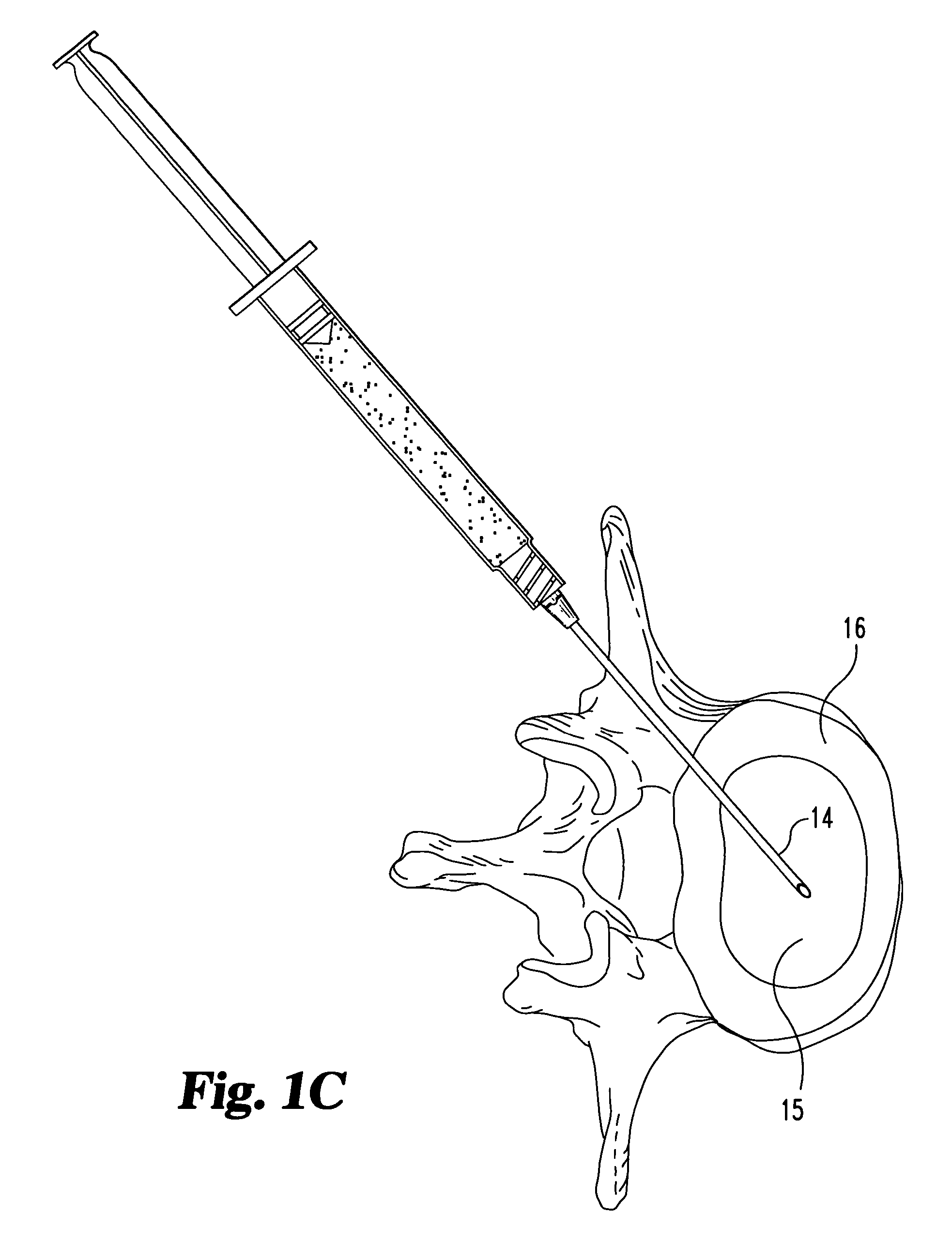
Compositions and methods for treating intervertebral discs with collagen-based materials
ActiveUS7744651B2Enhance the imagePrecise functionInternal osteosythesisLigamentsIntervertebral discInjected material
Owner:WARSAW ORTHOPEDIC INC
Preparation for promoting fibroblasts to secrete extracellular matrix components and preparation method thereof
InactiveCN101648010AImprove habitatEasy to useCosmetic preparationsPeptide/protein ingredientsCell-Extracellular MatrixHazardous substance
The invention discloses a preparation for promoting fibroblasts to secrete extracellular matrix components, and a preparation method and application thereof. The preparation comprises the components of A) the egg white of eggs, B) egg white lysozyme, and C) pyruvic acid or pyruvate, can also comprise ovotransferrin and / or glucose, and is obtained by mixing the components uniformly. The preparationcan serve as an externally used paste for skin in application, and can obviously stimulate the secretion of collagen and hyaluronic acid synthase in the fibroblasts of the skin and improve the resiliency and the moisture content of the skin. The preparation is derived from a biologic extractive technique, has no harmful substance residues, safe and high-efficiency use, and is different from the prior method for promoting the secretion of a single fibroblast ECM component; besides, the preparation has multiple effects, wide application range and extensive application prospect, and can be applied in the fields of the preparation of skin substitutes, the preparation of anti-aging skin care products, cosmetics, medical treatment and the like.
Owner:BEIJING ZHONGKE YINUO BIOTECH
Collagen-polysaccharide materials mimicking blood vessels, tissues and bones for medical, pharmaceutical and orthopedic applications, and processes for producing the same
Provided is a collagen-cellulose material containing, based on a total weight of the collagen-cellulose material: 1.0-9.0 wt. % of a collagen; 0.2-3.0 wt. % of cellulose or a derivative thereof; 0.5-6.5 wt. % of at least one acid selected from an inorganic acid, an organic acid, and mixtures thereof; and water. Also provided is a process for producing the collagen-cellulose material. Also provided is an artificial blood vessel containing the collagen-cellulose material which is in the form of a hollow tube, an artificial tissue containing the collagen-cellulose material which is in the form of a sheet, and an artificial bone containing the collagen-cellulose material which is in the form of a solid. Also provided is a medical device containing the collagen-cellulose material, as well as a process for testing phlebotomical, surgical or orthopedic instrumentation or practicing phlebotomical, surgical or orthopedic procedures using the medical device containing the collagen-cellulose material.
Owner:NITTA CASINGS
Method for preparing medical collagen protein powder
InactiveCN1814778AAvoiding the Risk of Carrying the BSE VirusOvercoming resource scarcitySurgeryPharmaceutical containersFiberProcedure Agents
This invention discloses a method for preparing medical collagen powder characterizing in applying purified pigskins as the raw material to be pre-processed, divided, frozen and dried, crushed, dipped in acid, then digested for 4-12h by 0.5-4.0wt% compound enzyme skin collagen process agent E to get a rough product to be refined, classified and dried to get the pure medical collagen protein powder.
Owner:成都维德医疗器械有限责任公司
Tissue sealant in which collagen and fibrin are mixed, and method for preparing same
InactiveUS20150320904A1High strengthReduce degradationPeptide/protein ingredientsInfusion syringesTissue sealantWeakness
The present invention relates to a tissue sealant in which collagen and fibrin are mixed and to a method for preparing same. To this end, the method of the present invention comprises the steps of: mixing a first material using fibrinogen and aprotinin; mixing a second material using thrombin, calcium chloride, and collagen; and preparing a third material by mixing the first material and the second material. The tissue sealant prepared by the method may supplement weaknesses, i.e. strength and degradability, of the fibrin sealant which is currently available in the commercial marketplace. Further, the tissue sealant of the present invention is cytophilic and activates blood platelets contained in blood so as to induce tissue regeneration. Thus, quality and reliability of products can be significantly improved so as to satisfy various needs of consumers who are users, thereby presenting good image.
Owner:SEWON CELLONTECH CO LTD
Methods and compositions for enhancing collagen, proteoglycan, and glutathione synthesis in the skin
InactiveUS20100160244A1Increase synthesisSustainable hydrationCosmetic preparationsBiocideAdditive ingredientIn vivo
A composition for application to the skin can stimulate the in vivo synthesis of collagen and proteoglycans and improve the appearance of the skin, increasing its elasticity and fullness. In general, a composition according to the present invention comprises: (1) an antioxidant compound in a quantity sufficient to enhance collagen synthesis in the skin; (2) an organic penetrant in which the antioxidant compound is soluble in a sufficient quantity that a concentration of the antioxidant compound sufficient to enhance collagen synthesis can be applied topically and penetrate the skin; (3) a mixture of essential amino acids or hydrolyzed whey protein; (4) a supplemental source of sulfur; and (5) a topical pharmaceutically acceptable carrier. The antioxidant compound can be lipoic acid or a lipoic acid analogue or derivative. The organic penetrant is preferably benzyl alcohol. Other ingredients, such as esters of tocopherol and ascorbic acid, can be included.
Owner:NIMNI MARCEL +1
Method Of Determining The Viability Of At Least One Cell
ActiveUS20140363840A1Microbiological testing/measurementBiological material analysisFlavin adenine dinucleotidePhosphate
This disclosure provides a method of determining the viability of at least one cell via quantification of nicotinamide adenine dinucleotide (phosphate) (NAD(P)H), flavin adenine dinucleotide (FAD), and / or collagen. The method includes the steps of contacting the at least one cell with light having a wavelength of from 700 to 900 nm, using two photon excitation, or from 335 to 400 nm, using one photon excitation, to induce an optical response from the NAD(P)H, FAD, and / or collagen and measuring the optical response. The method also includes the step of quantifying one or more of an amount, spatial localization, and / or time-dependent response of the NAD(P)H, FAD, and / or collagen utilizing the optical response.
Owner:RGT UNIV OF MICHIGAN
Treatment of disease with poly-n-acetylglucosamine nanofibers
InactiveUS20150024014A1Increase contentHigh expressionCosmetic preparationsOrganic active ingredientsFiberDecreased elasticity
Described herein are compositions comprising shortened fibers of poly-N-acetylglucosamine and / or a derivative thereof (“sNAG nanofibers”) and the use of such compositions in the treatment of various diseases, in particular, diseases associated with decreased tensile strength of tissue, decreased elasticity of tissue, increased collagen content or abnormal collagen content in tissue, abnormal alignment of collagen in tissue, and / or increased myofibroblast content in tissue.
Owner:MARINE POLYMER TECH
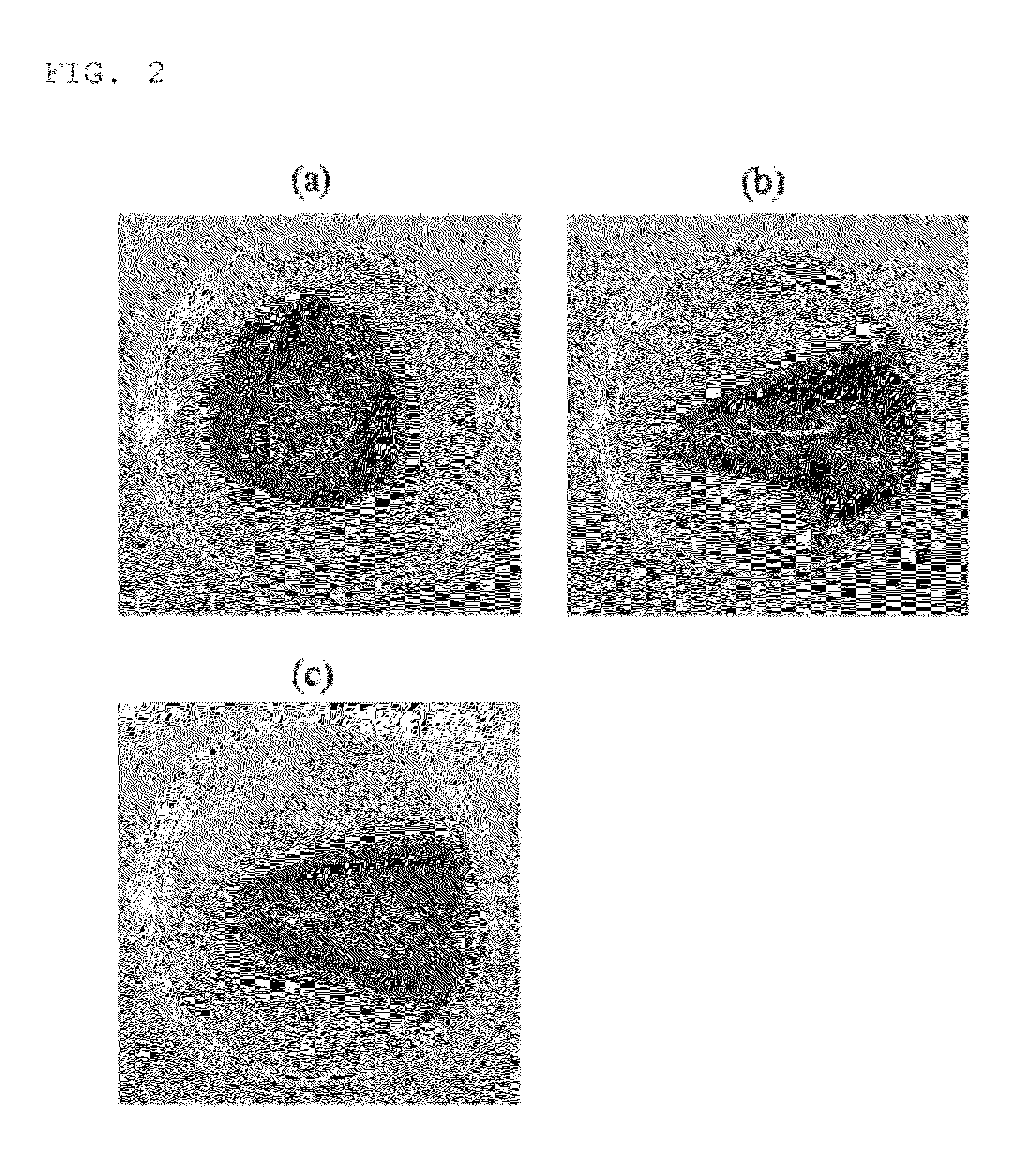
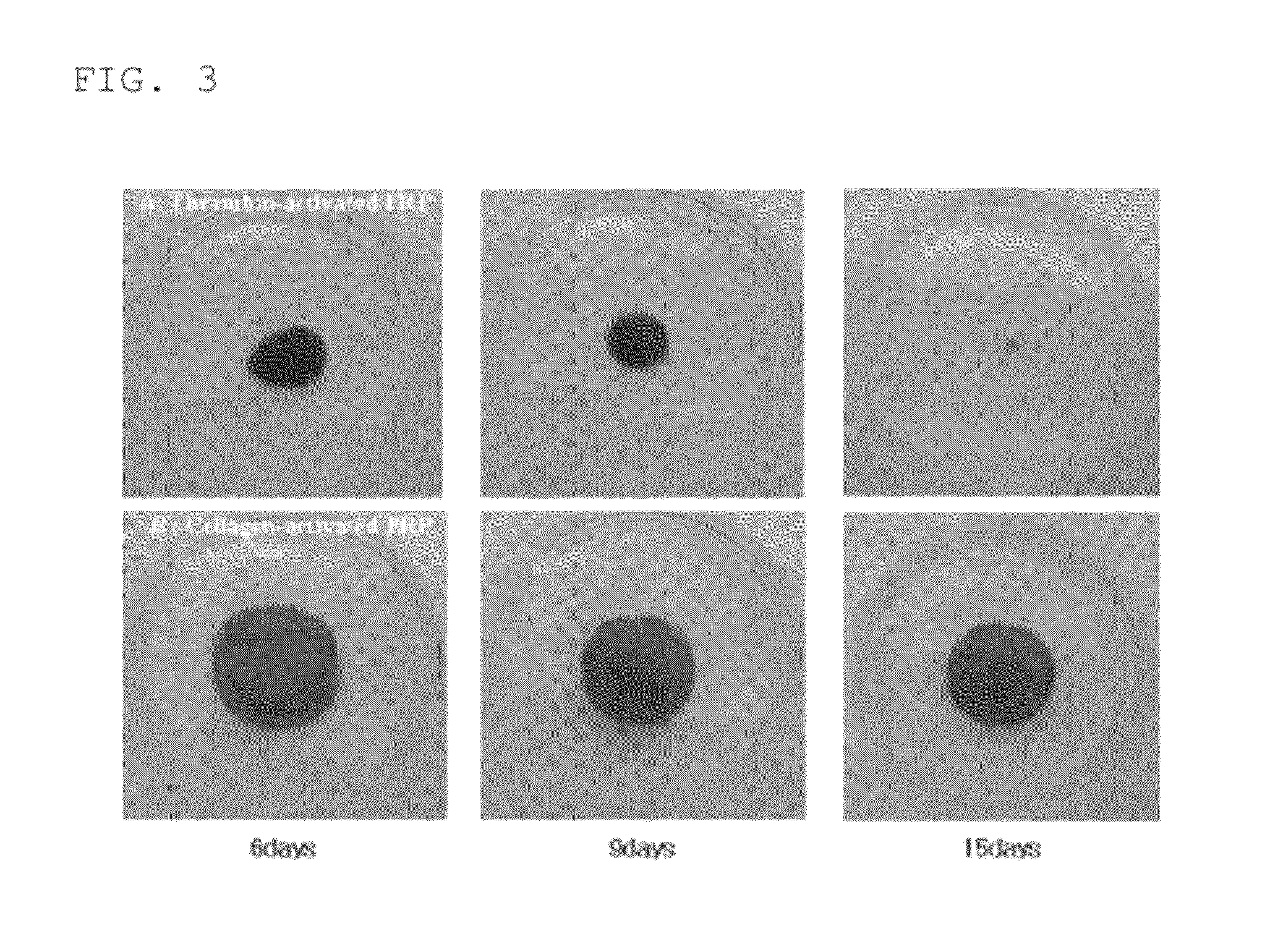
Composition for inducing tissue regeneration by activating platelet-rich plasma (PRP)
ActiveUS9011929B2Improve the problemImprove reactionPeptide/protein ingredientsMammal material medical ingredientsFiberFreeze-drying
The present invention relates to a composition for cartilaginous tissue repair and to a production method therefor. The present invention comprises the steps of: (a) dissolving freeze-dried fibrinogen in an aprotinin solution; (b) dissolving freeze-dried thrombin in a stabilizing solution; (c) mixing an enriched collagen solution with thrombin and the stabilizing solution; and installing the fibrinogen solution (a) to one side of a dual kit and the solution (c) containing the collagen to the other side, and then mixing and injecting into damaged cartilaginous tissue. In the present invention, which is constituted as described above, biomaterials such as collagen and fibrin are mixed so as to allow damaged cartilaginous tissue to be repaired to a state allowing transplantation onto the tissue, and efficient regeneration is induced, thereby making it possible to reduce surgery-related stress on people and animals while inducing relatively rapid and efficient cartilage repair and regeneration.
Owner:CELLONTECH
Anti-glycation facial mask
InactiveCN106691946AMaintain moisture contentHigh glossCosmetic preparationsToilet preparationsWrinkle skinSodium hyaluronate
The invention discloses an anti-glycation facial mask which comprises a mask base fabric (1) and a mask essence (2), wherein the mask essence comprises a water-soluble thickener (a), 0.1-20 wt% of a moisturizer (b), 0.1-10 wt% of an anti-glycation composition (c) and a water-bearing carrier (d); by using the water-soluble thickener, the mask essence has the viscosity of 1-10000 mPa.s; the moisturizer comprises polylol and sodium hyaluronate; and the anti-glycation composition comprises a pea extract and an Argania spinosa leaf extract. The anti-glycation facial mask can effectively inhibit the activities of metal proteinase and collagenase, promotes the synthesis of collagen, glycosaminoglycans (GAGs) and elastin, enables the skin to be tight and elastic and have fewer wrinkles, and thus, has favorable antiaging effects.
Owner:广东创美抗衰老研究有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com