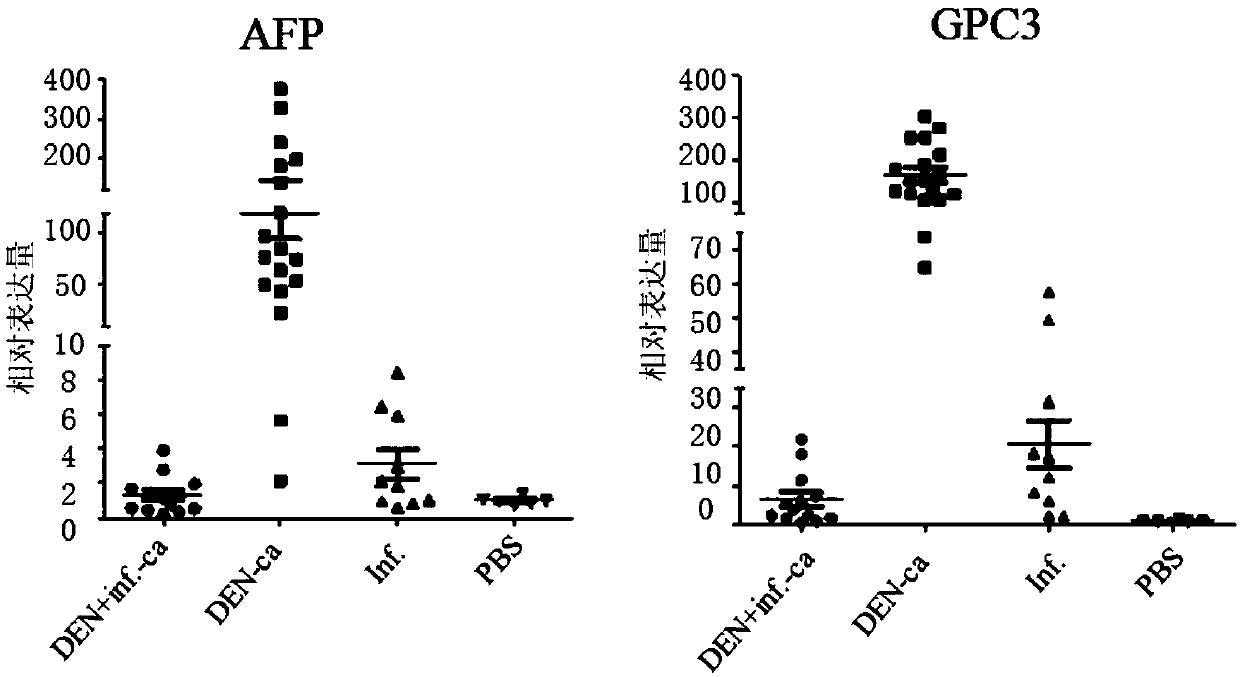
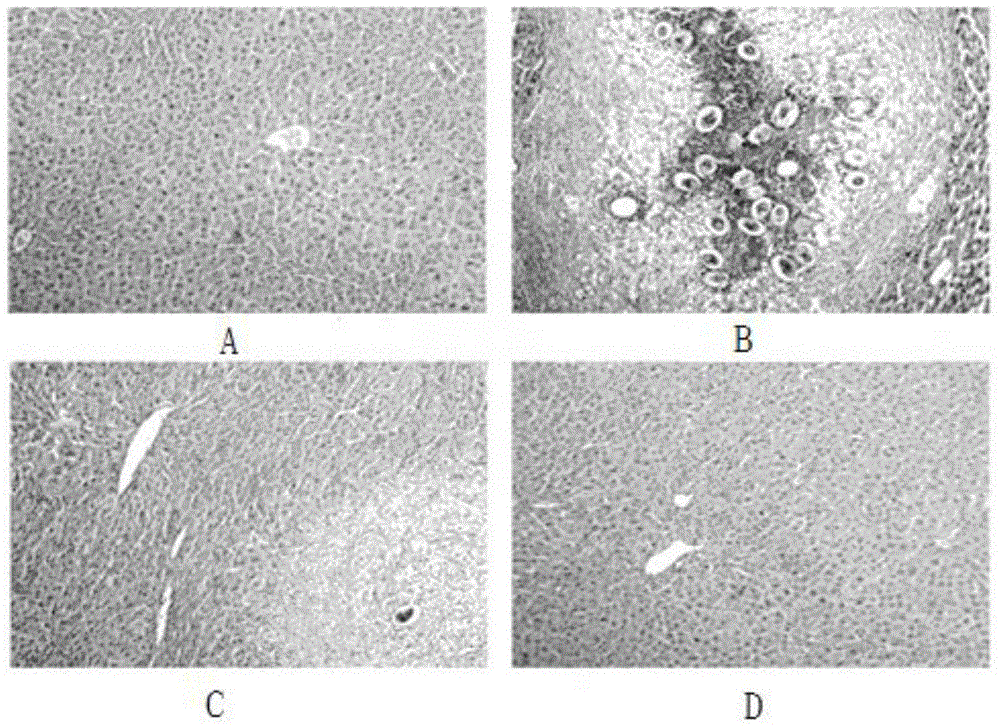
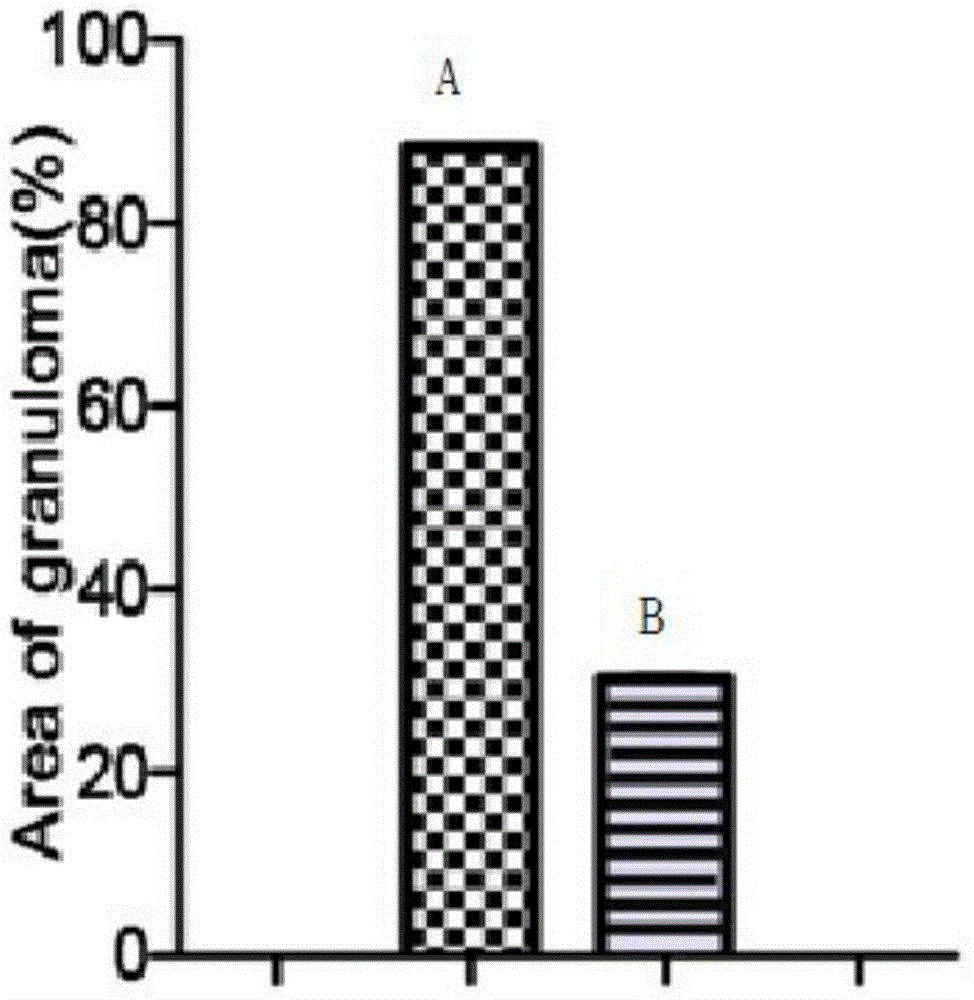
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Blood fluke infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Schistosome infectious oncomelania detection kit and detection method thereof
InactiveCN101457258ASuitable for useSimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceOncomelaniaBiology
A blood fluke infectious oncomelania detection kit and a detection method thereof belongs to the verminosis transmission medium detecting field. The invention provides a kit and a detection method for detecting blood fluke infectious oncomelania based on a loop-mediated isothermal DNA amplification technology (LAMP). According to the LAMP technology principle, six pairs of specific primers for amplifying a DNA fragment between the 20bp and 231bp of the schistosoma japonicum non-long terminal repeated inverse transcription transposon gene (AF412214) are designed. The LAMP method is constructed for detecting blood fluke gene in the body of the infectious oncomelania and the objective for distinguishing the blood fluke infectious oncomelania from the non-infectious oncomelania is reached. Comparing to the conventional blood fluke infectious oncomelania detection method the method of the invention has advantages of easy and fast operation, sensitive and specific without especial equipment and is suitable for bilharziasis controlling personnel use on site.
Owner:JIANGSU INST OF PARASITIC DISEASES
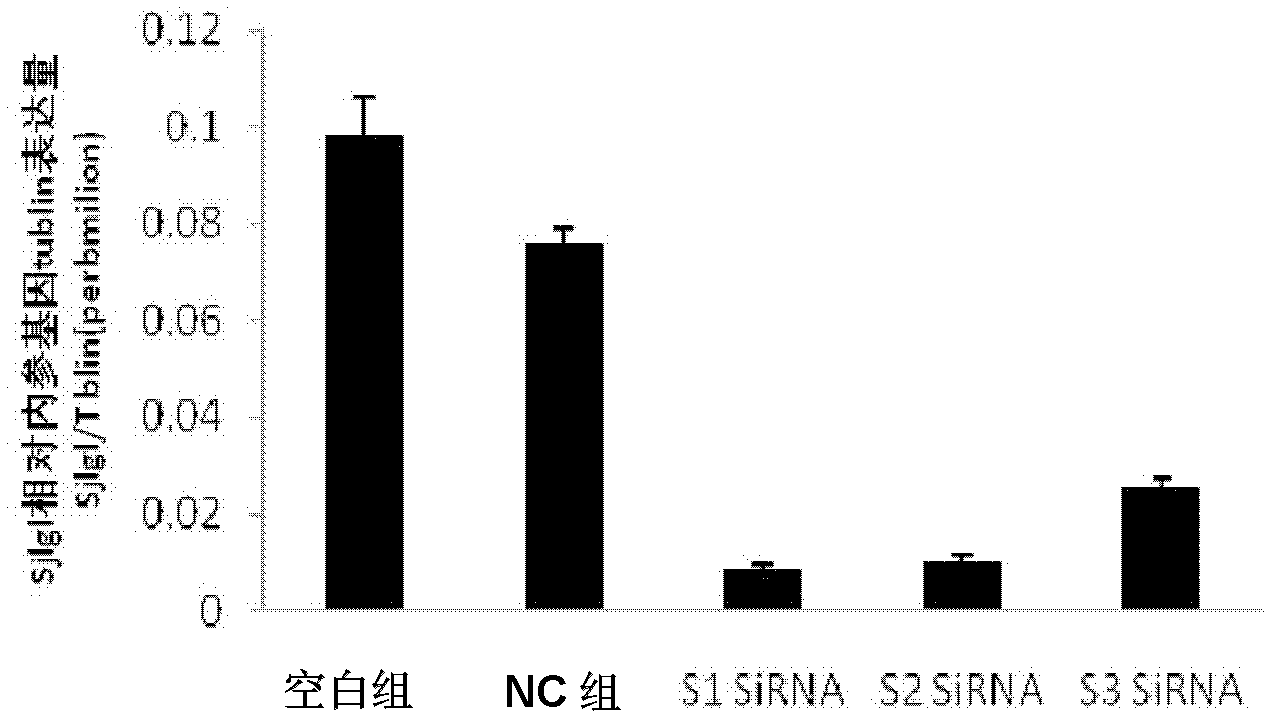
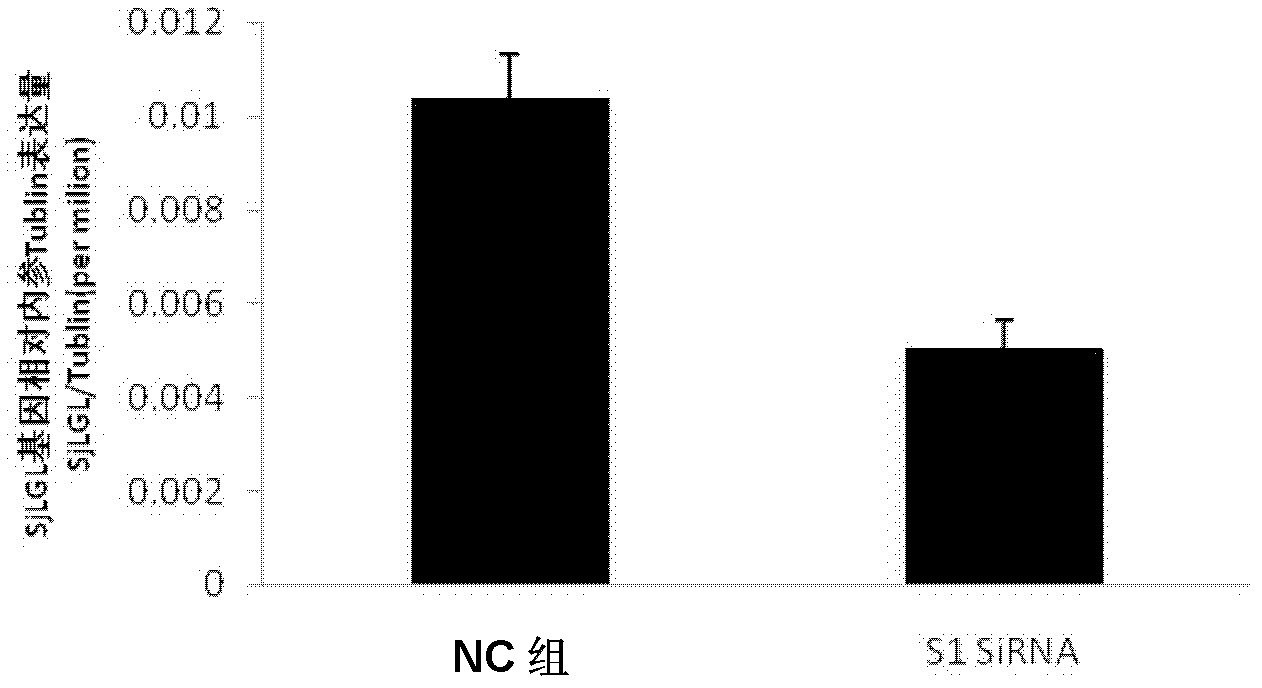
SiRNA of SjLGL gene of schistosoma japonica and use thereof
InactiveCN102220326ALower hatch rateGenetic material ingredientsAntiparasitic agentsNucleotideNucleotide sequencing
The invention discloses the siRNA of SjLGL gene of schistosoma japonica. The siRNA may be one pair or any more than two pairs of nucleotide sequences represented by SEQ ID No.1 and SEQ ID No.2, nucleotide sequences represented by SEQ ID No.3 and SEQ ID No.4, and nucleotide sequences represented by SEQ ID No.5 and SEQ ID No.6. The invention also discloses the use of the siRNA of the SjLGL gene of schistosoma japonica. The siRNA of the SjLGL gene of schistosoma japonica can obviously inhibit the transcription of the SjLGL gene, obviously lower the hatchability of the eggs of insects in livers of mice infected with schistosomes and is suitable for preparing medicines for treating schistosomiasis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
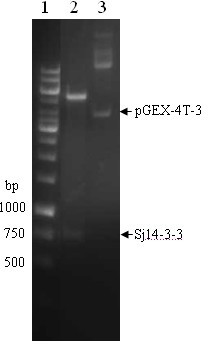
Anti-schistosoma japonicum Sj14-3-3 protein monoclonal antibody and application thereof in immune diagnosis
InactiveCN102180968AImprove accuracyIncrease positive rateImmunoglobulins against animals/humansFermentationProtein.monoclonalFluorescence
The invention discloses an anti-schistosoma japonicum Sj14-3-3 protein monoclonal antibody and application thereof in immune diagnosis, and belongs to the technical field of immune diagnosis of parasitic diseases. The monoclonal antibody against recombinant Sj14-3-3 protein is generated by hybridoma JYQ5C6-1 which has the collection number of CCTCCC201118. The monoclonal antibody against recombinant Sj14-3-3 protein is applied to the detection of an antibody used by schistosoma infected patients or circulating antigen Sj14-3-3 protein in animal blood in schistosoma infected immune diagnosis. The prepared anti-Sj14-3-3 protein molecular specific monoclonal antibody can be used for establishing various immunological diagnosis methods for detecting anti-Sj14-3-3 protein circulating antigens. The time fluorescence resolution method for detecting circulating antigen Sj14-3-3 protein, established by using the anti-Sj14-3-3 protein specific monoclonal antibody, can further improve the accuracy and the positive rate of detecting the schistosoma circulating antigen Sj14-3-3 protein, and has good application prospect.
Owner:JIANGSU INST OF PARASITIC DISEASES
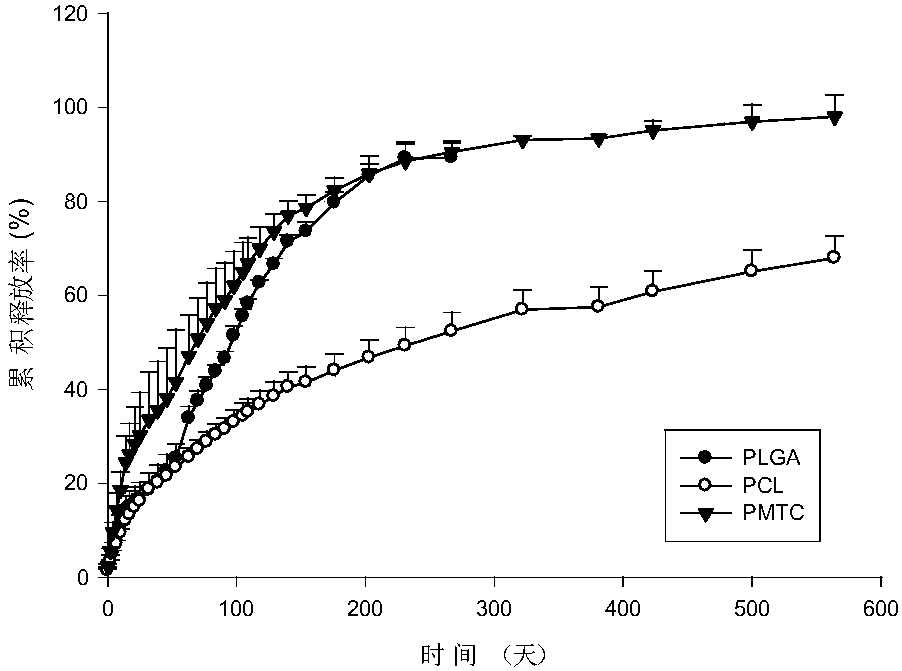
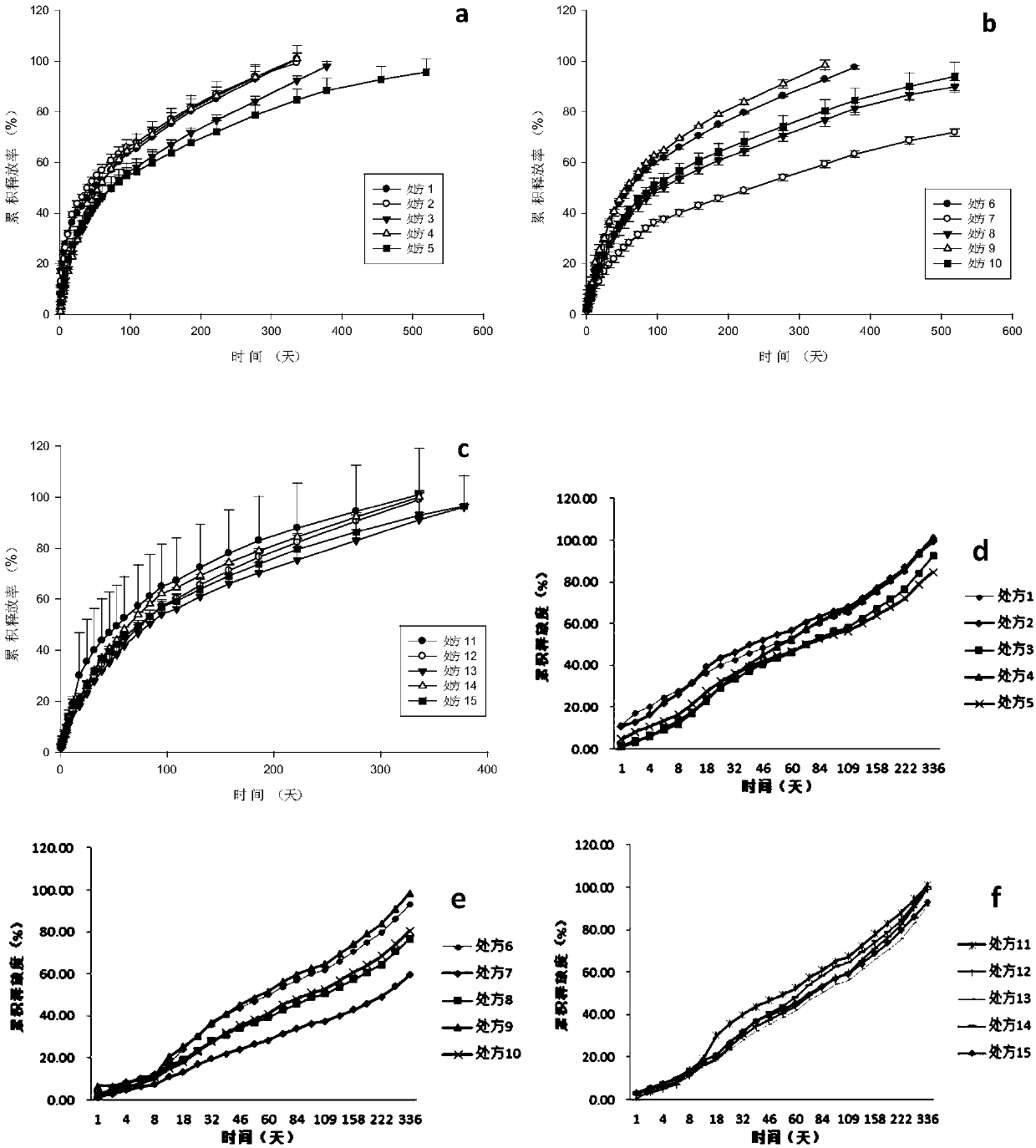
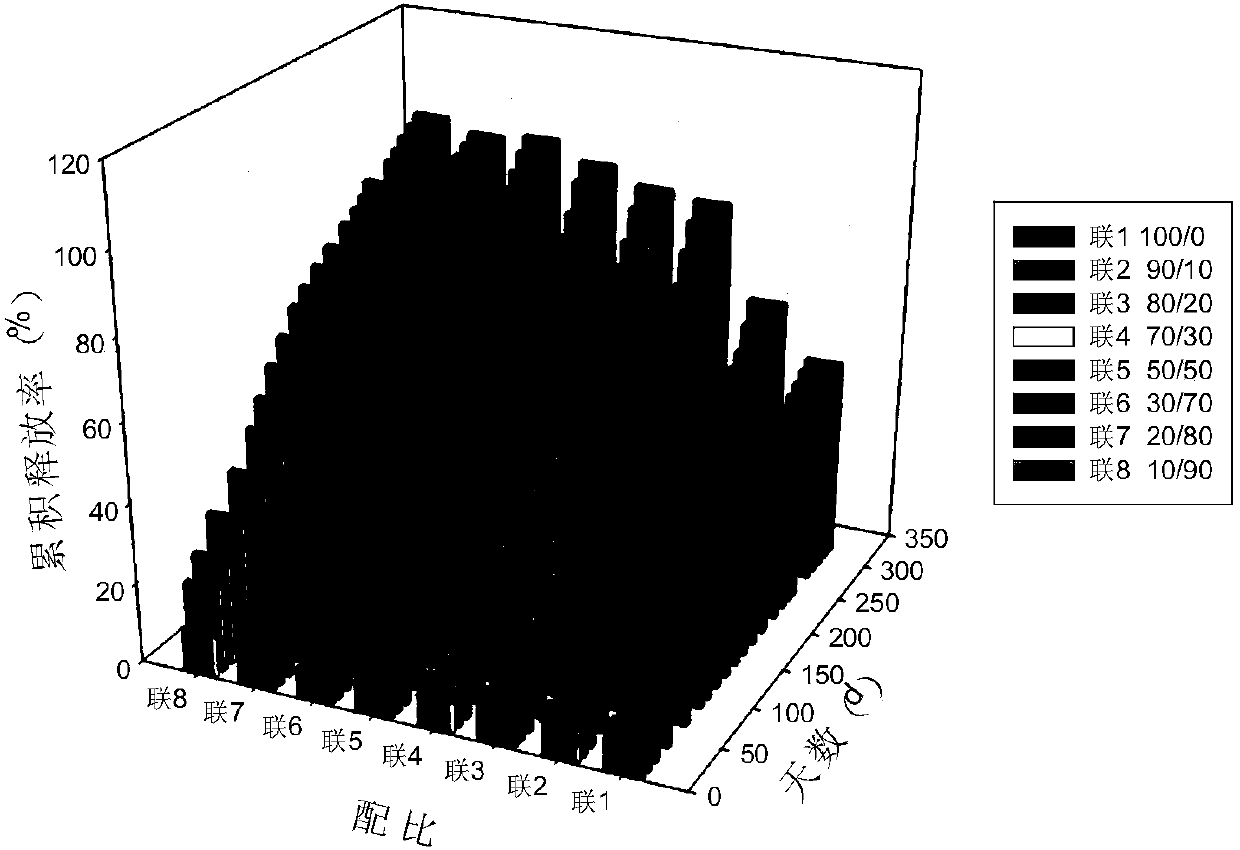
Anti-parasitical medicine in situ setting slow release injection and preparation method thereof
ActiveCN107811967AEasy to prepareImprove stabilityOrganic active ingredientsSolution deliveryAntiparasitic agentAnti parasitic
The invention discloses an anti-parasitical medicine in situ setting slow release injection and a preparation method thereof. The injection comprises the following components in ratio: 1-80g of an anti-parasitic disease medicine, 1-100g of a biodegradable high polymer material, 2-300mL of a dispersion medium and 0-50g of a solubilizer, wherein the content of the anti-parasitic disease medicine is1-800mg / mL. The injection can maintain long-term control effect when being medicated to a medicated object only once, the medicated object is protected from being infected by pathogens, propagation ofparasitic diseases can be economically and effectively controlled, material safety is high, prescription of a commercially available anti-parasitic disease medicine is simplified, compliance of the medicated object is improved, and control cost is reduced. Meanwhile, subcutaneous injection slow release injection is prepared by innovatively using a molluscicide, namely niclosamide, of the WHO, andthe effect of preventing schistosoma japonicum infection is achieved. The anti-parasitical medicine in situ setting slow release injection is simple in the preparation method, has good stability andis easy to use and popularize.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
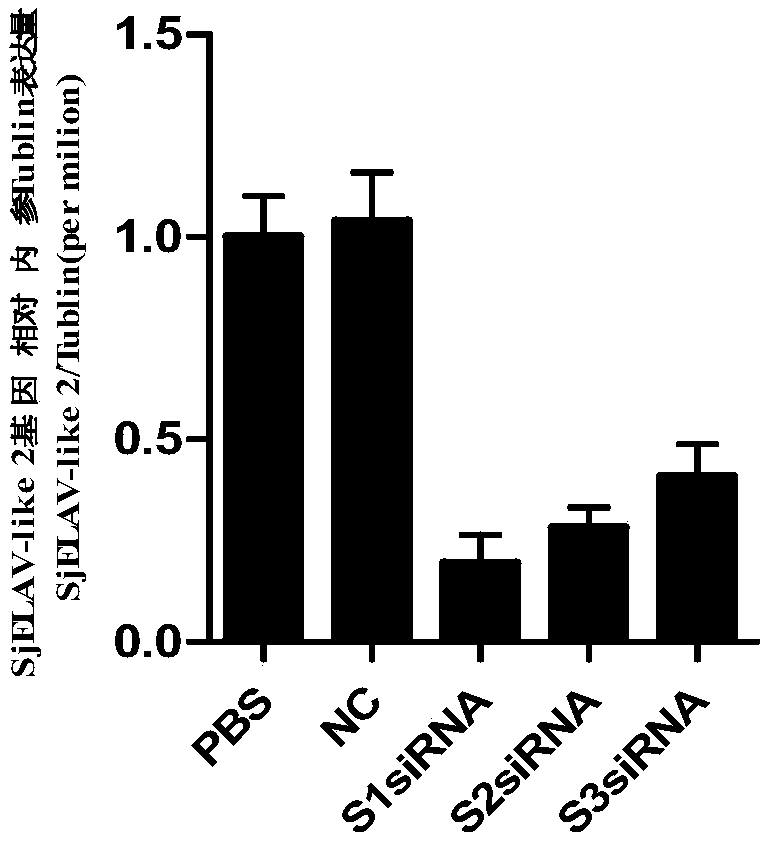
siRNA of schistosoma japonicum SjELAV-like 2 genes and application thereof
ActiveCN108795934ALower hatch rateSuitable for preparationOrganic active ingredientsAntiparasitic agentsDrugBlood fluke infection
The invention discloses siRNA of schistosoma japonicum SjELAV-like 2 genes. The siRNA is selected from one pair of a combination of more than optional two pairs as follows: nucleotide sequences shownas SEQ ID NO.1 and SEQ ID NO.2, nucleotide sequences shown as SEQ ID NO.3 and SEQ ID NO.4, and nucleotide sequences shown as SEQ ID NO.5 and SEQ ID NO.6. The invention further discloses the application of the siRNA of schistosoma japonicum SjELAV-like 2 genes. The siRNA of schistosoma japonicum SjELAV-like 2 genes disclosed by the invention is capable of obviously inhibiting transcription of the schistosoma japonicum SjELAV-like 2 genes and obviously reducing the liver egg hatching rate of schistosomiasis infected mice, and is applicable to preparation of drugs for treating schistosomiasis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
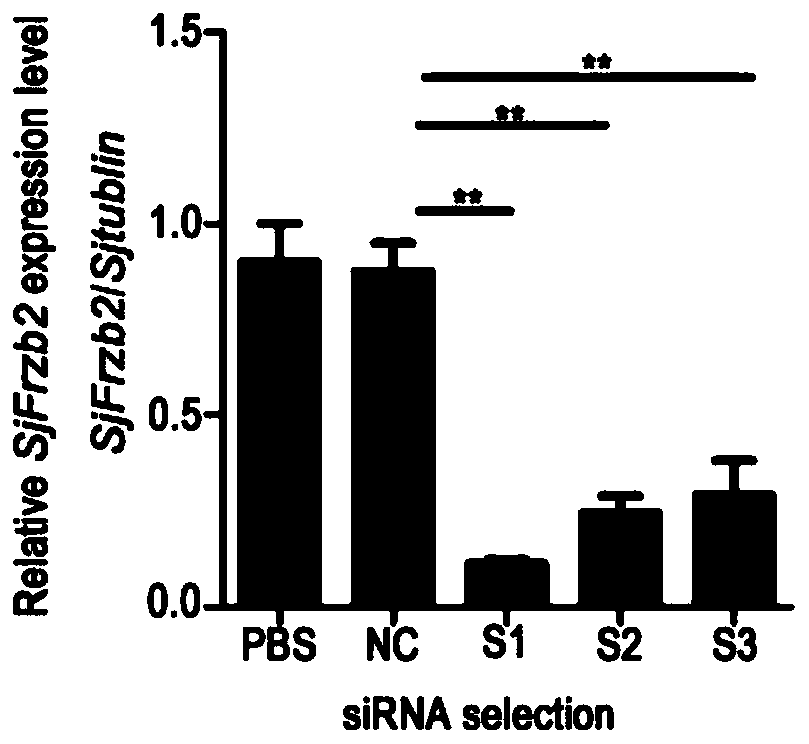
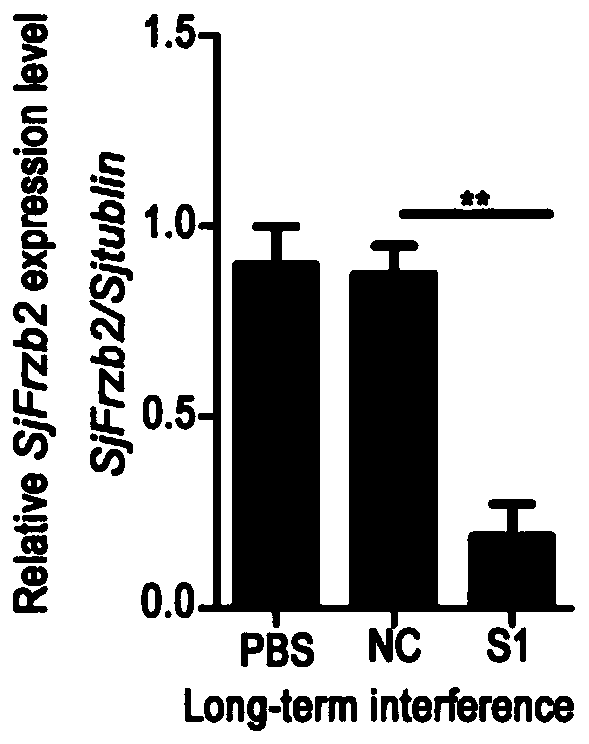
siRNA of schistosoma japonicum katsurada SjFrzb2 gene and application thereof
ActiveCN110564724ALower hatch rateSuitable for preparationOrganic active ingredientsAntiparasitic agentsNucleotide sequencingBiology
The invention discloses siRNA of a schistosoma japonicum katsurada SjFrzb2 gene. The siRNA has nucleotide sequences as shown in SEQ ID NO. 1 and SEQ ID NO. 2. The invention also discloses an application of the siRNA of the schistosoma japonicum SjFrzb2 gene. The siRNA of a schistosoma japonicum SjFrzb2 gene can obviously inhibit the transcription of the SjFrzb2 gene, the insect body number of schistosomiasis infected mice, the number of liver eggs, the liver egg contribution rate and the egg hatching rate of each female insect can be remarkably reduced, a certain pathological influence is alsocaused to the integument structure of the insect and the cell morphology of the gonad, and the product is suitable for being prepared into drugs for preventing and treating schistosomiasis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
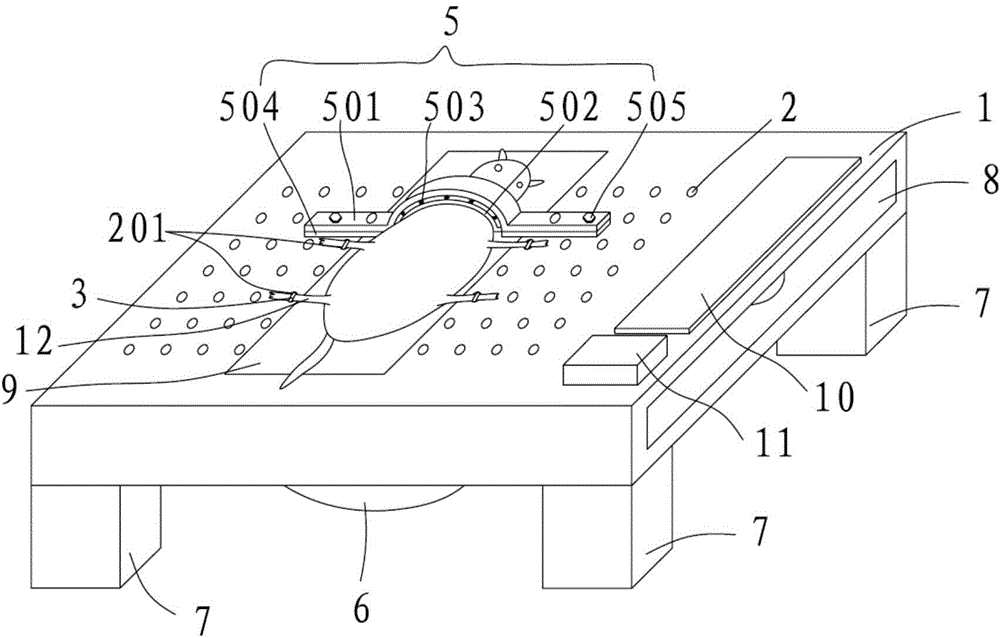
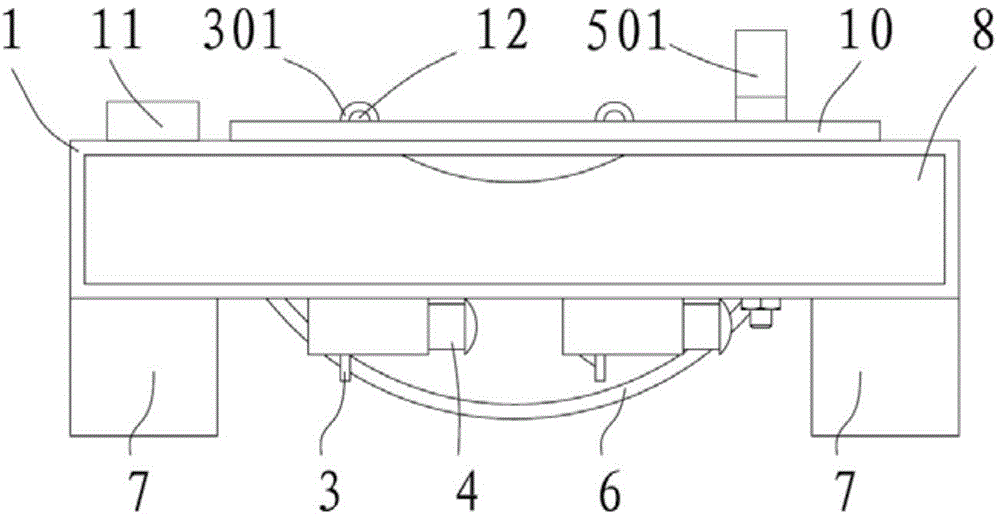
A mouse fixing device for schistosome infection experiments and an experiment method
The invention provides a mouse fixing device for schistosome infection experiments and an experiment method. The mouse fixing device for schistosome infection experiments comprises a base plate; the upper end surface of the base plate is provided with a working area used for placing a mouse; each of the two sides of the working area is provided with a plurality of holes, in which two holes are locating holes; elastic pull bands penetrate through the locating holes; the upper ends of the elastic pull bands are fixed to limbs of the mouse and the other ends penetrate through the locating holes and are fixed to the base plate via limiting members to press the limbs of the mouse to the base plate tightly; the base plate is also provided with a clamp used for fixing and pressing the neck of the mouse to the base plate. The mouse fixing device for schistosome infection experiments is simple in structure, can fix the four limbs and the neck of the mice of different sizes in the experimental process, can prevent the mice from get injured and prevent the mice from biting workers in the experimental process, and enables the workers to record the data of the mice in the schistosome infection experiment process timely and perform timed statistics on the data for progress of later experiments.
Owner:SUZHOU UNIV
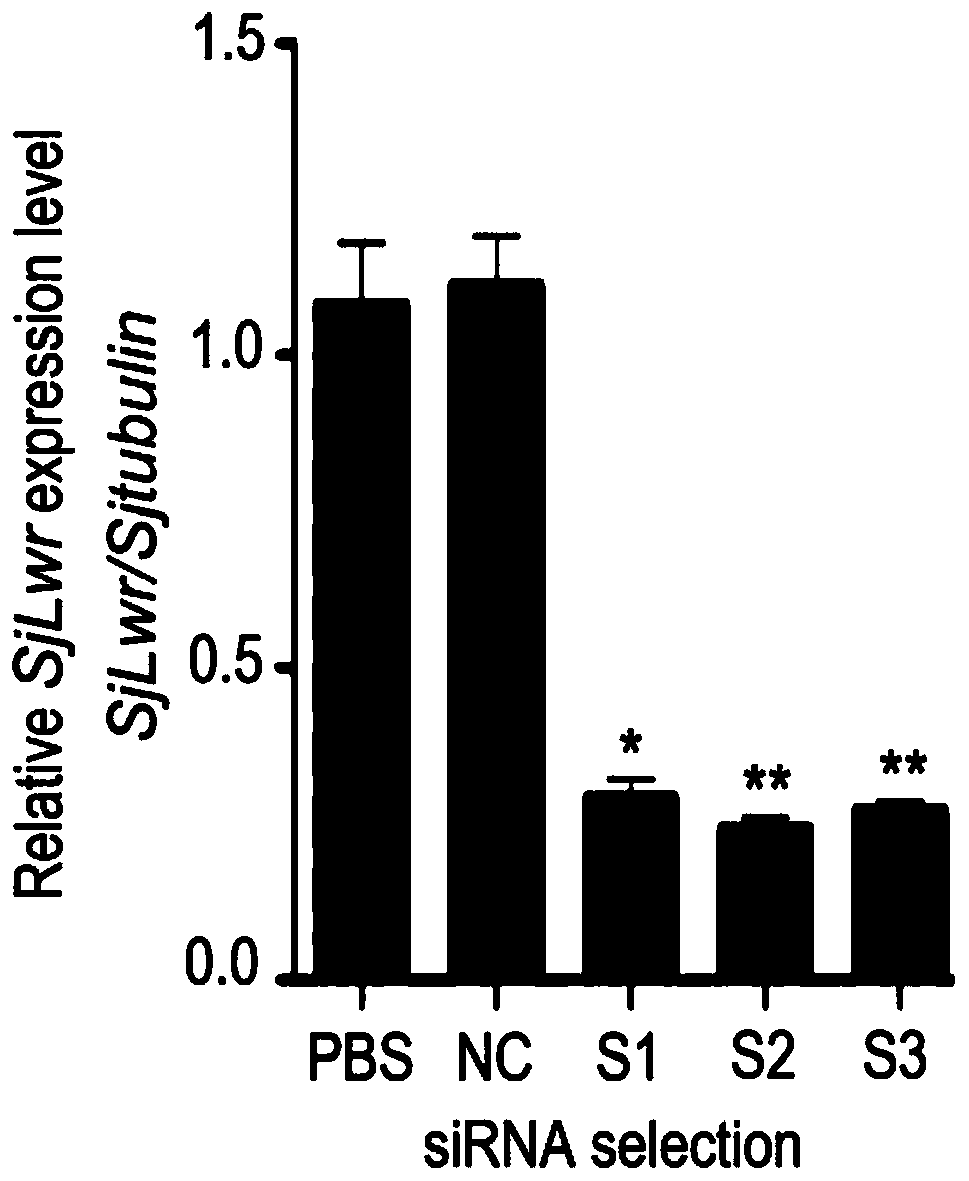
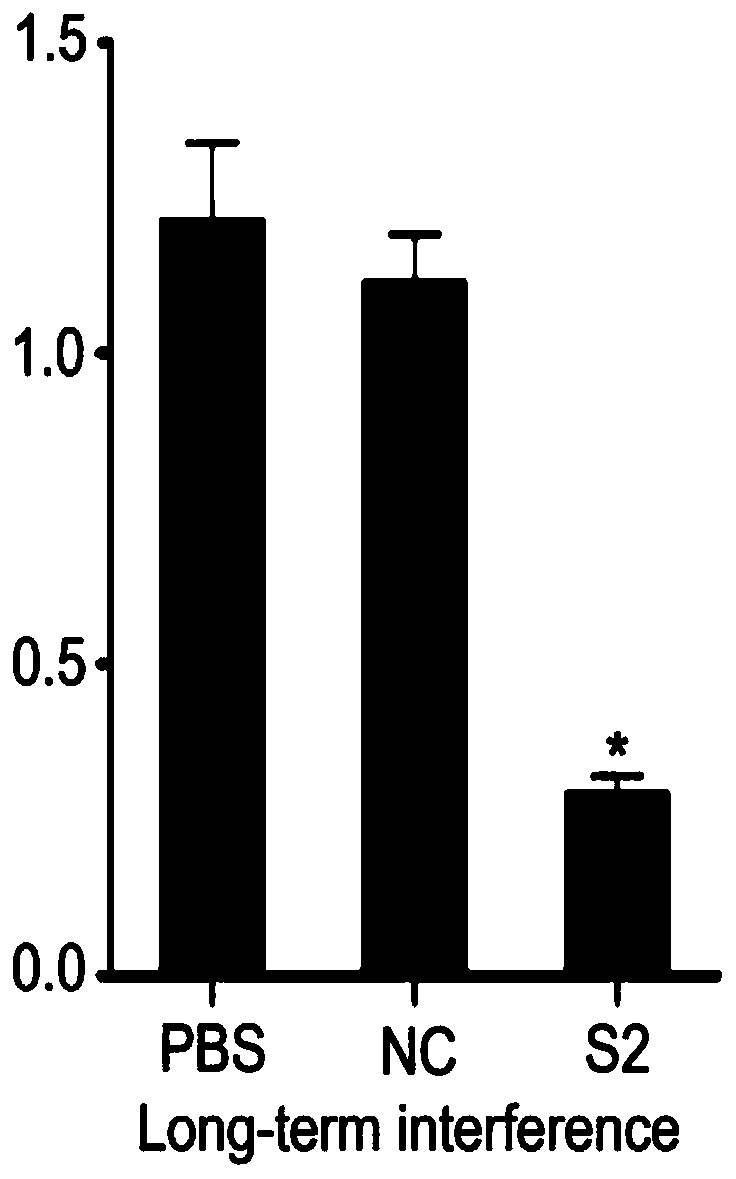
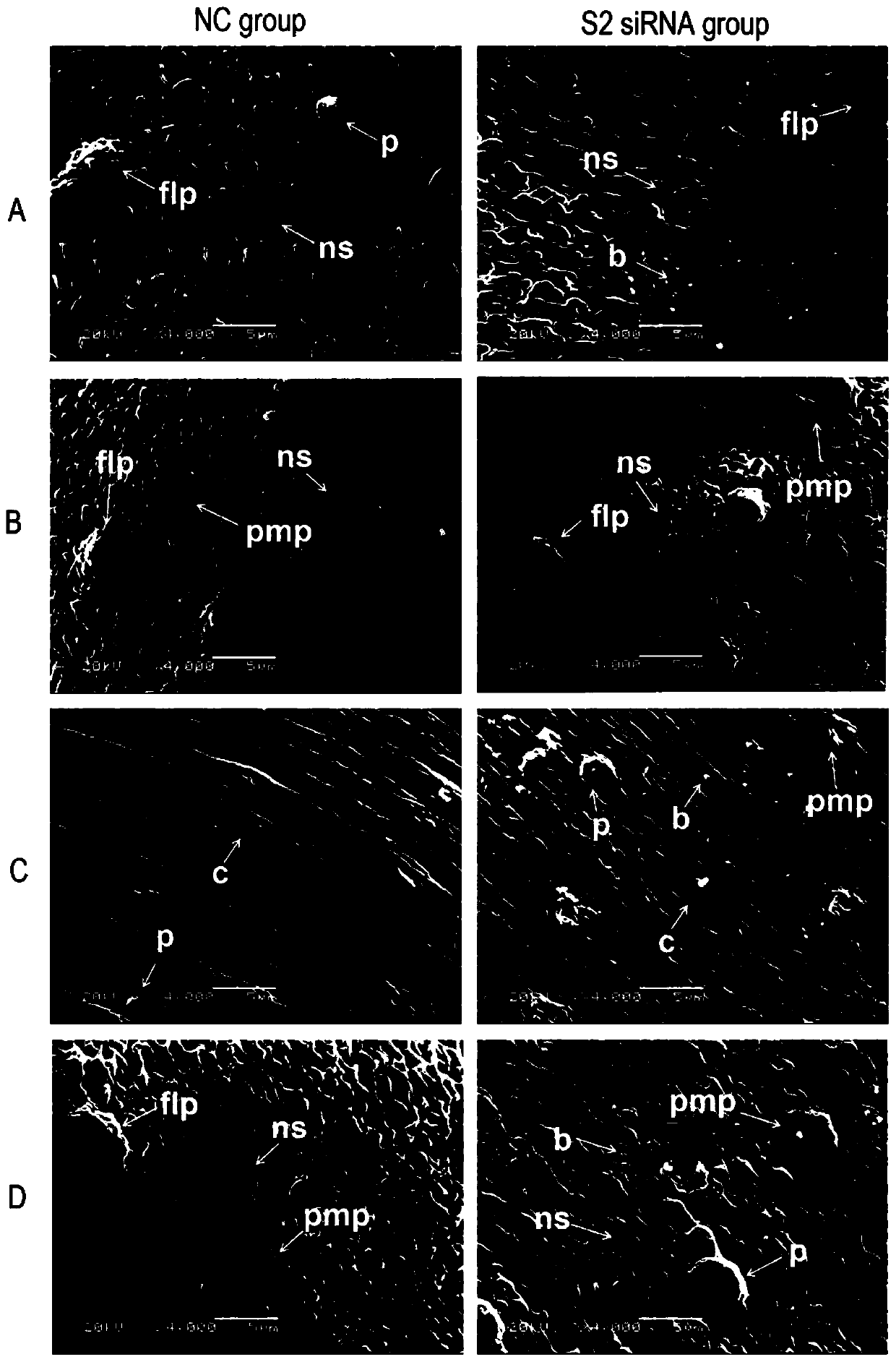
siRNA of schistosoma japonicum katsurada SjLwr gene and application thereof
PendingCN110564725ALower hatch rateOrganic active ingredientsAntiparasitic agentsNucleotide sequencingSchistosoma japonicum
The invention discloses siRNA of a schistosoma japonicum SjLwr gene. The siRNA has nucleotide sequences as shown in SEQ ID NO.1 and SEQ ID NO.2; or nucleotide sequences shown in SEQ ID NO.3 and SEQ IDNO.4, or nucleotide sequences shown in SEQ ID NO. 5 and SEQ ID NO. 6. The invention also discloses an application of the siRNA of the schistosoma japonicum SjLwr gene. The siRNA of the schistosoma japonicum katsurada SjLwr gene can obviously inhibit transcription of SjLwr gene, can obviously reduce schistosome infected mouse insect body number, liver egg number, and liver egg contribution rate and egg hatching rate of each female insect, has certain pathological influence on insect integument structure and gonad cell morphology, and is suitable for preparation of drugs for prevention and treatment of schistosomiasis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Q-PCR primer, identification method and kit for identifying schistosoma japonicum infected oncomelania
ActiveCN104651489AEasy to operateProgrammatic highMicrobiological testing/measurementDNA/RNA fragmentationOncomelaniaAgricultural science
The invention a Q.PCR primer for identifying schistosoma japonicum infected oncomelania, and also provides a method for identifying the schistosoma japonicum infected oncomelania by use of the primer. The PCR primer is reasonably designed according to the special A gene sequence of the schistosoma japonicum, and the PCR primer is combined with reverse transcription, PCR amplification and amplification curve interpretation to identify whether the oncomelania is of positive infection of the schistosoma japonicum; the identification method has the advantages of simple operation, high sequencing, quick identification, accurate result and the like; the defects of high time and labor consumption, poor repeatability, large man-made interference factor and the like of a traditional microscopic dissection and identification method are avoided.
Owner:JIANGSU INST OF PARASITIC DISEASES
Hybridoma for secreting anti-recombinant schistosoma japonica enolase specific monoclonal antibody as well as preparation method and application of hybridoma
InactiveCN104611296AImprove accuracyIncrease positive rateTissue cultureVector-based foreign material introductionMicroorganismElisa method
The invention relates to a hybridoma for secreting an anti-recombinant schistosoma japonica enolase specific monoclonal antibody as well as a preparation method and application of the hybridoma, and belongs to the technical fields of gene engineering, preparation of monoclonal antibodies and immunologic diagnosis. The hybridoma JYG-1 capable of secreting the anti-recombinant schistosoma japonica enolase (Sj Enolase protein) specific monoclonal antibody is preserved in the Common Microbiology Center of CCCCM (China Committee for Culture Collection of Microorganisms) and has the preservation number of CGMCC NO. 9910. The hybridoma JYG-1 can secrete the anti-recombinant schistosoma japonica enolase (Sj Enolase) specific monoclonal antibody JYGENmb-1. The monoclonal antibody JYGENmb-1 can be combined with Sj Enolase protein specificity of schistosoma japonica, a sandwich ELISA method for detecting Sj Enolase protein is provided, and the monoclonal antibody can be applied to establishing various immunological methods for detecting Sj Enolase protein in schistosoma japonica infected human and animal blood and excreting secretion.
Owner:JIANGSU INST OF PARASITIC DISEASES
Semi-automatic mouse fixing apparatus for schistosome infection tests
A semi-automatic mouse fixing apparatus for schistosome infection tests belongs to the technical field of schistosomiasis prevention and treatment and comprises eight distance regulating slots, a mark area, an organic glass sheet, clips, a pin, silica gel adaptable to the human body and an elastic rubber band which are connected mutually. The eight distance regulating slots and the mark area are arranged on the organic glass sheet, and the silica gel adaptable to the human body is attached to the clips. The semi-automatic mouse fixing apparatus for schistosome infection test can fix a mouse quickly while avoiding hurting the mouse, thereby bringing convenience to schistosome infection test on the mouse conveniently and quickly.
Owner:JIANGSU INST OF PARASITIC DISEASES
Metronidazole ethanolamine spray as blood flukedisease preventing and treating medicine for farm cattle and its preparing method
InactiveCN1985813AEasy to prepareEasy to useOrganic active ingredientsAerosol deliveryDiseaseAlcohol
The present invention is niclosamide ethanolamine spray as blood fluke disease preventing and treat medicine for farm cattle and its preparation process. The present invention prepares the niclosamide ethanolamine spray through dissolving niclosamide ethanolamine in 0.5-5 weight portions in cosolvent in 5-10 weight portions, adding penetant in 0.5-2 weight portions and emulsifier in 1-5 weight portions, adding anhydrous alcohol via stirring to 100 weight portions and obtain the niclosamide ethanolamine spray. The niclosamide ethanolamine spray is sprayed to the skin of farm cattle to form one lipophilic protecting medicine layer capable of preventing farm cattle against blood fluke disease in 15-30 days.
Owner:JIANGSU INST OF PARASITIC DISEASES
Floating sentry mouse cage for site test of schistosomiasis infection
InactiveCN102823506APrevent invasionSatisfy flotation requirementsAnimal housingEngineeringBlood fluke infection
The invention relates to a floating sentry mouse cage for site test of schistosomiasis infection, comprising a cage body, wherein the cage body is internally provided with a plurality of air permeable separation plates so that the cage body is divided into a plurality of compartments; the tops of the compartments are movably connected with one-way door covers; two ends of the cage body are fixedly connected with foam floats; and the top of the cage body is connected with a cage pulling rope. The floating sentry mouse cage has the beneficial effects as follows: by means of the design of grid-structure cage body and the compartments, a plurality of mice can be dispersedly placed at fixed points in one test, so that the sample amount is large and the test result is more correct; by means of the design of the foam floats, the floating requirement of the tested cage body is well satisfied and the phenomenon that the cage is sunk and the mice escape is avoided, so that the smooth going of the test is ensured; an antirust lead wire material is applied, so that the product is antirust and durable and then the test cost is saved; and by means of the snap joint design of the door covers, the mice are prevented from escaping and the invasion from water snakes in water to the mice is prevented.
Owner:JIANGSU INST OF PARASITIC DISEASES
Schistosomiasis electrochemistry sensing quick determination kit, detection method and preparation method of kit
ActiveCN103197059AStrong specificityHigh sensitivityMaterial electrochemical variablesAntigenPhosphate
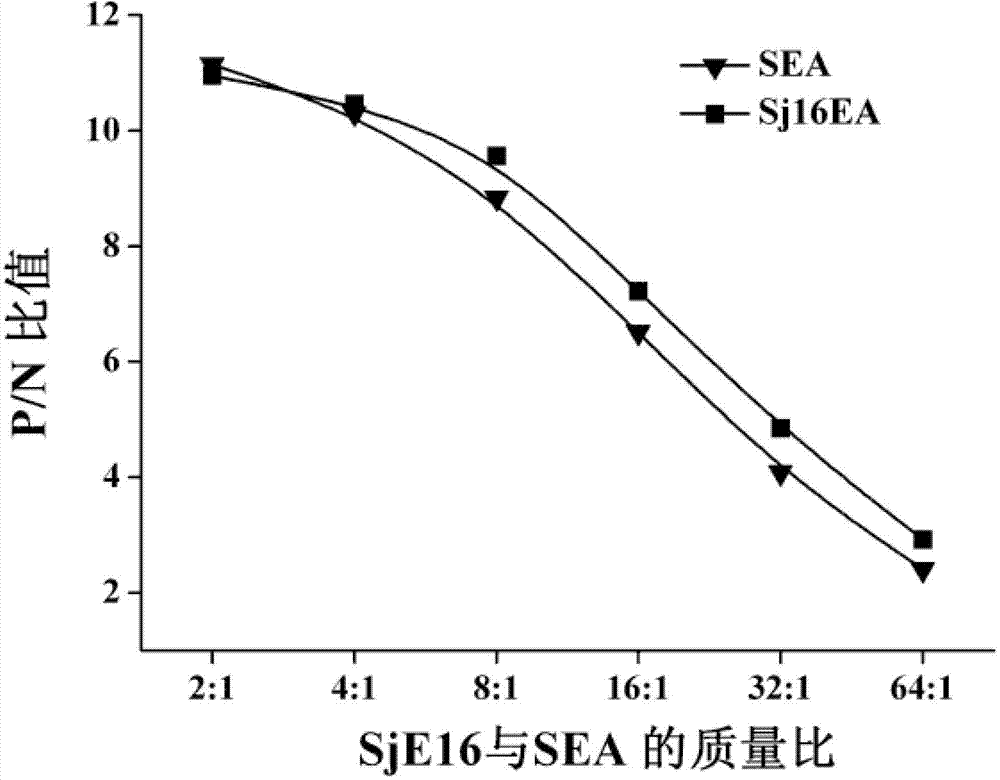
The invention provides a schistosomiasis electrochemistry sensing quick determination kit, a detection method and a preparation method of the kit. The kit comprises an electrochemistry sensing array, an enzyme conjugate operating fluid, an enzyme substrate, a negative comparison product, a positive comparison product, a sample diluent and a scrubbing solution, wherein the electrochemistry sensing array comprises a printing carbon electrode and a surface-co-assembled schistosome antigen layer and an adsorbed sealing agent layer; a schistosome antigen is the mixture of SjE16 and SEA, an enzyme conjugate working solution is a second-antibody BSA (bovine serum albumin) solution containing a horseradish peroxidase mark, and the enzyme substrate is the mixture of tetramethyl benzidine and hydrogen peroxide; and the negative comparison product is normal blood serum, the positive comparison product is schistosome infection blood serum, and the sample diluent and the scrubbing solution are phosphate buffer solutions containing surface active agents. The kit provided by the invention has the advantages that the preparation is simple, the cost is low, the multi-sample analysis is performed, detection sensitivity, specificity and reproducibility are good, and the schistosome electrochemistry sensing quick determination kit and the detection method are hopefully used for screening and monitoring schistosome patients in an affected area.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
Schistosome infectious oncomelania detection kit and detection method thereof
InactiveCN101457258BSuitable for useSimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceOncomelaniaA-DNA
A blood fluke infectious oncomelania detection kit and a detection method thereof belongs to the verminosis transmission medium detecting field. The invention provides a kit and a detection method for detecting blood fluke infectious oncomelania based on a loop-mediated isothermal DNA amplification technology (LAMP). According to the LAMP technology principle, six pairs of specific primers for amplifying a DNA fragment between the 20bp and 231bp of the schistosoma japonicum non-long terminal repeated inverse transcription transposon gene (AF412214) are designed. The LAMP method is constructedfor detecting blood fluke gene in the body of the infectious oncomelania and the objective for distinguishing the blood fluke infectious oncomelania from the non-infectious oncomelania is reached. Comparing to the conventional blood fluke infectious oncomelania detection method the method of the invention has advantages of easy and fast operation, sensitive and specific without especial equipmentand is suitable for bilharziasis controlling personnel use on site.
Owner:JIANGSU INST OF PARASITIC DISEASES
Schistosomiasis vaccine compositions and methods of use
ActiveUS20110091507A1Limiting schistosomiasisImprove educationProtozoa antigen ingredientsPeptidesAntigenDNA construct
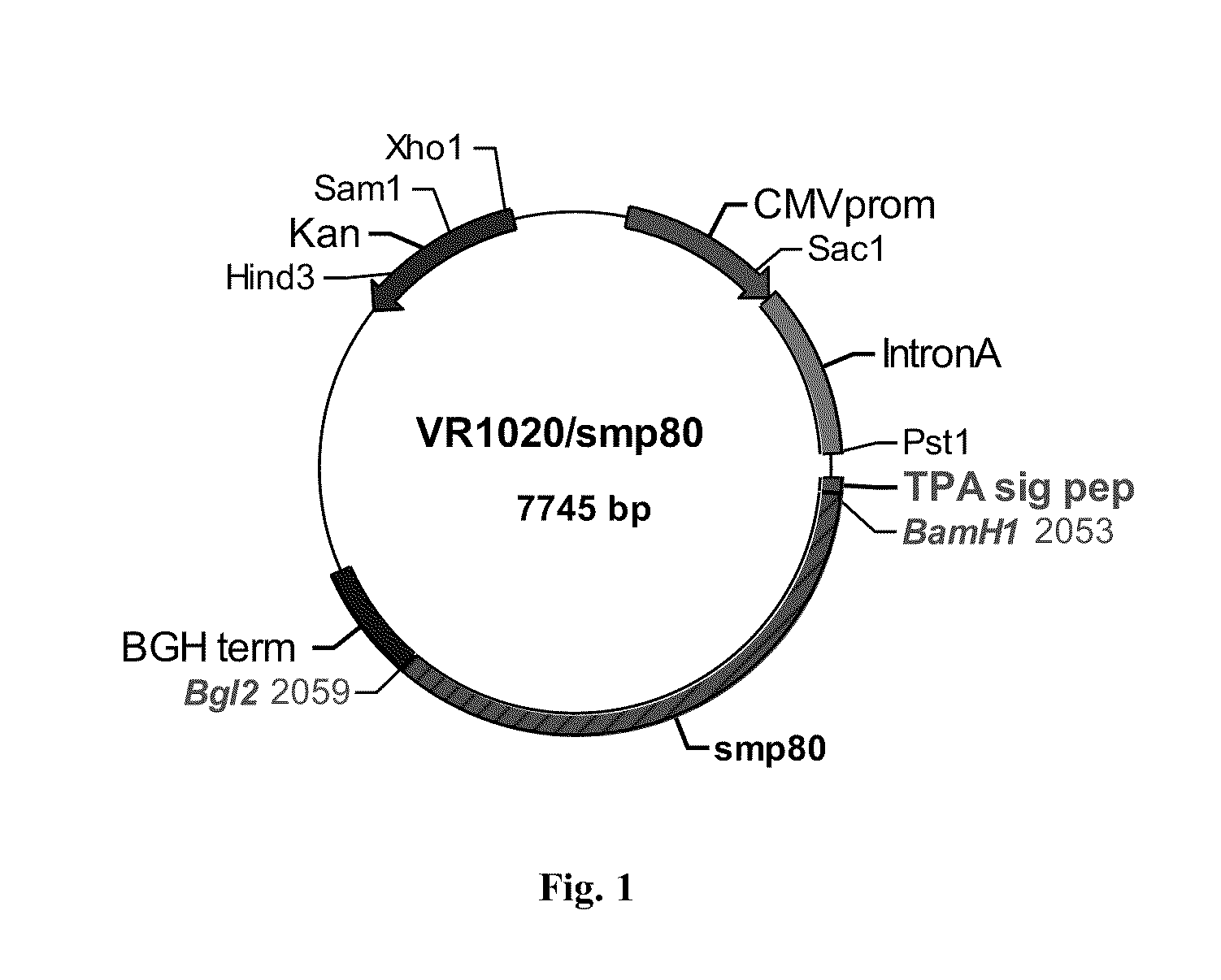
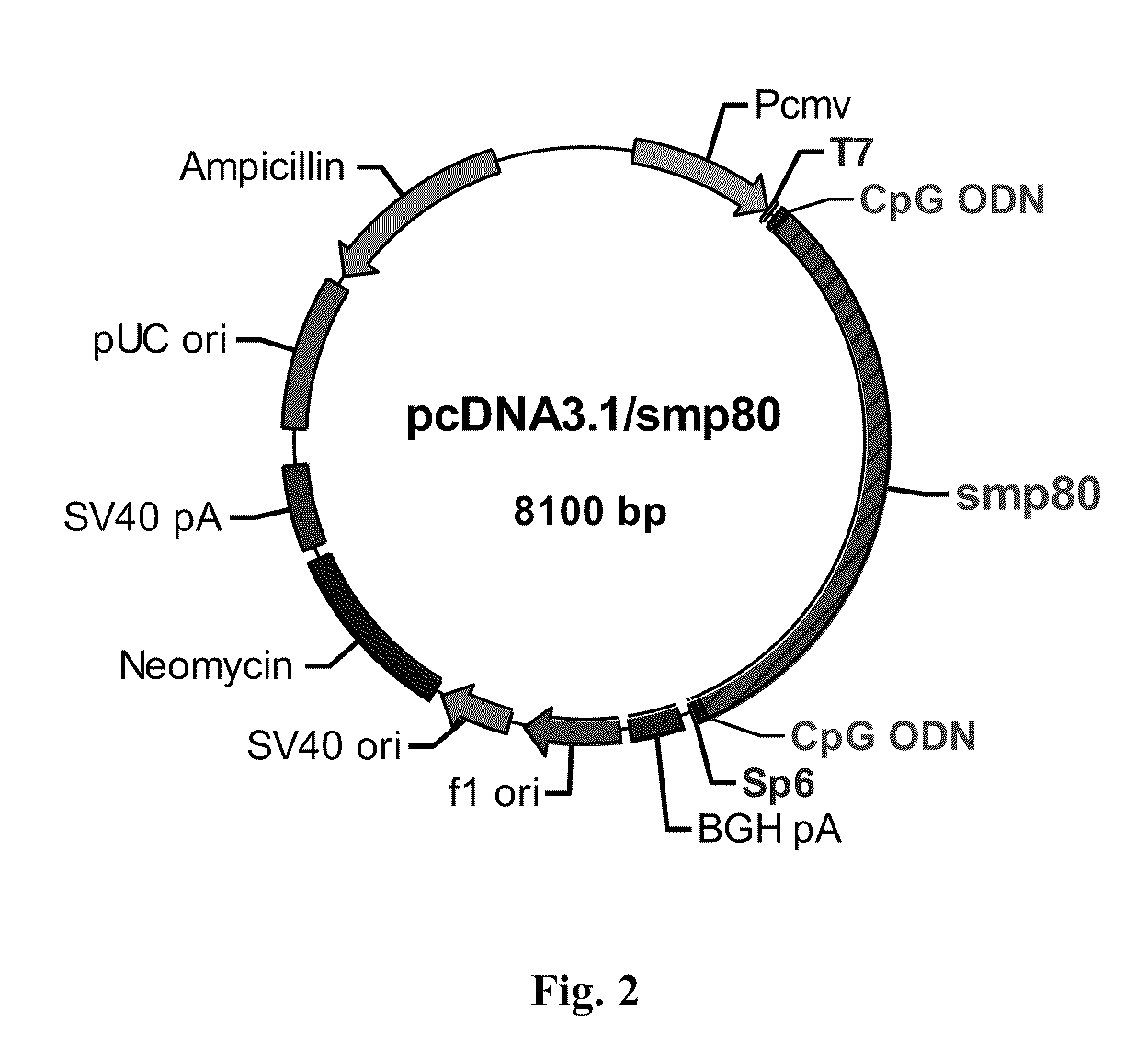
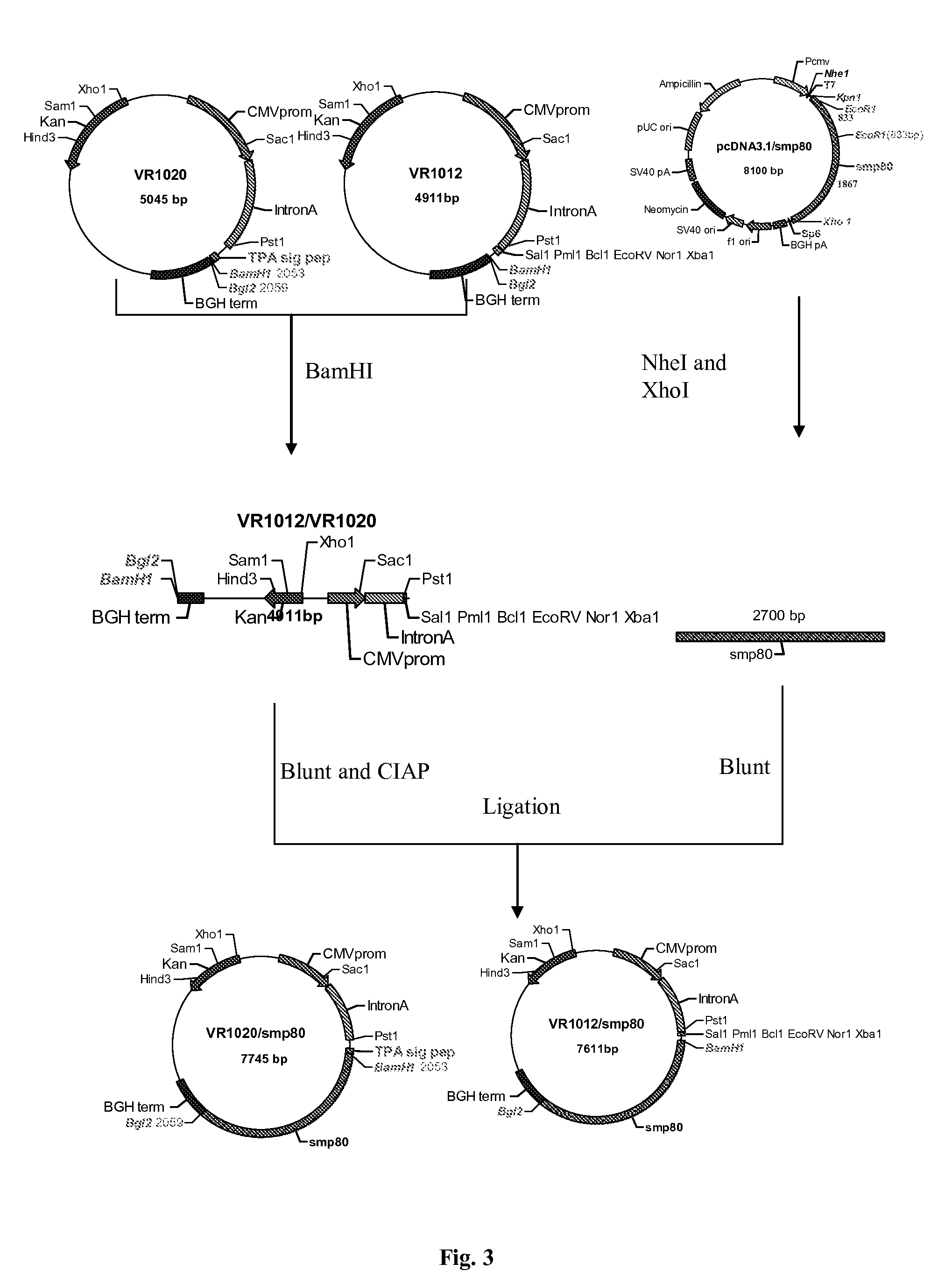
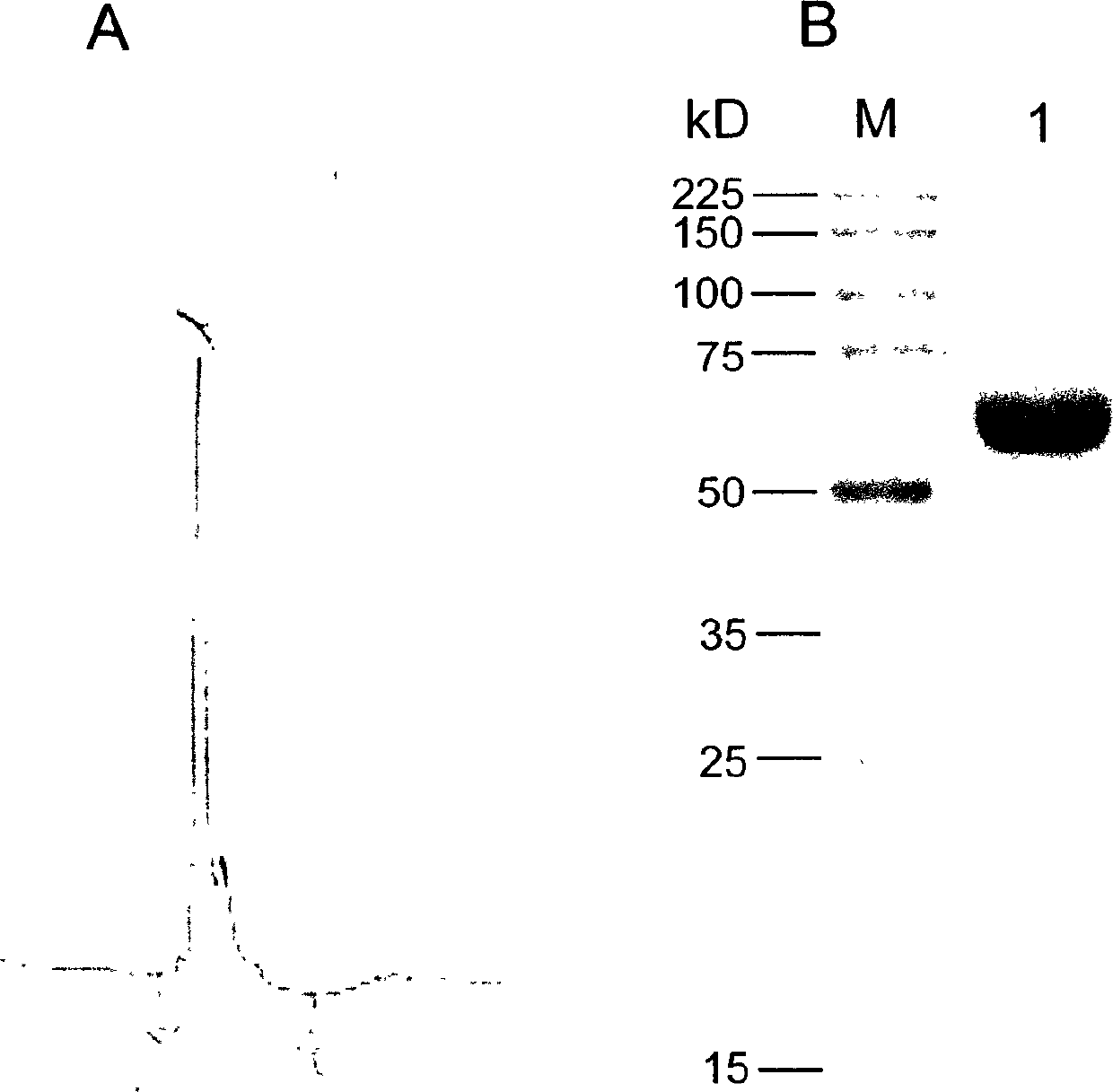
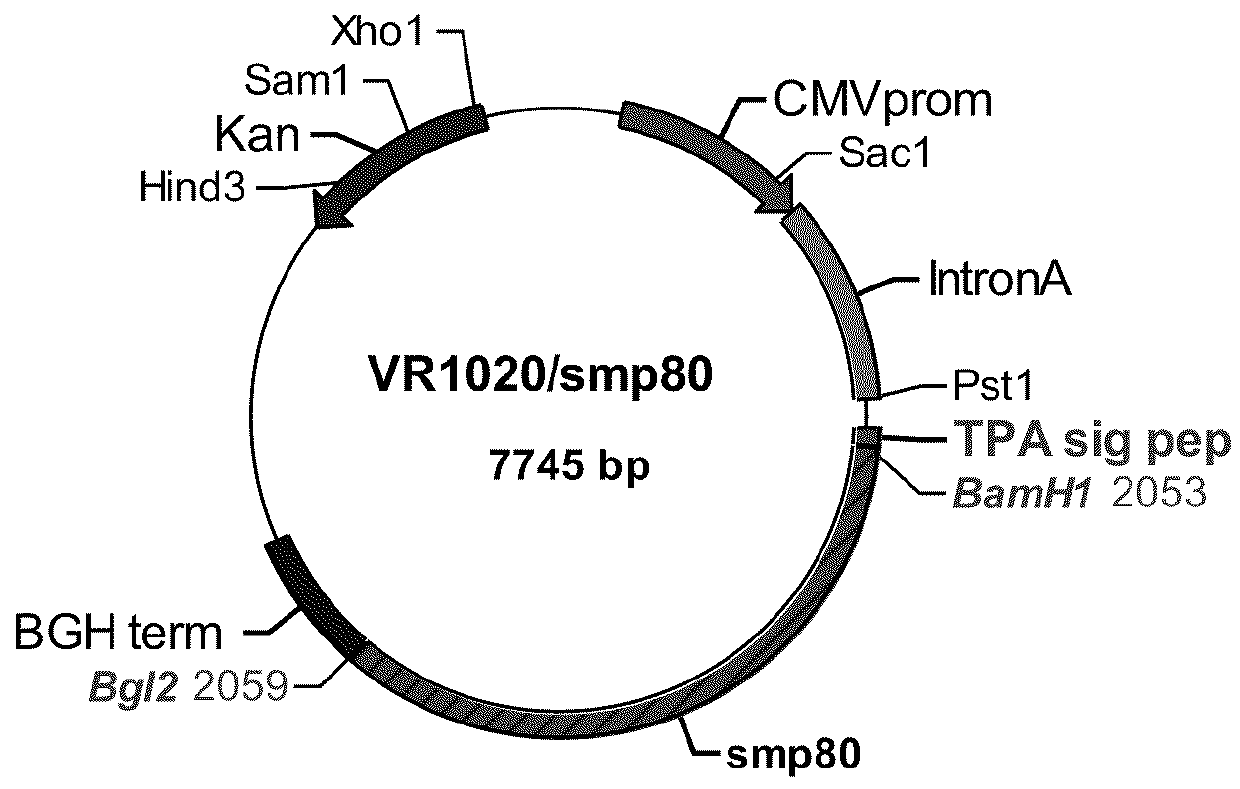
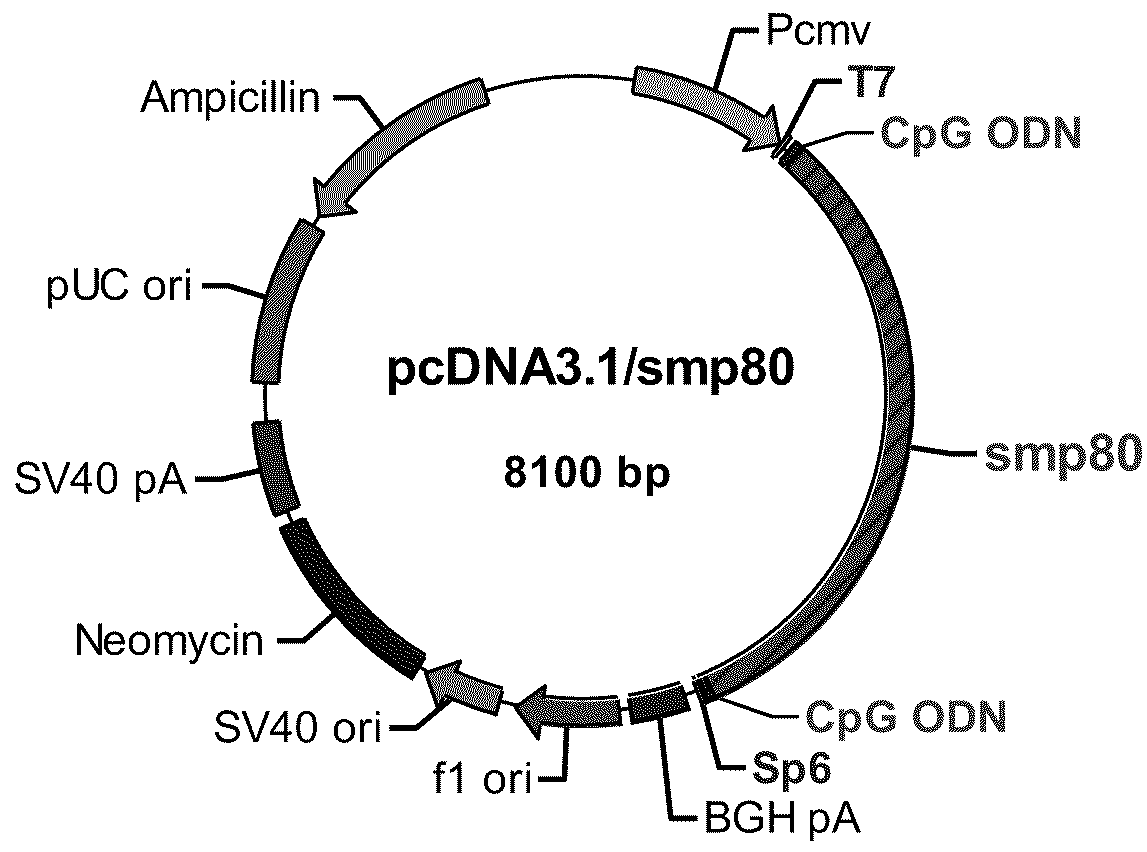
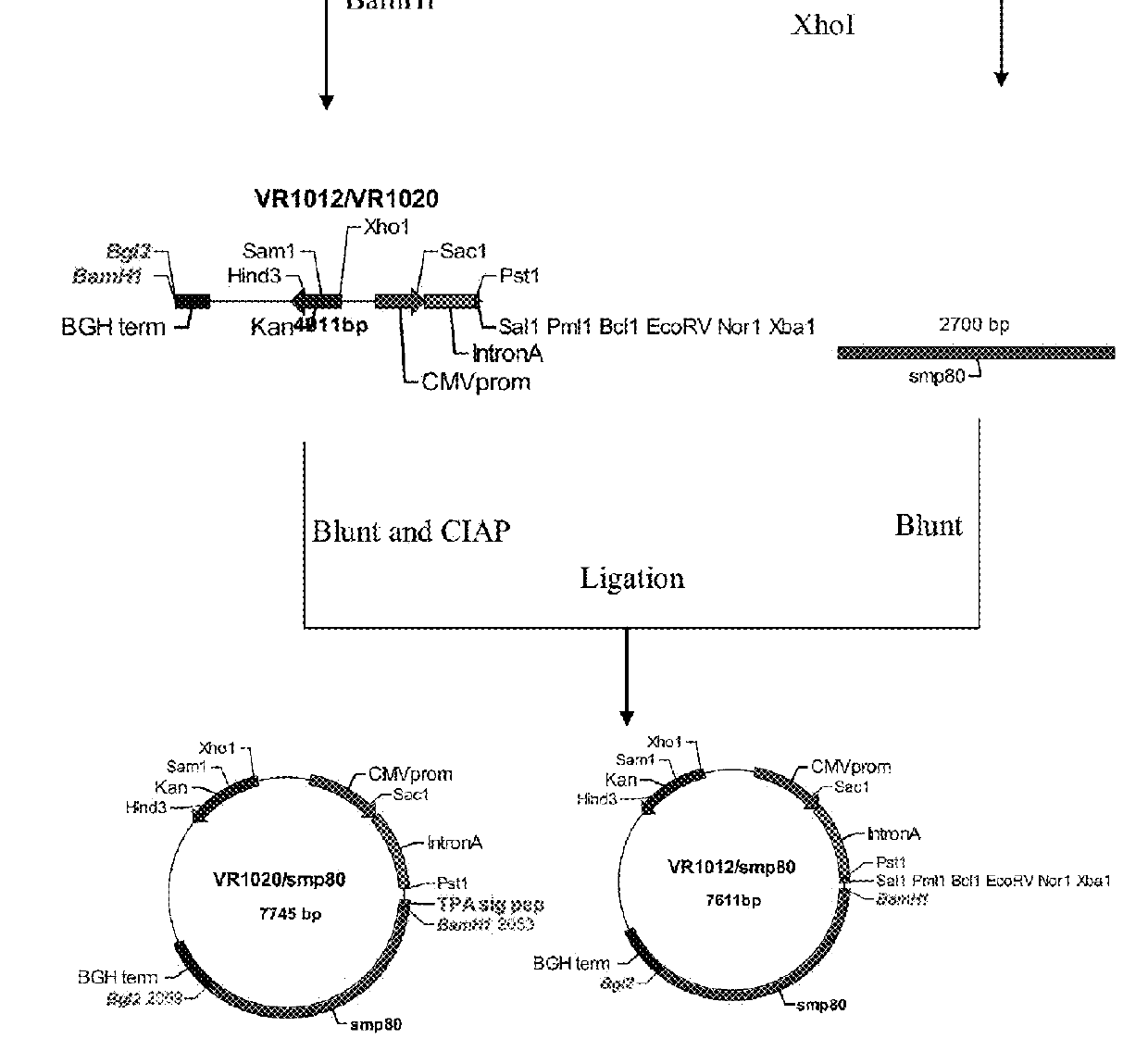
No effective vaccine exists for the devastating parasitic disease of Schistosomiasis. The present invention focuses on Sm-p80, a functionally important antigen of Schistosoma mansoni that plays a pivotal role in the schistosome immune evasion process. When used in a novel vaccine formulation, Sm-p80 demonstrates consistent immunogenicity, protective potential, and antifecundity effects. Two novel DNA constructs were made for immunization purposes. Sm-p80 coding sequence was cloned into VR 1020. Additionally, Sm-p80 coding sequence was cloned into pcDNA3.1 with flanking CpG motifs on each end of the Sm-p80 sequence. When used in different vaccine formulations, both of the constructs demonstrate the superior antifecundity and anti-worm effects of Sm-p80, which has great potential as an important vaccine candidate for the reduction of the morbidity associated with schistosome infection.
Owner:TEXAS TECH UNIV SYST
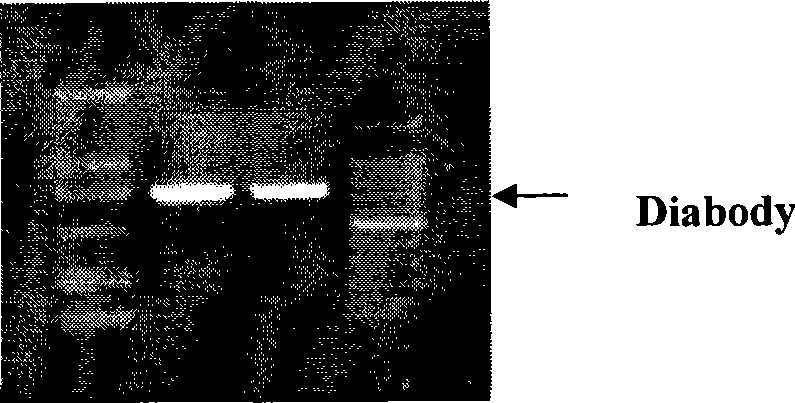
Monoclonal antiidiotypic antibody NP30 single-specificity double-chain antibody of Japanese blood fluke, preparation method and uses thereof
InactiveCN101367876AAlleviate host immune pathological damageInduced allergic reactionImmunoglobulins against animals/humansAntibody ingredientsGenetic engineeringDouble strand
The present invention relates to a schistosoma japonicum monoclonal anti-idiotypic antibody NP30 monospecific diabody and a preparation method and applications thereof., which belong to the field of genetic engineering technology and immunotherapy drug preparation. The preparation method utilizes the genetic engineering technology to prepare the schistosoma japonicum monoclonal anti-idiotypic antibody NP30 monospecific diabody and adopts overlap PCR to amplify diabody gene VH-linker-VL (diabody, D for short), the length of linker chooses five amino acid residues, the sequence is GGGGS, and two scFv molecules are forced to mutually form VH-VL pairing, and bound together by a non-covalent bond to form a dipolymer, which is called diabody. The diabody can be used to treat acute schistosome infection and immunoprotection against schistosomiasis.
Owner:NANJING MEDICAL UNIV
Long-acting artesunate drug for preventing schistosome infection and preparation method thereof
ActiveCN103405779AMaintain insecticidal activityExtended half-lifeOrganic active ingredientsPharmaceutical non-active ingredientsHalf-lifeTherapeutic effect
The invention discloses a long-acting artesunate drug for preventing schistosome infection and a preparation method thereof. According to the method, a low immunogenicity GST-NSP protein is adopted, a chemical coupling method is employed to couple artesunate (AS) having an effect with the GST-NSP protein so as to prepare an (AS) n-GST-NSP conjugate. While maintaining the adolescent schistosome killing activity of the AS, the conjugate prolongs the half-life period of the AS in a host, not only has an early treatment effect on schistosome infection, and also has a function of preventing schistosome infection, thus providing a new means for preventing schistosome infection of human and a variety of reservoir animal hosts, and controlling the epidemics of schistosomiasis. The drug provided in the invention has good application prospects the pharmacy field, including treatment and prevention of schistosomiasis.
Owner:JIANGSU INST OF PARASITIC DISEASES
Application of tea polyphenol and derivatives thereof in prevention of schistosoma japonicum katsurada infection
InactiveCN103768254AAvoid infectionPrevention of Percutaneous InfectionOrganic active ingredientsAntiparasitic agentsCutaneous infectionsPolyphenol
The invention discloses application of tea polyphenol and derivatives thereof in prevention of schistosoma japonicum katsurada infection, and belongs to the field of medical technology and preventive medicine. The tea polyphenol and derivatives thereof can be used for preventing schistosoma japonicum katsurada infection, in particular for destruction of epigallocatechin-3-gallate to the structure of cercaria in affected water, weakening the skin penetrating function of cercaria, and preventing per-cutaneous infection of schistosoma japonicum katsurada.
Owner:JIANGHAN UNIVERSITY
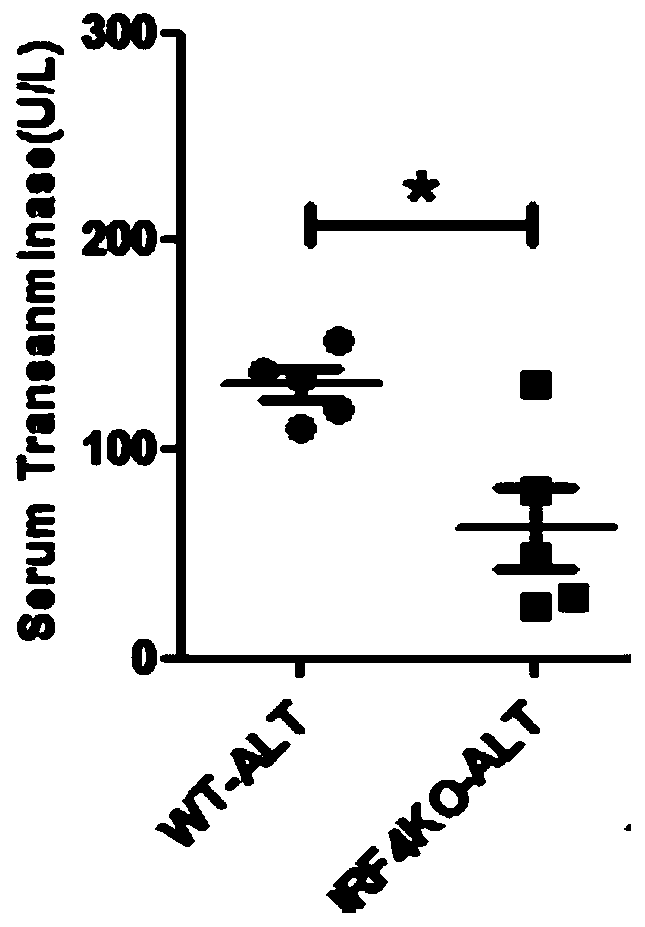
Application of IRF4 gene in resisting schistosoma infection
ActiveCN110227160AImprove liver functionRelieve disease symptomsAntiparasitic agentsPharmaceutical active ingredientsLiver functionBiology
The invention discloses application of an IRF4 gene in resisting schistosoma infection, in particular to application of the IRF4 gene in preparing or screening a medicament for resisting the schistosoma infection. Meanwhile, the invention also discloses a medicament for resisting the schistosoma infection. The medicament comprises an agent capable of inhibiting or silencing the expression of the IRF4 gene. The invention utilizes IRF4 gene knock-out mice and wild mice to carry out experiments, finds that the IRF4 gene deletion can effectively inhibit the formation of liver granuloma of the micewith schistosoma infection, and obviously improves the liver function of the mice with schistosoma infection. The invention provides a new treatment target for resisting the schistosoma infection, provides a new thought for preparing and screening the medicament for resisting the schistosoma infection, and has great medicinal prospect.
Owner:GUANGZHOU MEDICAL UNIV
Preventing and curing Schistosomiasis mansoni by inhibiting Trk receptors on female Schistosoma
Schistosomiasis mansoni is caused by flukes called Schistosoma(es) that enters the human body through the skin in Schistosoma infested waters. The Schistosomes travel from the skin into human blood vessels where they mate, produce antigen containing eggs that travel from the blood vessels into the small intestines, where they are released in the human feces. Male and female Schistosome mates in human blood vessels, male Schistosomes secrete a protein called TGR β protein to the Trk receptor sites on the females Schistosomes membranes. The process stimulates the formation of chemical SmInAct in female Schistosomes, a chemical necessary for the female Schistosomes to produce eggs. This novel technique describes new methods to inhibit Trk receptor sites on female Schistosome membranes using Trk inhibitor agent to prevent TGR β proteins from binding to the Trk receptor sites. Thus, preventing SmInAct from being created in female Schistosomes, preventing production of eggs and Schistosomiasis.
Owner:LANGLOIS RAHME GABRIEL
Schistosoma japonicum chymotrypsin-like protease (SjCTRL) as well as preparation method and application thereof
ActiveCN105153290AAntiparasitic agentsInvertebrate antigen ingredientsChymotrypsin-Like ProteasesEukaryotic plasmids
The invention discloses a Schistosoma japonicum chymotrypsin-like protease (SjCTRL), a protein with an amino acid sequence shown in the SEQ ID NO.2, or a protein which has the same function and is formed through replacement, deletion or insertion of one or more amino acids for the protein. Besides, the invention further discloses a preparation method of the SjCTRL. The preparation method comprises steps such as transmembrane structure removed gene sequence amplification of the SjCTRL, recombinant plasmid construction and identification, induction expression and purification of the SjCTRL and the like, and the immunoprotection of the SjCTRL in anti-schistosoma-infection of mice is evaluated. It is preliminarily verified that the SjCTRL can reduce the number of adults of C57BL / 6 mice infected by Schistosoma japonicum and the number of eggs in livers to a certain extent and can serve as a target of a potential anti-schistosoma vaccine.
Owner:FUDAN UNIV +1
Related gene and protein for preventing and treating Japanese blood fluke infection, and its uses
The invention discloses a protein sequence for killing Japanese Schistosoma and preventing and treating Japanese Schistosoma infection and the corresponding gene sequence and its usage. The protein sequence uses affinity chromatography, polyacrylamide gel electrophoresis; dialysis, freeze drying and protein amino end test sequence to separating obtain the blood serum albumin from oriental hamster unguent. It uses polyase chain reaction technology to obtain oriental hamster blood serum albumin gene sequence. The gene and the decorated mutant insert into the eukaryon expression holder in the animal body, which can be used in preventing and treating the gene of Japanese Schistosoma infection.
Owner:CENT SOUTH UNIV
Schistosomiasis vaccine compositions and methods of use
ActiveUS9248169B2Protozoa antigen ingredientsAntiparasitic agentsDNA constructSchistosomiasis vaccine
No effective vaccine exists for the devastating parasitic disease of Schistosomiasis. The present invention focuses on Sm-p80, a functionally important antigen of Schistosoma mansoni that plays a pivotal role in the schistosome immune evasion process. When used in a novel vaccine formulation, Sm-p80 demonstrates consistent immunogenicity, protective potential, and antifecundity effects. Two novel DNA constructs were made for immunization purposes. Sm-p80 coding sequence was cloned into VR 1020. Additionally, Sm-p80 coding sequence was cloned into pcDNA3.1 with flanking CpG motifs on each end of the Sm-p80 sequence. When used in different vaccine formulations, both of the constructs demonstrate the superior antifecundity and anti-worm effects of Sm-p80, which has great potential as an important vaccine candidate for the reduction of the morbidity associated with schistosome infection.
Owner:TEXAS TECH UNIV SYST
Schistosomiasis electrochemistry sensing quick determination kit, detection method and preparation method of kit
ActiveCN103197059BReduce dosageIncreased sensitivityMaterial electrochemical variablesAntigenPhosphate
The invention provides a schistosomiasis electrochemistry sensing quick determination kit, a detection method and a preparation method of the kit. The kit comprises an electrochemistry sensing array, an enzyme conjugate operating fluid, an enzyme substrate, a negative comparison product, a positive comparison product, a sample diluent and a scrubbing solution, wherein the electrochemistry sensing array comprises a printing carbon electrode and a surface-co-assembled schistosome antigen layer and an adsorbed sealing agent layer; a schistosome antigen is the mixture of SjE16 and SEA, an enzyme conjugate working solution is a second-antibody BSA (bovine serum albumin) solution containing a horseradish peroxidase mark, and the enzyme substrate is the mixture of tetramethyl benzidine and hydrogen peroxide; and the negative comparison product is normal blood serum, the positive comparison product is schistosome infection blood serum, and the sample diluent and the scrubbing solution are phosphate buffer solutions containing surface active agents. The kit provided by the invention has the advantages that the preparation is simple, the cost is low, the multi-sample analysis is performed, detection sensitivity, specificity and reproducibility are good, and the schistosome electrochemistry sensing quick determination kit and the detection method are hopefully used for screening and monitoring schistosome patients in an affected area.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
siRNA of schistosoma japonicum SjELAV-like 1 genes and application thereof
ActiveCN108795935ASuitable for preparationOrganic active ingredientsAntiparasitic agentsNucleotidePlant Germ Cells
The invention discloses siRNA of schistosoma japonicum SjELAV-like 1 genes. The siRNA has nucleotide sequences shown as SEQ ID NO.3 and SEQ ID NO.4. The invention further discloses the application ofthe siRNA of schistosoma japonicum SjELAV-like 1 genes. The siRNA of schistosoma japonicum SjELAV-like 1 genes disclosed by the invention is capable of obviously inhibiting transcription of the schistosoma japonicum SjELAV-like 1 genes and obviously reducing the number of liver loaded eggs and egg hatching rate of schistosomiasis infected mice, causes certain damages to the tegumental structure ofschistosoma and causes morphological abnormality of germ cells, and is applicable to preparation of drugs for treating schistosomiasis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
A kind of pharmaceutical composition for preventing and treating acute schistosomiasis infection
ActiveCN105663134BImprove insecticidal effectMorphologically normalOrganic active ingredientsAntiparasitic agentsCutaneous infectionsTherapeutic effect
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Schistosoma japonicum infection, and application of components of schistosoma japonicum to prevention and treatment of human tumors
InactiveCN109536497AGrowth inhibitionInhibit migrationOrganic active ingredientsMammal material medical ingredientsSchistosoma speciesHuman tumor
The invention relates to the use of schistosoma japonicum infection, and an application of components of schistosoma japonicum to the prevention and treatment of human tumors. The schistosoma japonicum components include but are not limited to eggs, miRNAs, exosomes, and proteins. The present invention shows for the first time that the schistosoma japonicum components have an effect of resisting avariety of host tumors including but not limited to liver cancer. The schistosoma japonicum and the components thereof come into play by inducing host-specific and non-specific immune responses, or modulating tumor-associated genes and signaling pathways, or directly killing tumor cells. The schistosoma japonicum and the components thereof have application value in the prevention and treatment ofhuman tumors.
Owner:TONGJI UNIV
Schistosomiasis protective agent used for cattle, namely niclosamide spraying agent and preparation method thereof
InactiveCN102247323AEasy to prepareEasy to operateOrganic active ingredientsAerosol deliveryAlcoholPhysical well being
The invention relates to a schistosomiasis protective agent used for cattle, namely niclosamide spraying agent and a preparation method thereof, belonging to the technical field of protective agent medicines used for preventing cattle from being infected by schistosomiasis. In parts by mass, 0.5-5 parts of niclosamide is dissolved into 5-20 parts of cosolvent, 0.5-2 parts of penetrant and 1-5 parts of emulsifier are added, then absolute ethyl alcohol is added under the stirring condition to prepare solution, and the total mass of the solution is 100 part, thus the niclosamide spraying agent is obtained. The spraying agent is sprayed onto the skin of cattle to form a medicine protective layer, the cattle can be effectively prevented from being infected by schistosome in 15-30 days, spread of schistosomiasis is controlled, and human health is protected while sustainable development of animal husbandry is promoted. The niclosamide spraying agent provided by the invention is simple in preparation method, is convenient to use and can prevent and control prevalence of schistosomiasis among people while the cattle is protected from being infected by schistosome.
Owner:JIANGSU INST OF PARASITIC DISEASES
Medicine composition for preventing acute schistosome infection
ActiveCN105663134AImprove insecticidal effectMorphologically normalOrganic active ingredientsAntiparasitic agentsCutaneous infectionsTreatment effect
The invention discloses a medicine composition for preventing acute schistosome infection and belongs to the technical field of medicines. The medicine composition is composed of genistein and praziquantel according to the weight ratio of 25-75:500. The medicine composition, compared with the praziquantel, has better treatment effects. The medicine composition can recover normal liver tissue shape, eliminate granuloma, greatly reduce inflammatory cells and ensure normal P65 protein expression. The medicine composition has excellent insecticide effects on schistosoma japonicum and can be used for preventing and treating acute schistosoma japonicum per-cutaneous infection.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com