Medicine composition for preventing acute schistosome infection
A composition and technology of schistosomiasis, applied in the field of medicine, can solve the problems of ineffective worm eggs and poor curative effect, and achieve good insecticidal effect, prevention and treatment of percutaneous infection, and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Effect of the pharmaceutical composition of the present invention on liver morphology in mice with acute Schistosoma japonicum infection.
[0033] Experimental method: get 35 6-8 week old BALB / c male mice and group by random number table, be respectively: normal control group, praziquantel control group (500mg / kg), medicine low dose group of the present invention (dye lignin 25 mg / kg+praziquantel 500 mg / kg), the drug high dose group of the present invention (genistein 75 mg / kg+praziquantel 500 mg / kg). Except for the mice in the normal control group, the mice in the other groups were infected with 18±2 cercariae by the abdominal application method, and at the fifth week after the infection of Schistosoma japonicum, the normal group was given normal saline, and the rest of the groups were given 500 mg / kg praquine After two days of ketone, the corresponding dose of genistein was administered, and the mice were treated at the sixth week, and the liver was collecte...
Embodiment 2
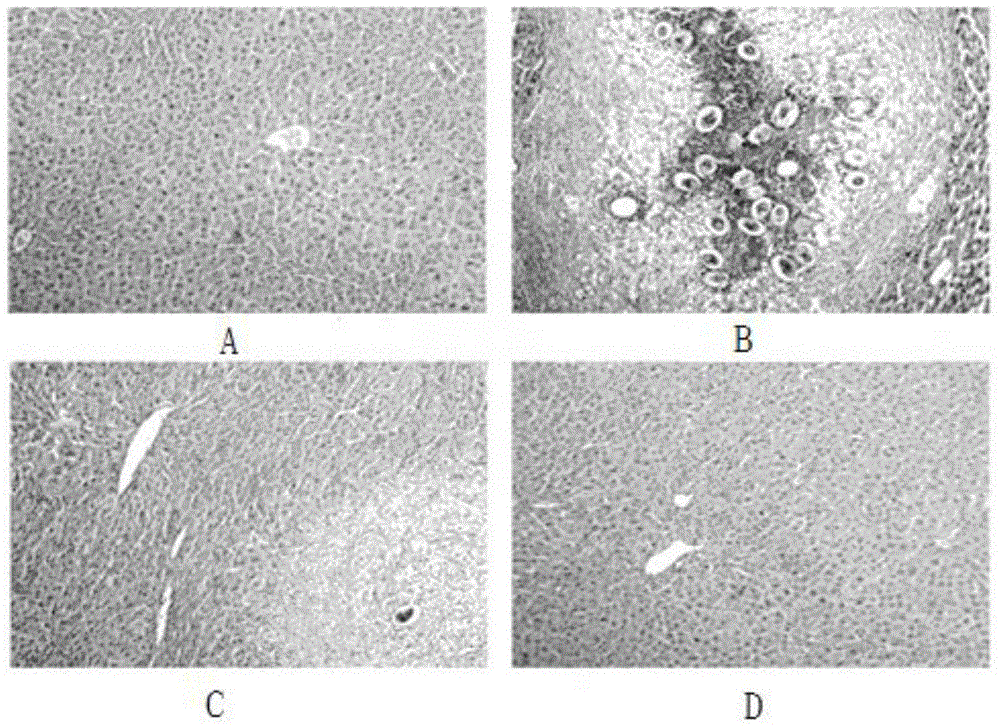
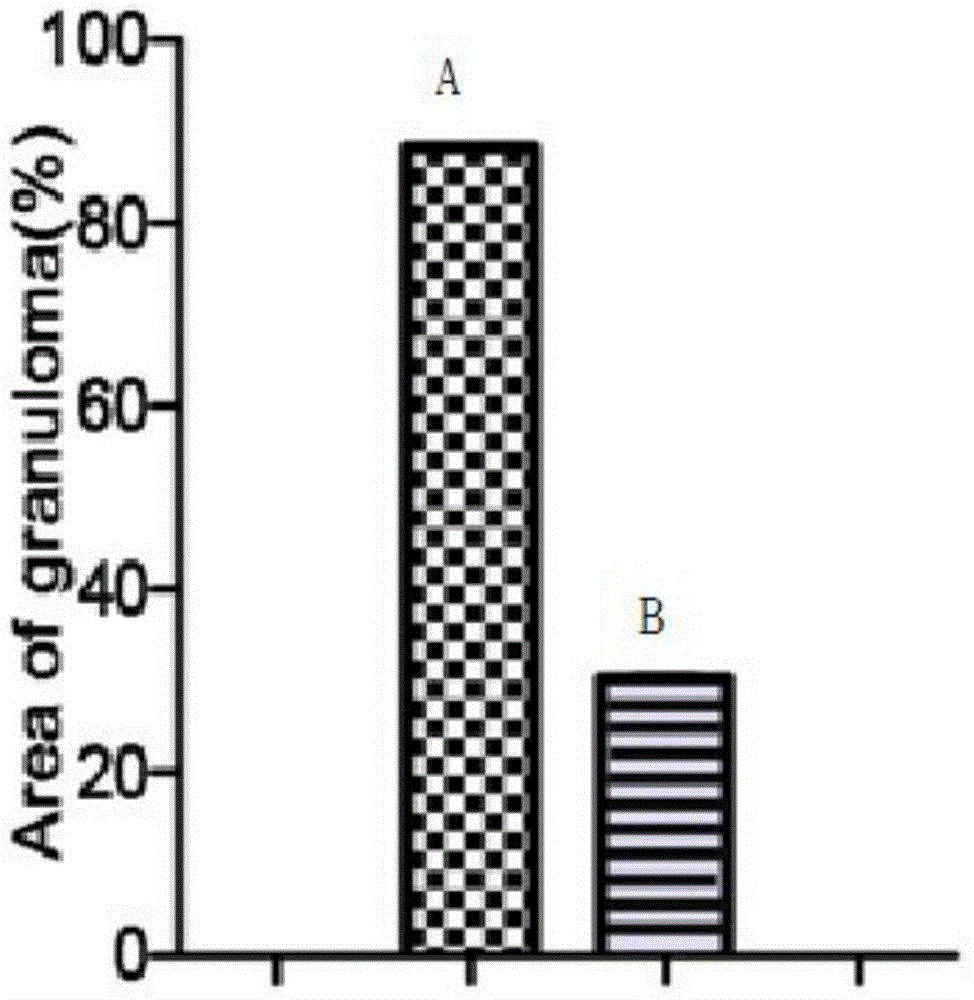
[0034] Example 2 Effect of the pharmaceutical composition of the present invention on granuloma formation caused by acute Schistosoma japonicum infection.
[0035] Experimental method: the pretreatment is the same as in Example 1. Liver tissue from the same part of the liver was fixed with 4% paraformaldehyde, dehydrated and transparent (dehydrated with 70% ethanol, 80% ethanol, 90% ethanol, 95% ethanol for 3 hours each, dehydrated with absolute ethanol Ⅰ and absolute ethanol Ⅱ for each 2h, xylene I and xylene II transparent for 20 minutes each, paraffin I and paraffin II soaked in wax for 40 minutes each), embedding and sectioning, the section thickness is 4 μm, oven-dried the sections at 50°C, carried out HE staining, and sealed the slides. Sealed with permanent gum and observed pathologically.
Embodiment 3
[0036] Example 3 Immunohistochemical observation of the effect of the pharmaceutical composition of the present invention on the expression of P65 protein in liver tissue of mice with acute Schistosoma japonicum infection.
[0037] Paraffin sections were dewaxed to water; 3% H 2 o 2 Incubate at room temperature for 5-10min, wash with distilled water; soak in PBS for 5min; block with 5-10% normal goat serum (diluted in PBS), incubate at room temperature for 10min; Appropriately diluted biotin-labeled secondary antibody (1% BSA-PBS dilution), incubate at 37°C for 10-30min; wash with PBS, 5min×3 times; drop appropriate diluted horseradish enzyme-labeled streptavidin; 37°C Incubate for 10-30min; rinse with PBS, 5min×3 times; develop color with chromogen (DAB); fully rinse with tap water, counterstain, and mount the slide.
[0038] Experimental method: the pretreatment is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com