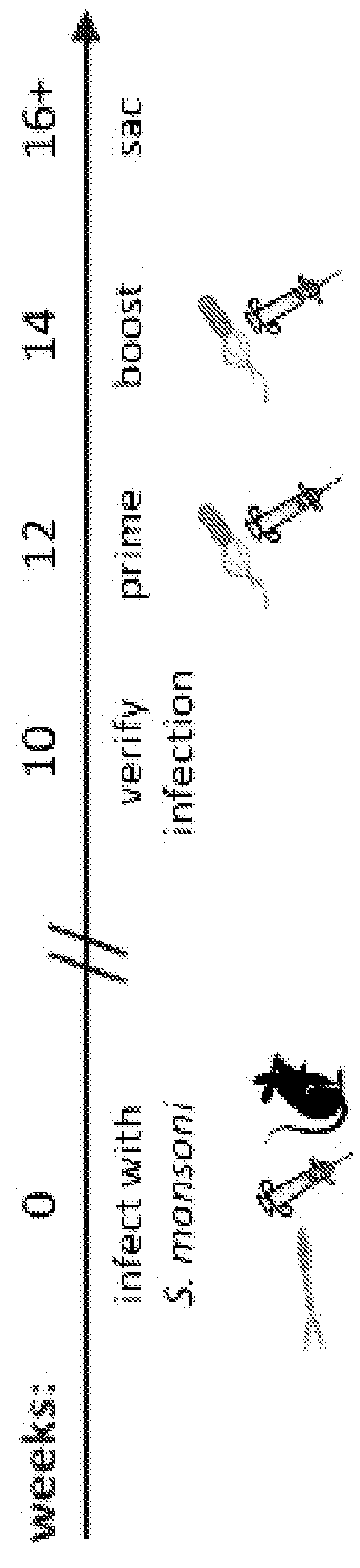
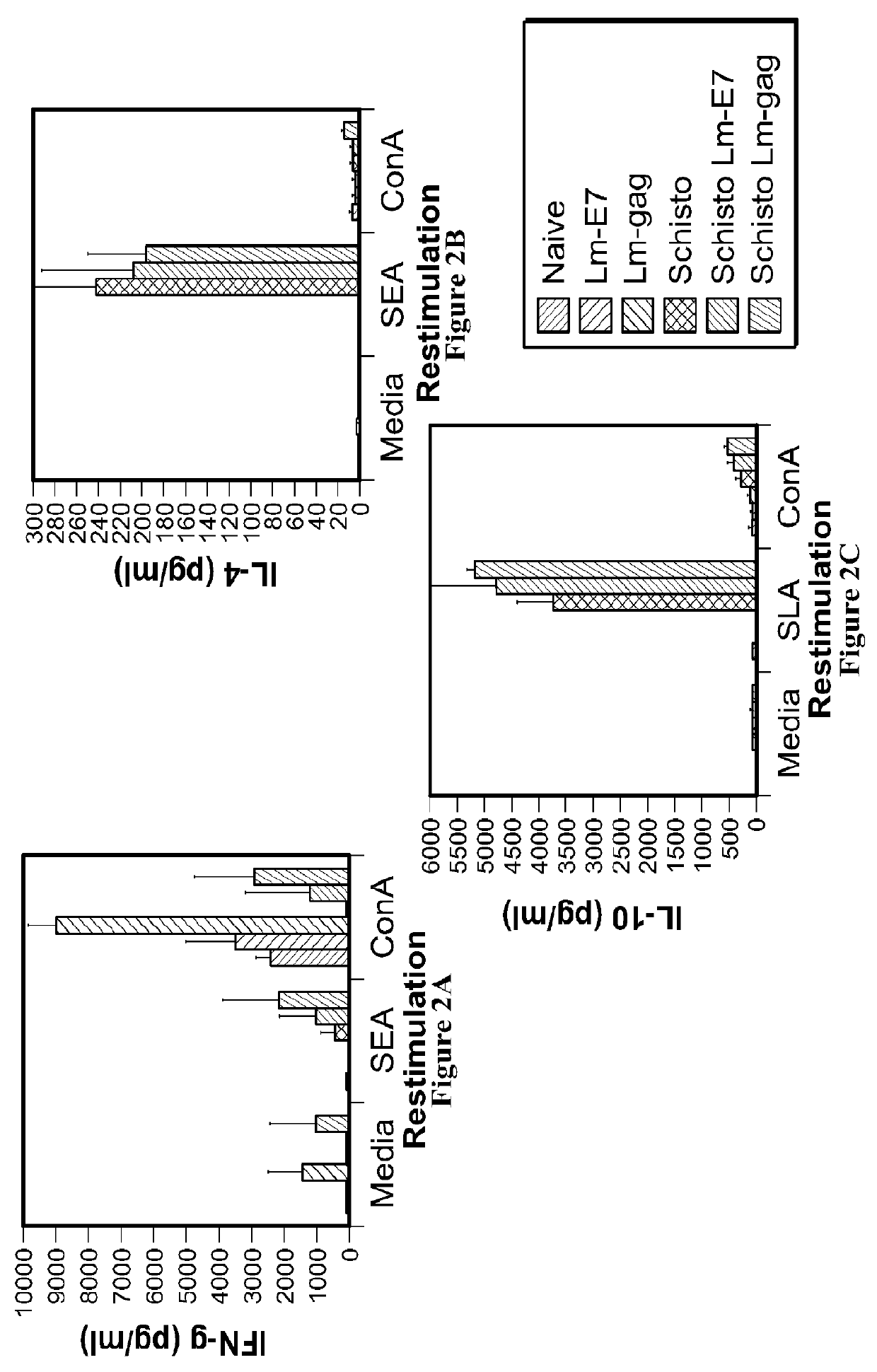
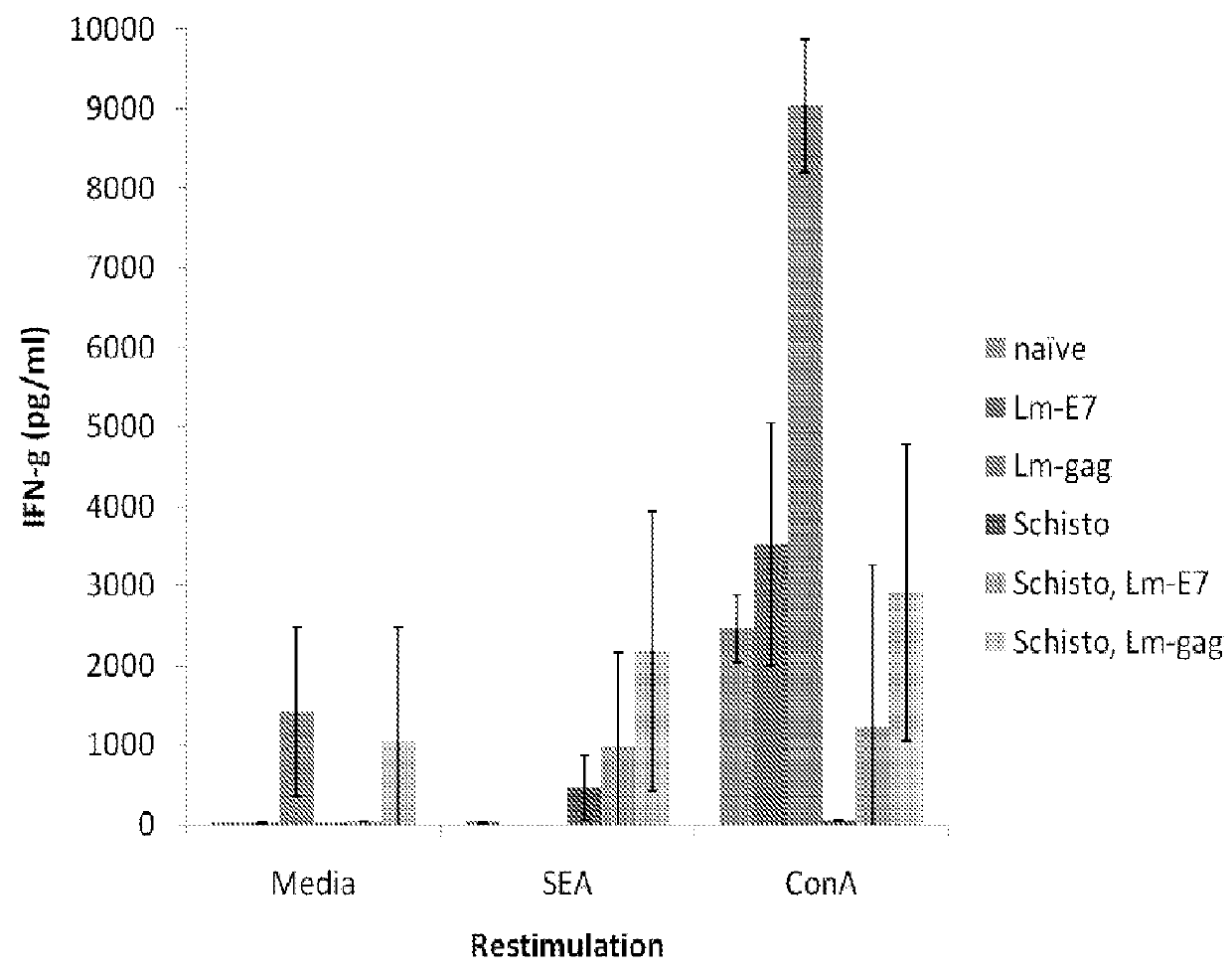
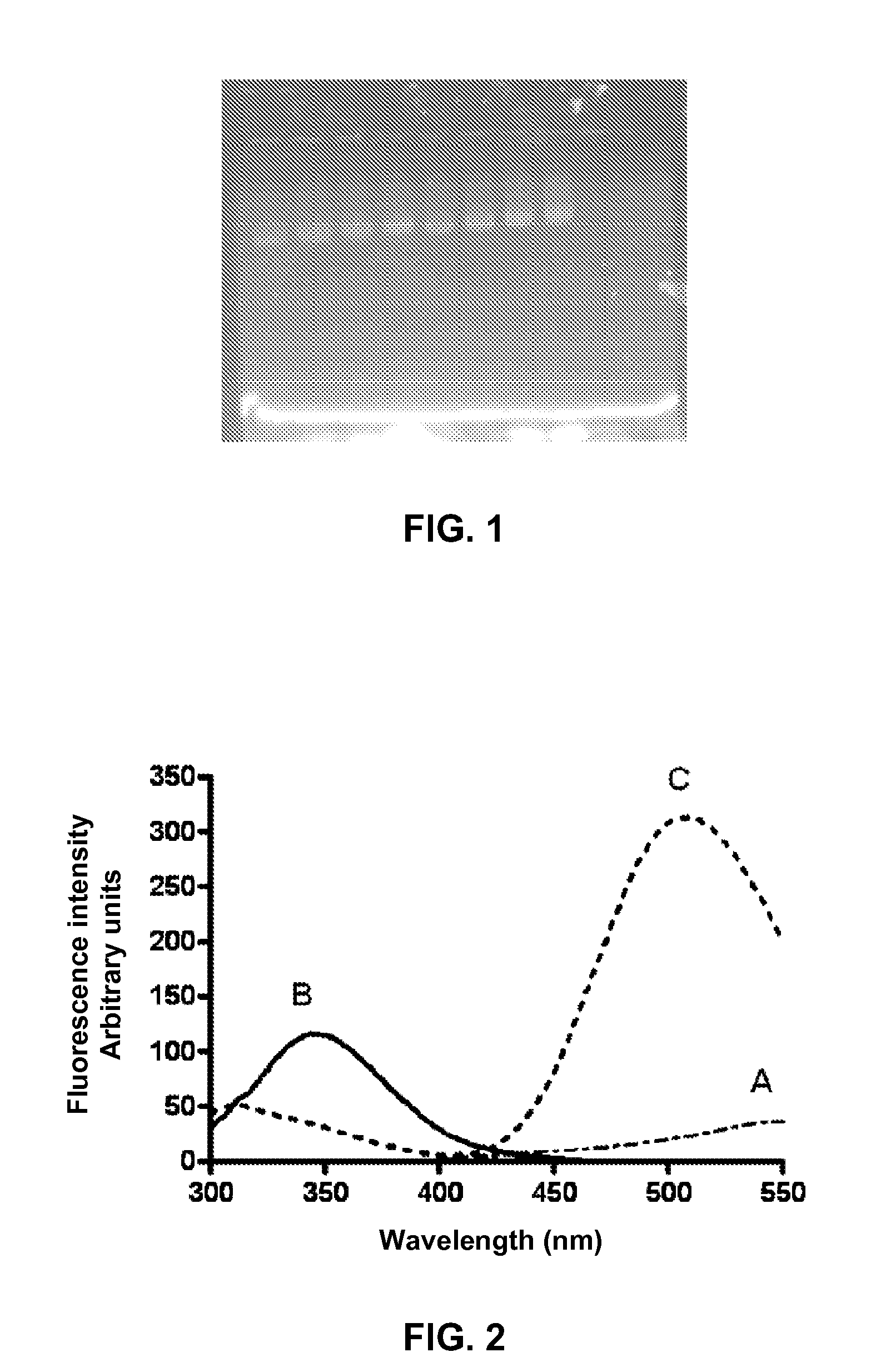
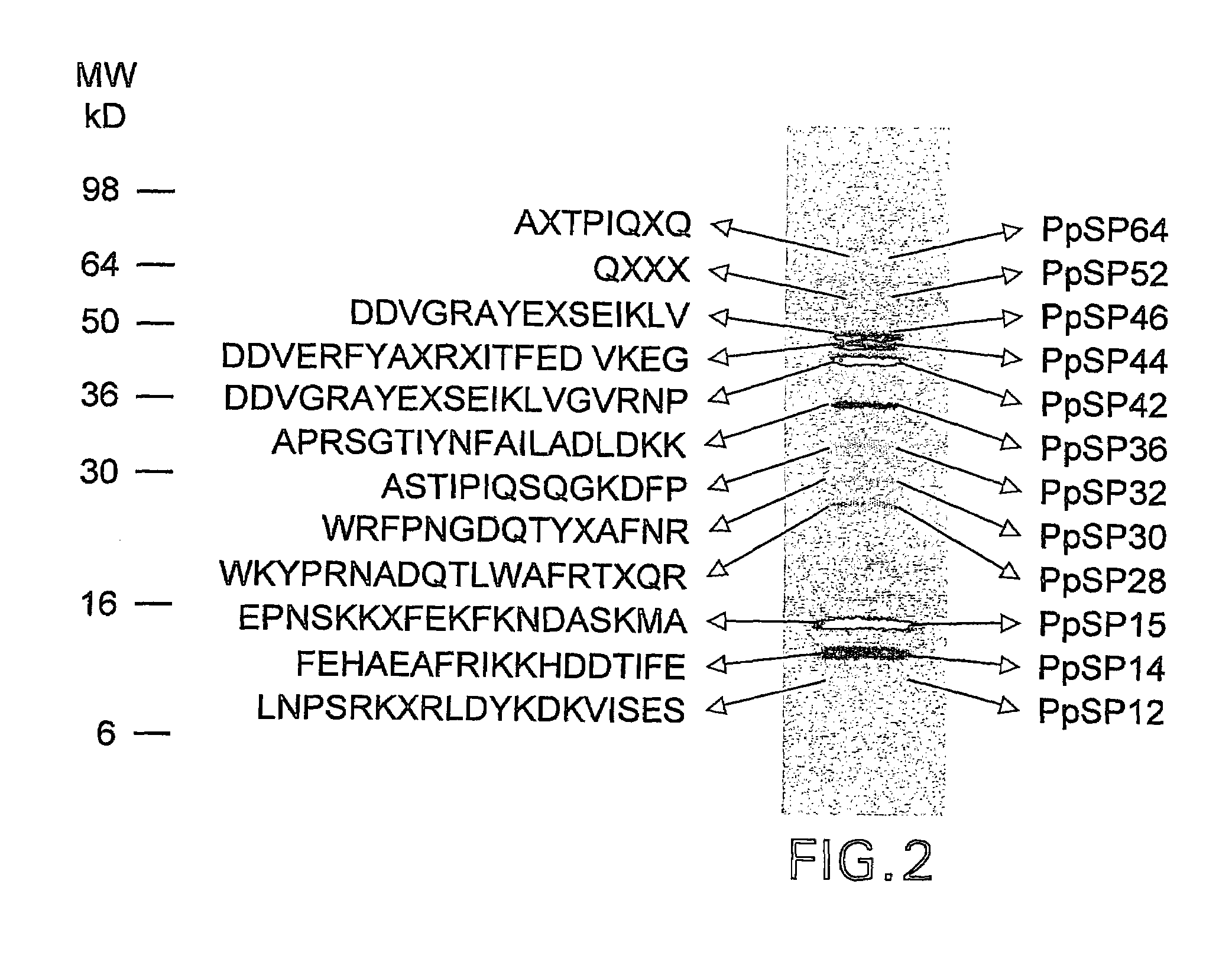
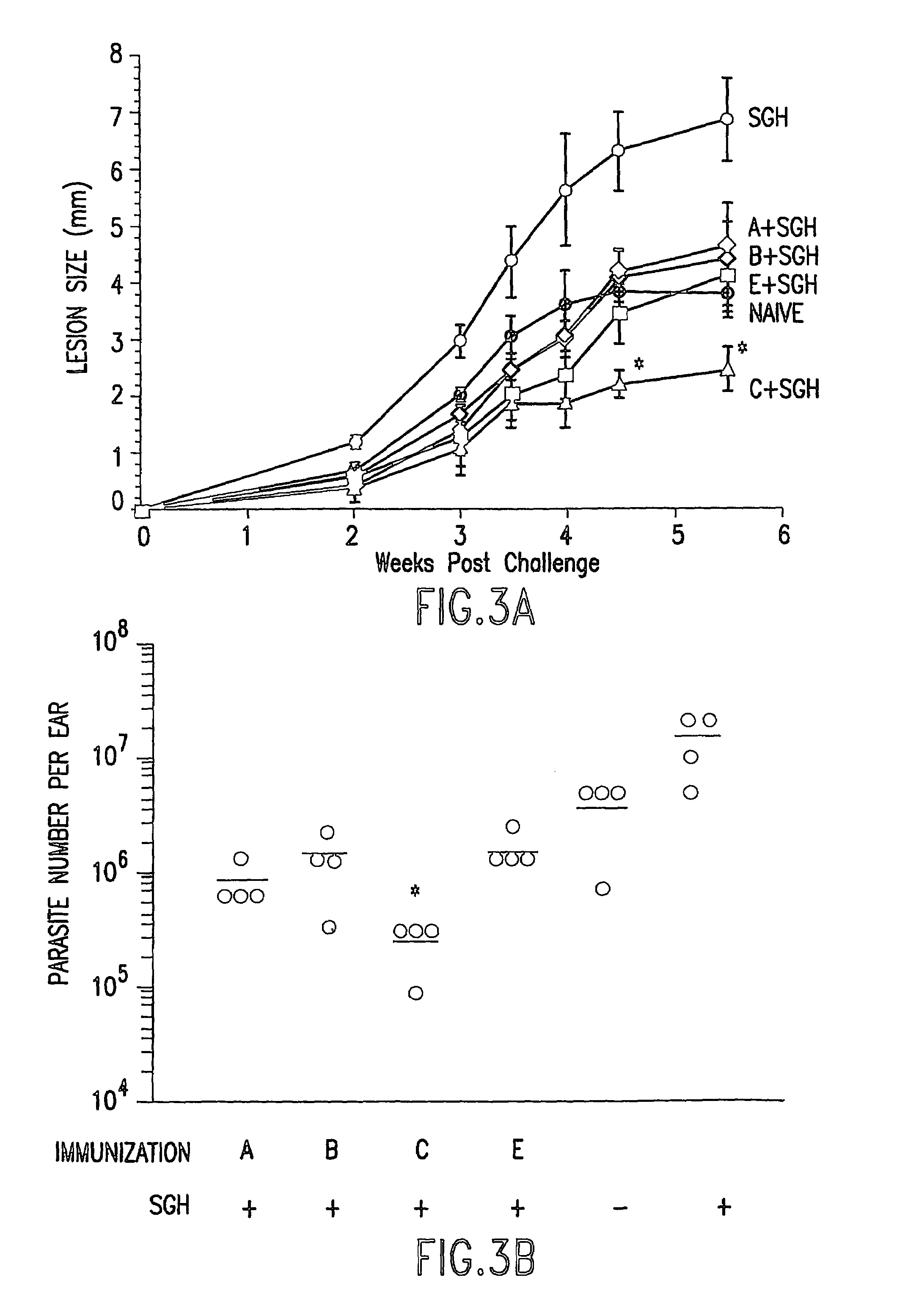
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
193results about "Invertebrate antigen ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
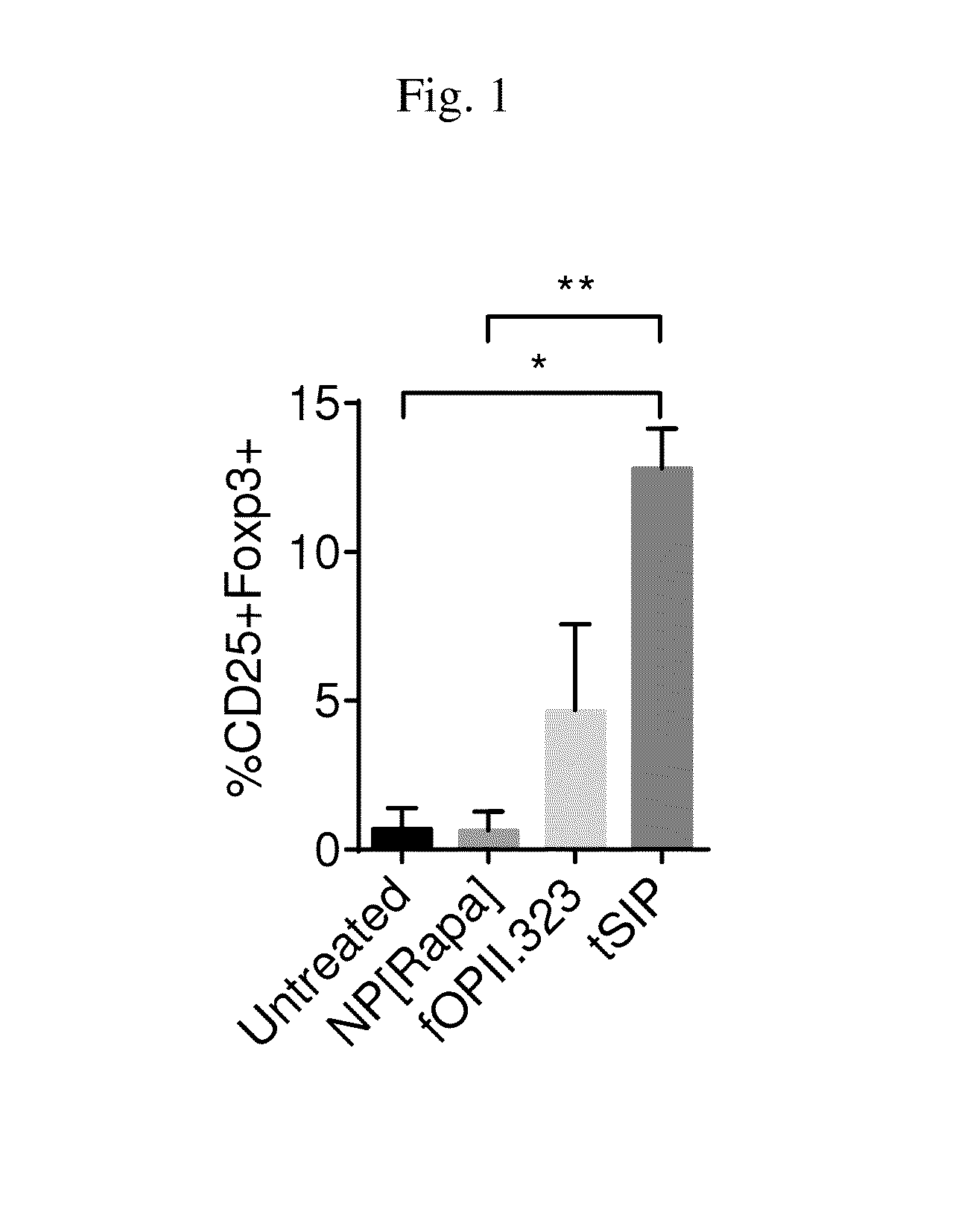
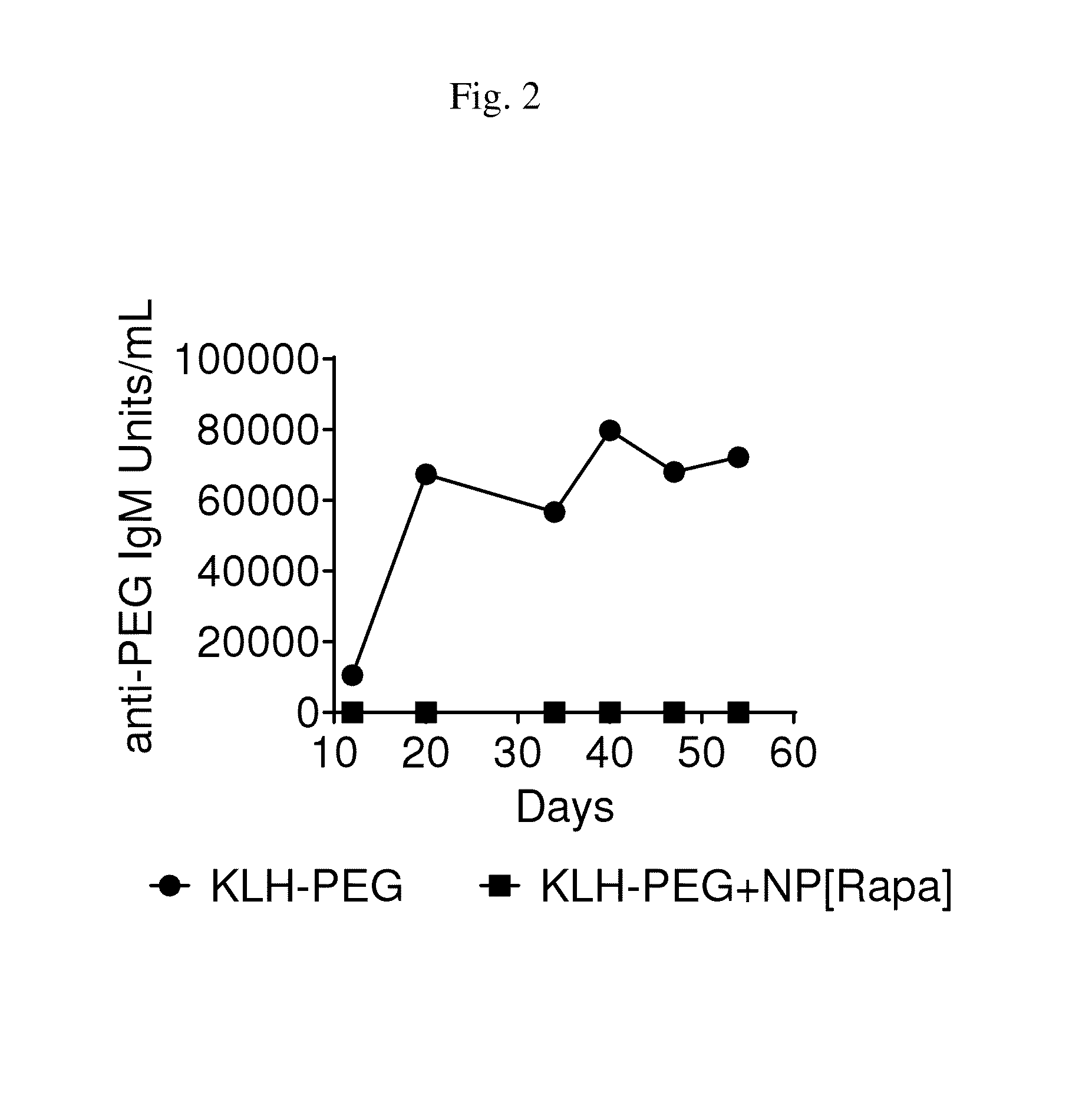
Methods and compositions related to synthetic nanocarriers with rapamycin in a stable, super-saturated state
PendingUS20160128987A1Induce immune toleranceDurable immune toleranceBiocideGranular deliveryPolyesterNanocarriers
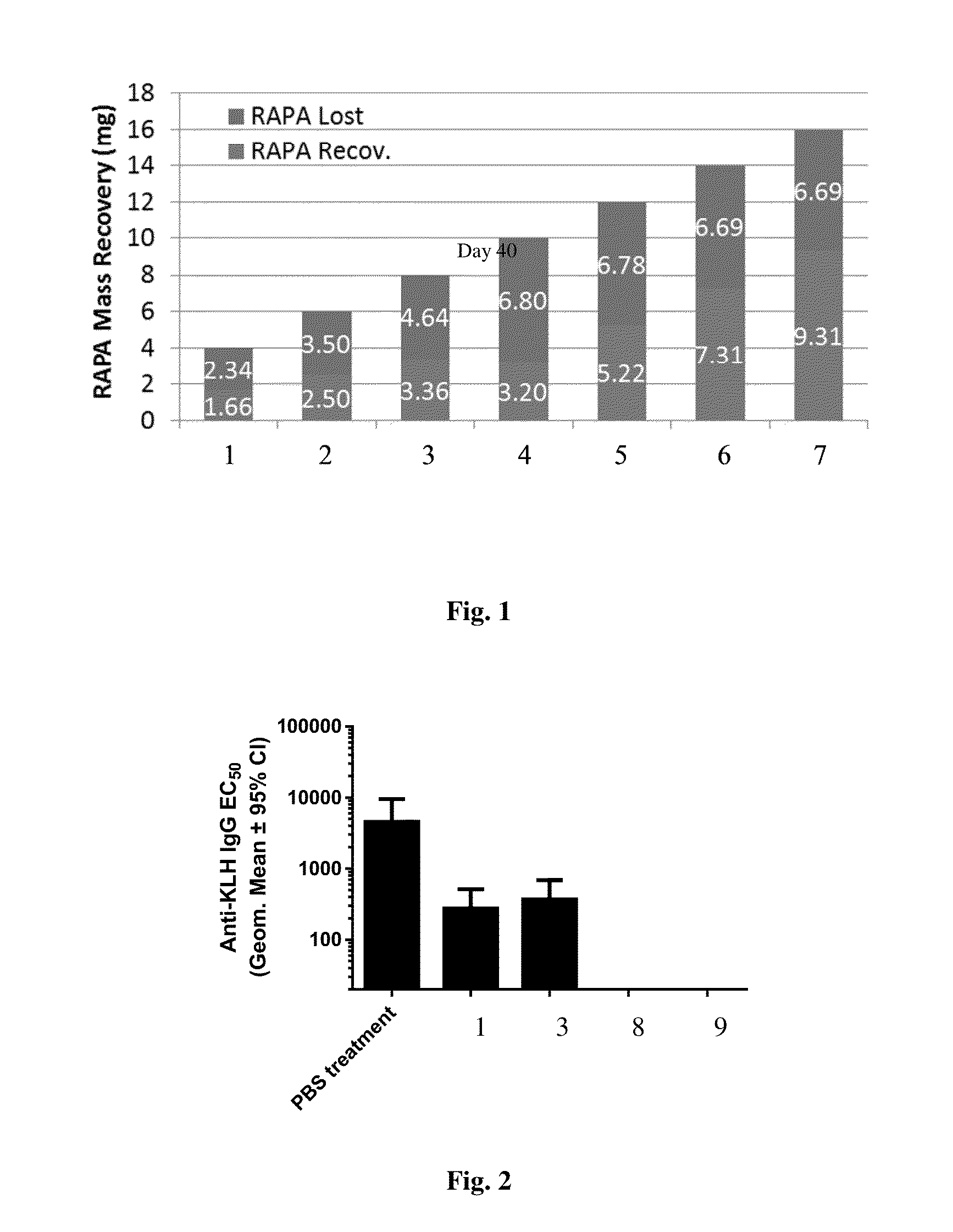
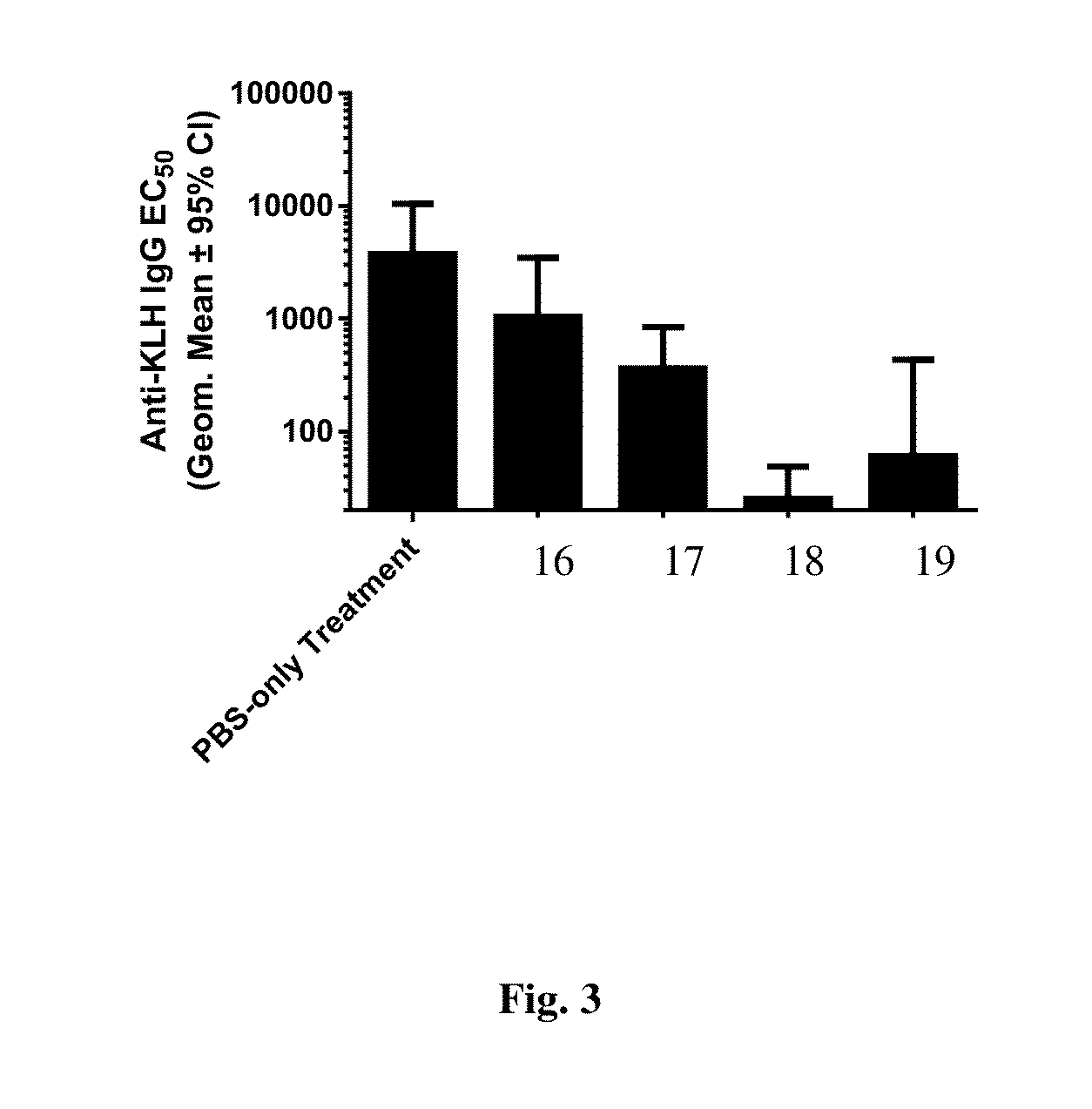
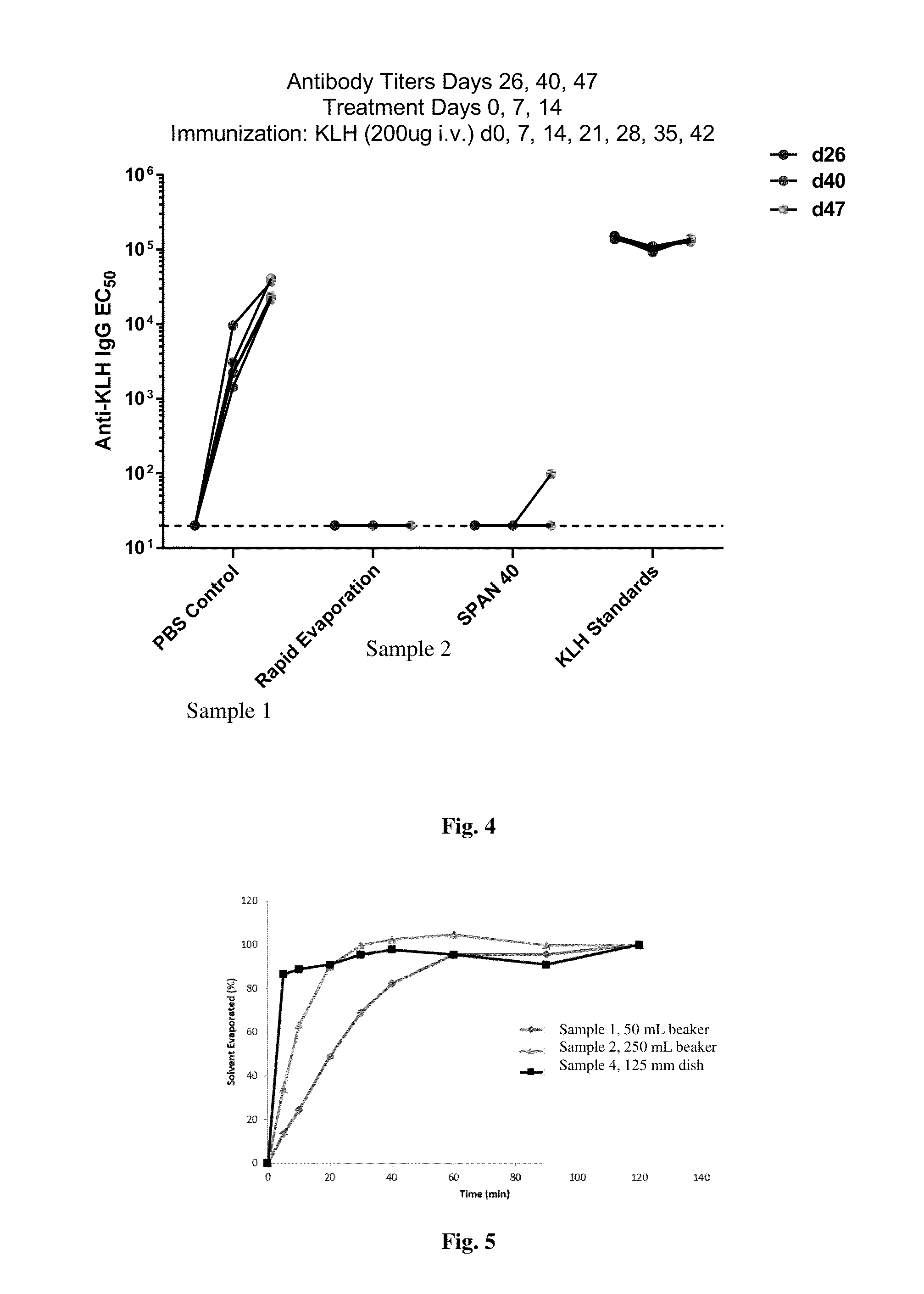
Disclosed are compositions and methods that provide synthetic nanocarriers that comprise hydrophobic polyester carrier material and rapamycin that is in a stable, super-saturated amount. In some embodiments, the synthetic nanocarriers are also initially sterile filterable. In other embodiments, the rapamycin is present in the synthetic nanocarrier compositions in an amount that is less than 50 weight % rapamycin / hydrophobic polyester carrier material in the composition.
Owner:SELECTA BIOSCI
Hookworm vaccine
InactiveUS7303752B2Worm burdenBlood lossProtozoa antigen ingredientsSnake antigen ingredientsAntigenVaccination
Preparations which elicit an immune response to hookworm antigens and which may be utilized as hookworm vaccines are provided. In addition, a method of increasing the effectiveness of vaccinations against infectious diseases in patients infected with hookworm is provided. The method involves chemically treating the hookworm infestation prior to administering the vaccine.
Owner:GEORGE WASHINGTON UNIV THE
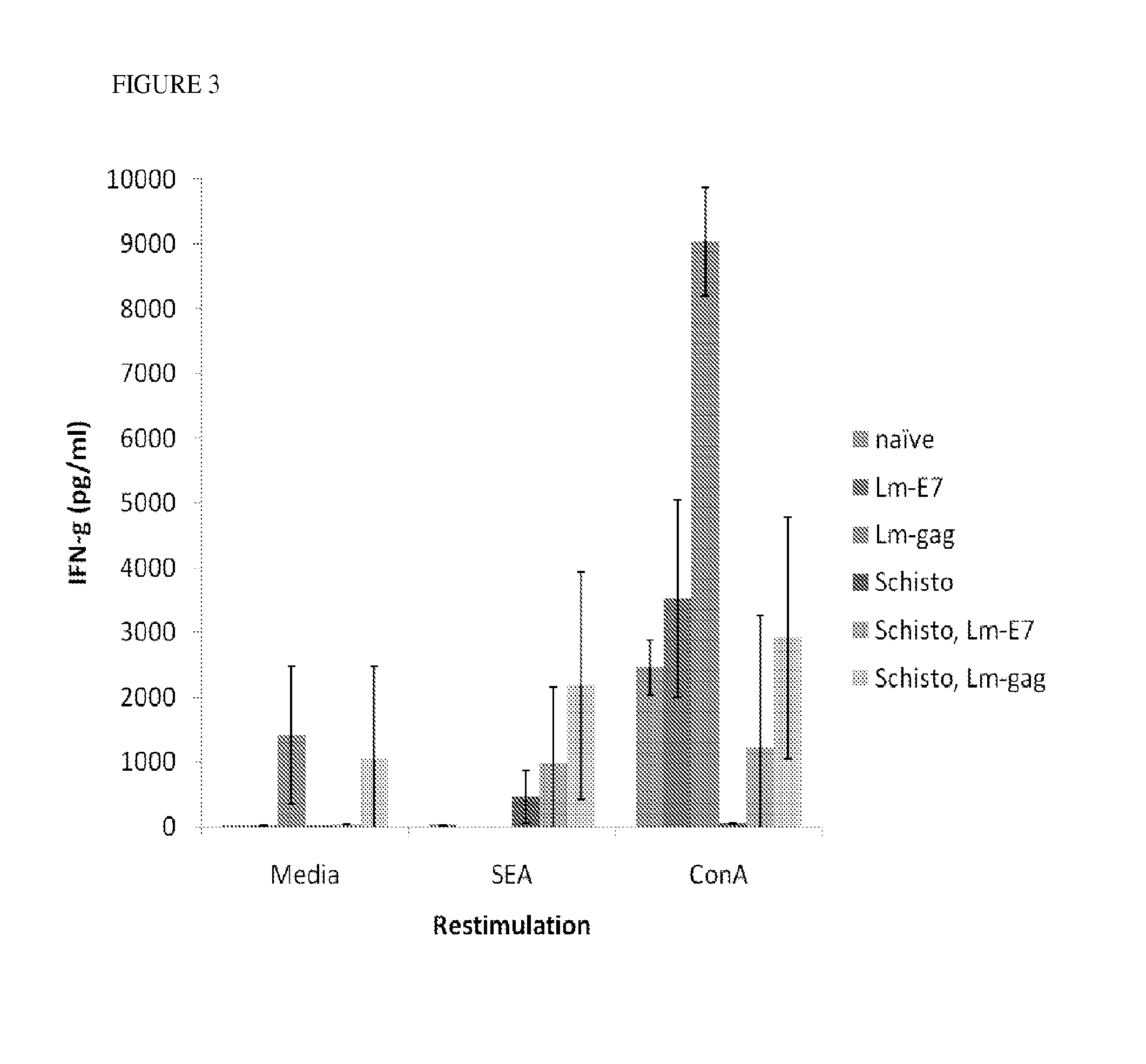
Use of listeria vaccine vectors to reverse vaccine unresponsiveness in parasitically infected individuals
This invention relates to methods of using a Listeria vaccine vector to induce a Th1 immune response in subjects having persistent Th2 immune response profiles.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Tolerogenic synthetic nanocarriers and therapeutic macromolecules for reduced or enhanced pharmacodynamic effects
ActiveUS20140328854A1Reduced pharmacodynamically effectiveLow effective dosePowder deliveryOrganic active ingredientsTolerabilityNanocarriers
Owner:SELECTA BIOSCI
Methods and compositions for enhancing cd4+ regulatory t cells
PendingUS20140335186A1Increase the number ofOrganic active ingredientsPowder deliveryRegulatory T cellCD4 antigen
Owner:SELECTA BIOSCI
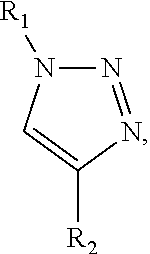
Cationic lipid compound, composition containing same and application
ActiveCN114044741AOrganic active ingredientsNervous disorderPharmaceutical medicineOrganic chemistry
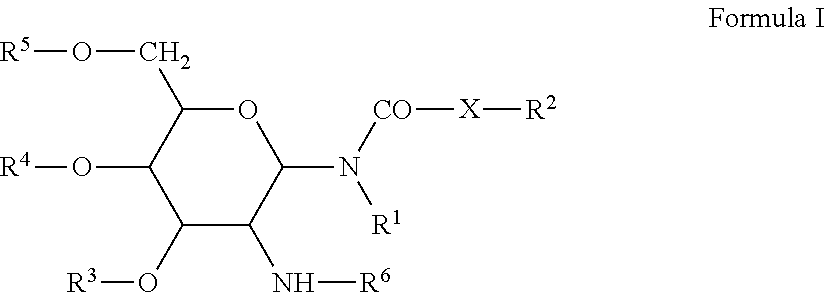
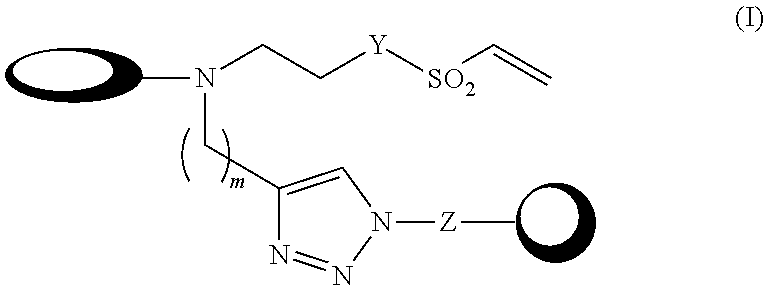
The present disclosure provides a compound of formula (I) or an N-oxide, solvate, pharmaceutically acceptable salt or stereoisomer thereof. Compositions comprising the aforementioned compounds and the use for delivering therapeutic or prophylactic agents are also provided.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
Oil-based adjuvants
The instant invention provides various formulations comprising combinations of immunostimulating oligonucleotides, polycationic carriers, sterols, saponins, quaternary amines, TLR-3 agonists, glycolipids, and MPL-A or analogs thereof in oil emulsions, use thereof in preparations of immunogenic compositions and vaccines, and use thereof in the treatment of animals.
Owner:ZOETIS SERVICE LLC
Multivalent vaccine for filariasis
ActiveCN103298485AProtozoa antigen ingredientsPeptide/protein ingredientsWAS PROTEINMultivalent Vaccine
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Use of Listeria vaccine vectors to reverse vaccine unresponsiveness in parasitically infected individuals
This invention relates to methods of using a Listeria vaccine vector to induce a Th1 immune response in subjects having persistent Th2 immune response profiles.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Double-labelling agents based on vinyl sulphone
The invention relates to labelling agents containing a compound with two labelled molecules and a vinyl sulphone group. The invention also relates to the compounds, the method for obtaining these and the uses thereof in the marking of biomolecules and, more specifically, proteins.
Owner:UNIV DE GRANADA
Multivalent Vaccine for Filariasis
The present invention is a multivalent vaccine for immunizing an animal against filariasis. In some embodiments, the antigens of the multivalent vaccine are protein-based, DNA-based, or a combination thereof.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Cooperia vaccine
The present invention relates to nucleotide sequences encoding Cooperia antigens, as well as to recombinant DNA molecules containing such nucleotide sequences and host cells expressing these nucleotide sequences. The invention further relates to Cooperia proteins, to methods for the production of the proteins, nucleotide sequences, recombinant DNA molecules and hosts. Furthermore, the invention relates to vaccines which induce protective immunity against infection by parasitic nematodes such as species of the genus Cooperia and to methods for preparing such a vaccine.
Owner:UNIV GENT
Coding gene of haemaphysalis qinghaiensis aquaporin (AQP) and application of coding gene
The invention discloses a coding gene of haemaphysalis qinghaiensis aquaporins (AQPs) and an application of the coding gene. The coding gene of the AQPs can be used for preparing novel anti-tick medicines and anti-tick vaccines. A sequence of the coding gene of the AQPs is shown as SEQ ID NO:1. The invention also provides specific primers cloned by the coding gene of the AQPs, wherein sequences ofthe specific primers are shown as SEQ ID NO:2 and SEQ ID NO:3; the invention also provides a method for cloning the coding gene of the AQPs, and the cloned gene product can be also applied to in-vitro recombinant protein expression and antibody preparation; and an obtained antibody can be used for preparing the novel anti-tick medicines and the anti-tick vaccines. The invention has important theoretical significance an potential application value on prevention and control of ticks. The coding gene of the haemaphysalis qinghaiensis aquaporins (AQPs) and the cloning method of the coding gene provided by the invention are applicable to systematic researches of subtype, varieties, expression distributin, protein structures, biological functions and the like of the AQPs.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Vaccination of animals to elicit a protective immune response against tick infestations and tick-borne pathogen transmission
ActiveUS8722063B2Reduces and eliminates infestationReduce morbidityFungiBacteriaVaccinationProtein isolate
Two antigenic and immunogenic proteins of the cattle tick, Rhipicephalus microplus, and the genes encoding these proteins, are effective for eliciting a protective immune response that controls and prevents infestations of bovines and other livestock by the tick. The proteins isolated from the cattle tick include an aquaporin protein and a TC5777 gut membrane protein. Each of the proteins elicit an immunoprotective response in livestock to the cattle tick, and can be formulated and administered as vaccines. Alternatively, the isolated DNA sequences which encode these proteins can be incorporated into nucleic acid constructs which could be utilized as DNA vaccines. The nucleic acid constructs can also be used for the transformation of cells and the production of recombinant proteins. Induction of the protective immune response controls and prevents infestations of the treated animals with the tick, thereby protecting them against tick-borne pathogen transmission.
Owner:US SEC AGRI
Lipoglycan compositions and methods of treating parasitic infections
InactiveUS7063848B2Extended durationImprove the level ofOrganic active ingredientsBiocideProtection sexOrganism
A composition for and a method of eliciting in a vertebrate a protective immune response against an eukaryotic parasite are disclosed. The method includes administering to the vertebrate a composition having a carrier group coupled to an oligosaccharide obtained from a lipoglycan found on the surface of an eukaryote. The composition is administered in an amount sufficient to elicit a protective immune response against the parasite.
Owner:UNIV OF MASSACHUSETTS
Anti-arthropod vector vaccines method of selecting and uses thereof
ActiveUS7388089B2Preventing LeishmaniasisPeptide/protein ingredientsImmunoglobulinsArthropod VectorLeishmaniasis
The present invention provides methods of selecting and uses of anti-arthropod vector vaccines to prevent Leishmaniasis. The present invention also provides compositions for vaccines to prevent Leishmaniasis.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Protein having antigen activity against liver flukes
ActiveCN103459414AImprove featuresHigh sensitivityProtozoa antigen ingredientsImmunoglobulinsTrue positive rateADAMTS Proteins
The present invention relates to a protein having antigenic activity against liver flukes, and more particularly, to an antigenic protein having enhanced sensitivity and specificity for the diagnosis and treatment of the liver flukes by producing proteins having antigenic activity against the liver flukes. The antigenic protein includes sequences selected from all or parts of amino acid sequences which are selected from the group consisting of SEQ ID Nos. 1 to 4 of the present invention.
Owner:韩国中央大学校产学协力团
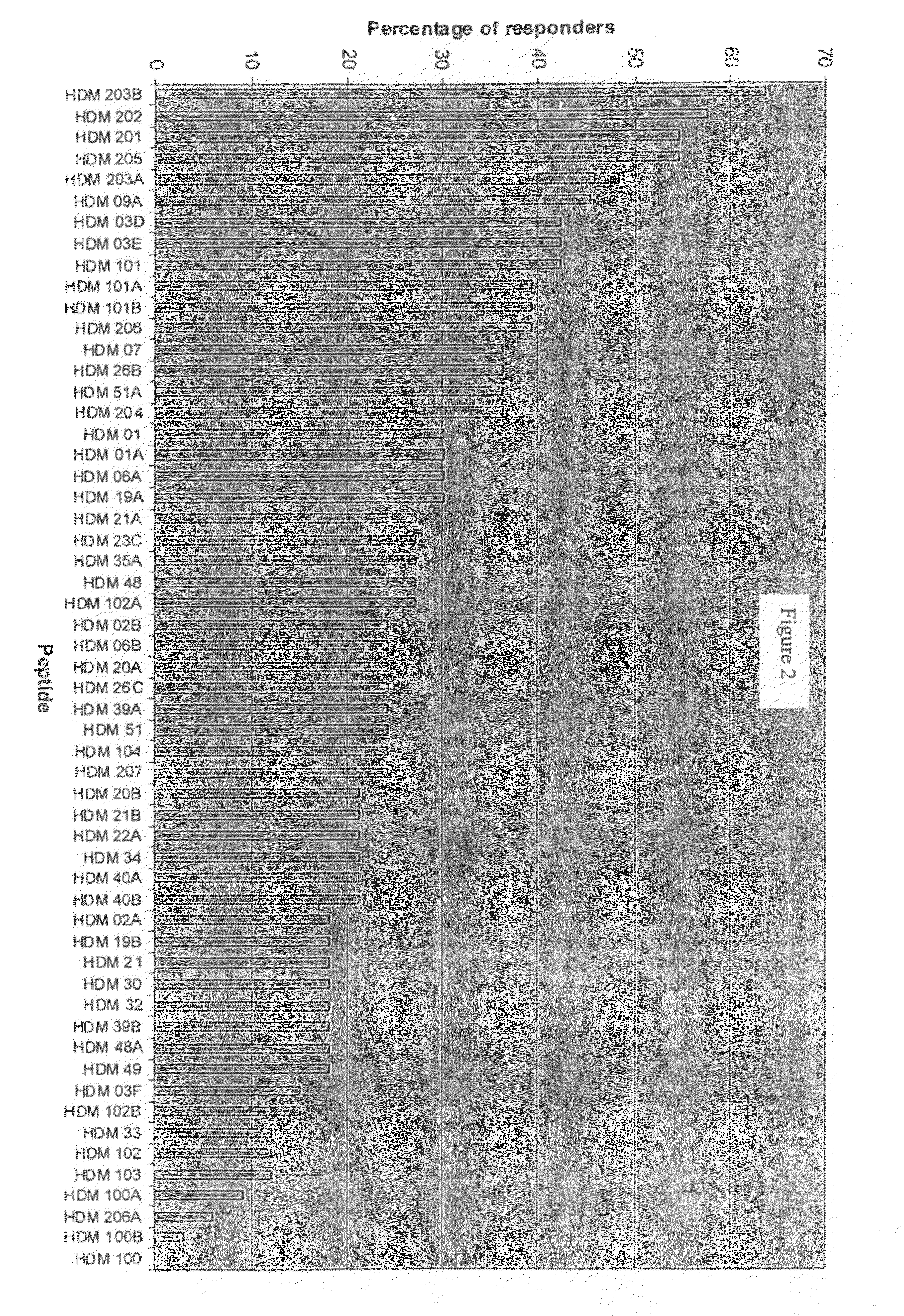
Peptide for vaccine
ActiveUS20100260805A1Wide coverageBroad utilityPeptide/protein ingredientsAntibody mimetics/scaffoldsAllergyTGE VACCINE
The present invention relates to compositions comprising peptides for preventing or treating allergy to house dust mites, and in particular to optimal combinations of peptides for preventing or treating said allergy.
Owner:CIRCASSIA
Vaccine against infectious disease
InactiveUS20050123556A1Low production costEasy to operateAntibacterial agentsSsRNA viruses positive-senseInfectious DisorderVirus
Owner:MERIAL LTD
Vaccine composition for controlling ectoparasite infestations
ActiveUS20130273095A1Enhance immune responseHarm viabilityProtozoa antigen ingredientsPeptide/protein ingredientsChemical synthesisRibosomal protein
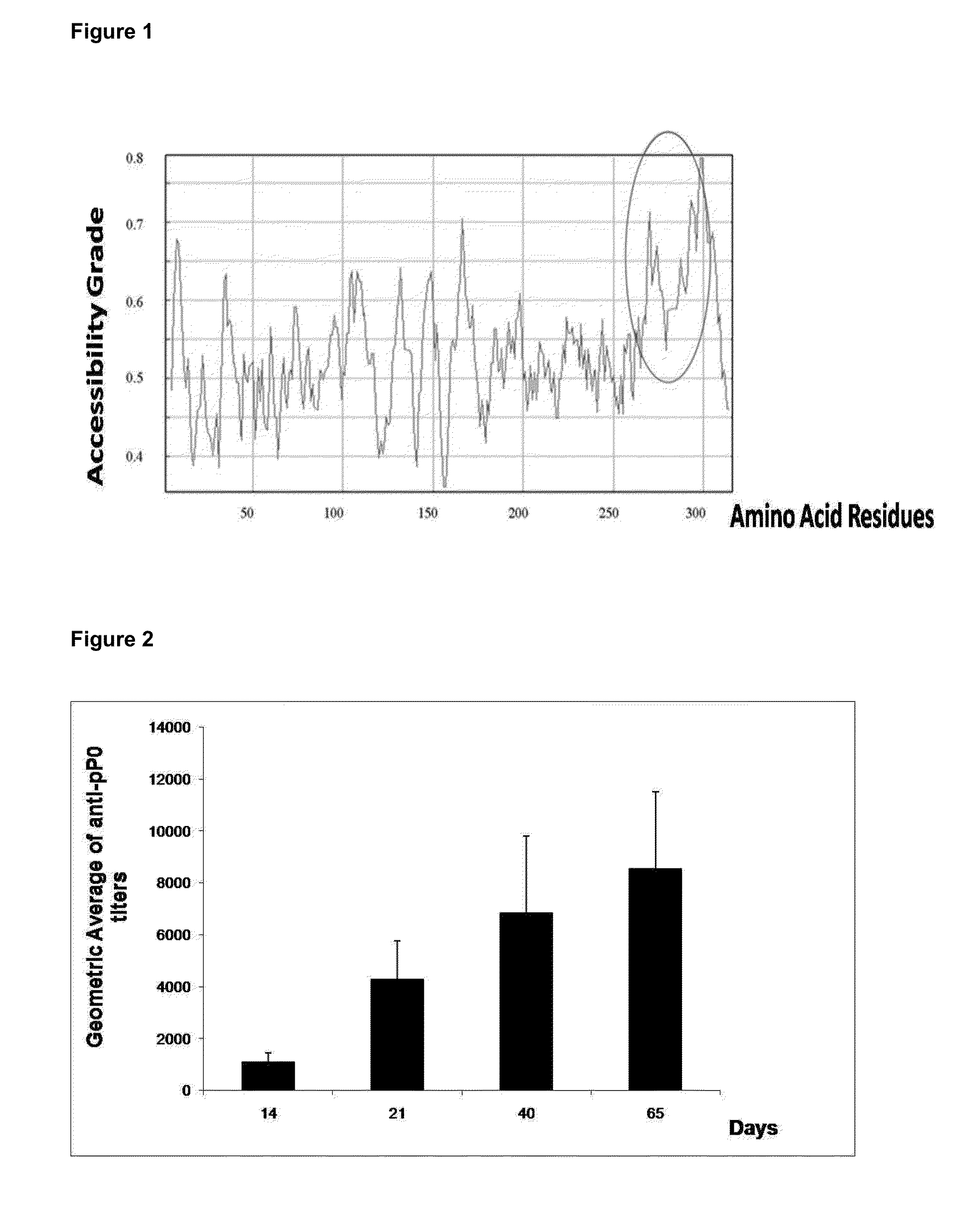
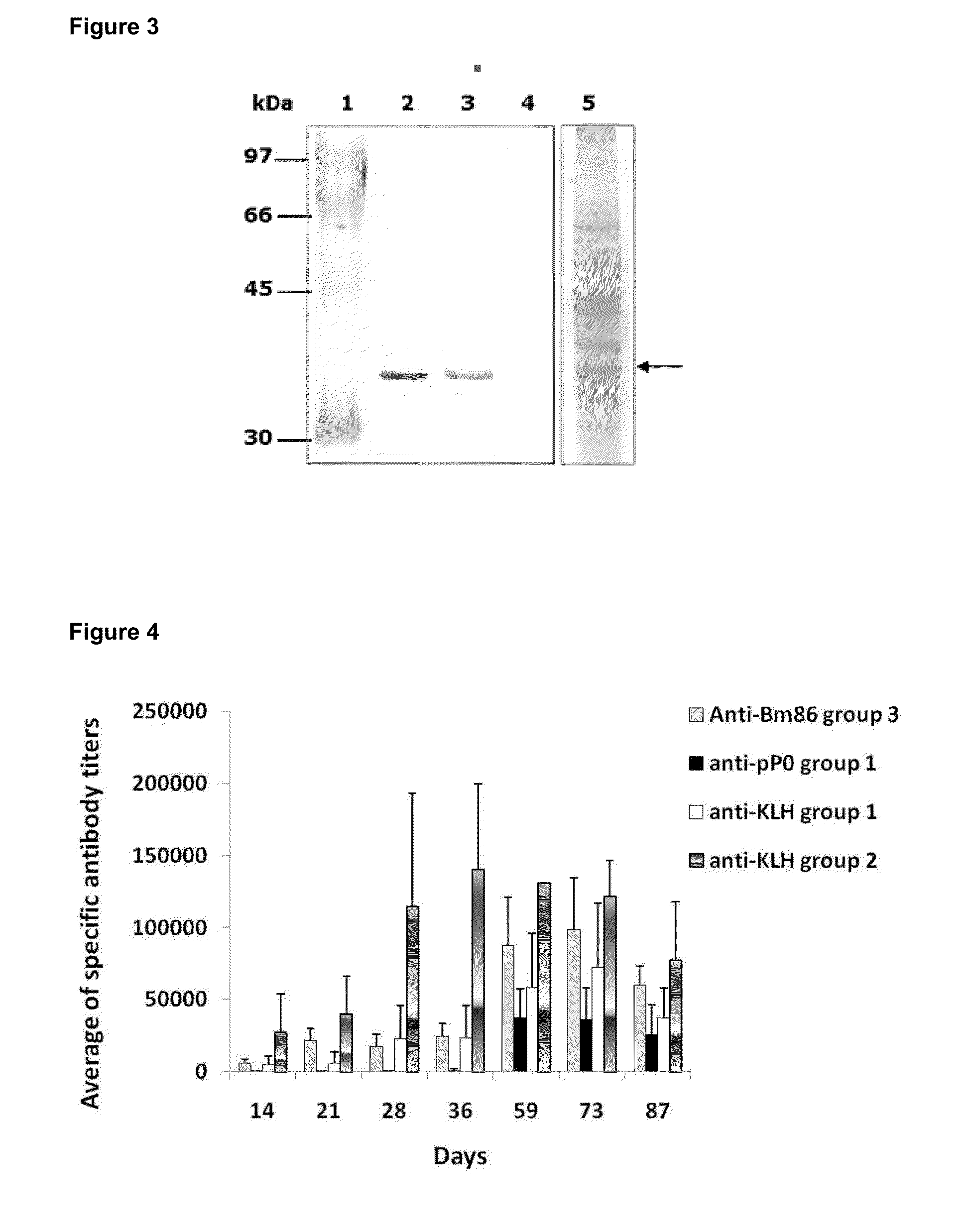
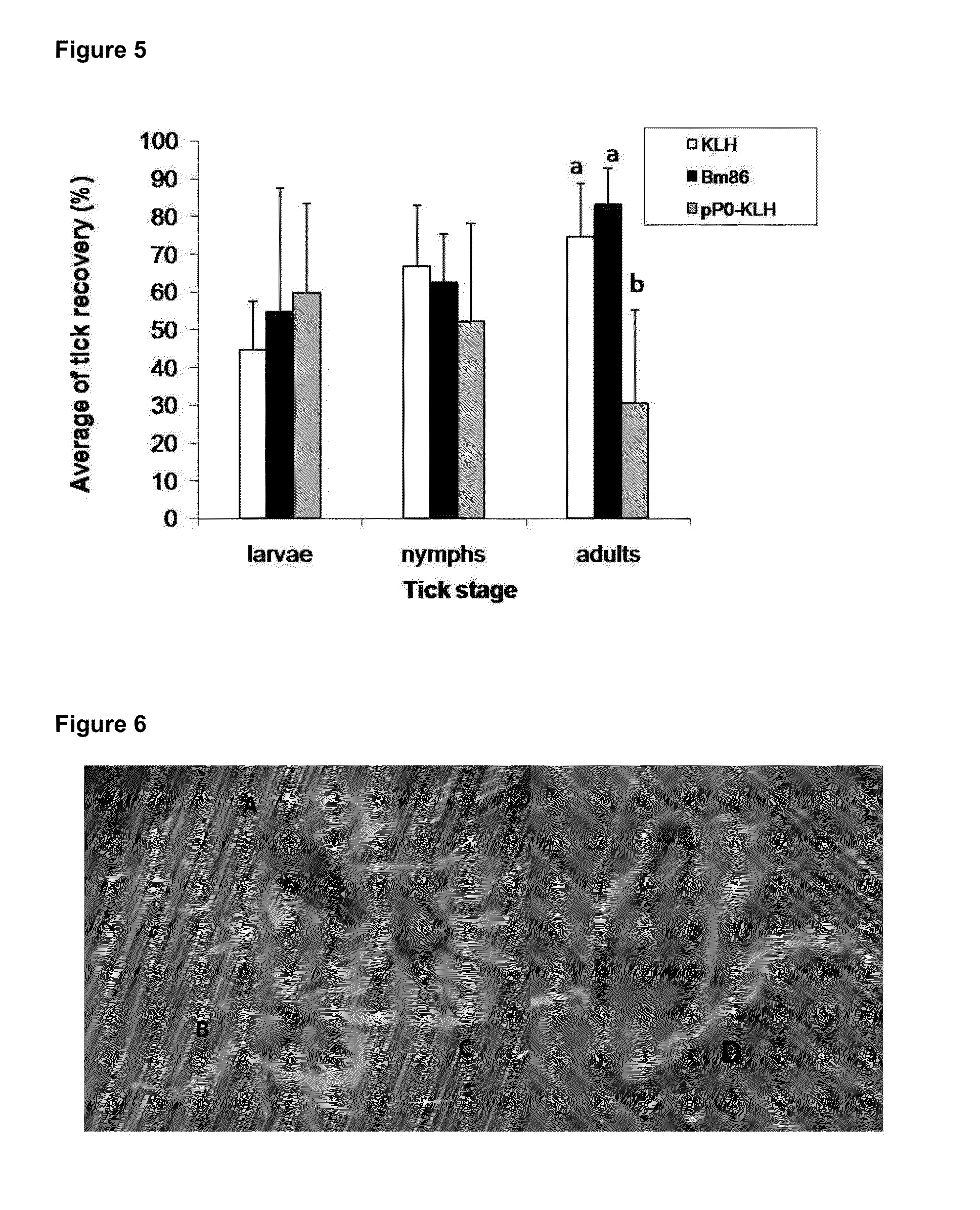
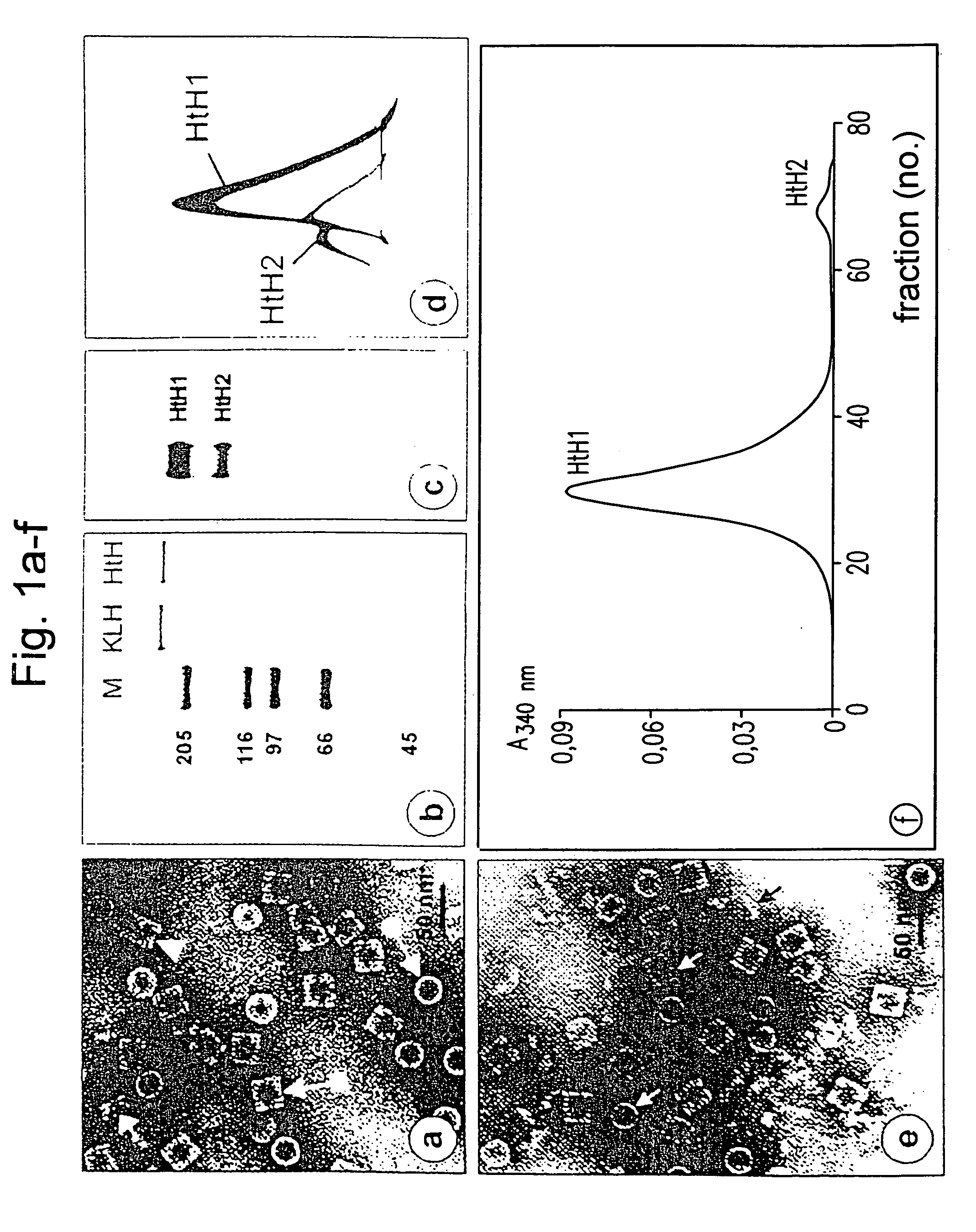
The present invention relates to the use of a peptide of P0 ribosomal protein in the manufacture of a vaccine composition to control of ectoparasite infestations and therefore the transmission of their associated pathogens. This peptide is located between 267 and 301 amino acids of the P0 protein, and can be obtained by recombinant means or by chemical synthesis. This peptide can be fused to a carrier protein or peptide, or an immuno-carrier and be included in an oily formulation. The formulations comprising the peptide vaccine confer protection against ticks and ectoparasites known as “sea lice” without generating autoimmunity in the host organism. Among these ticks may be mentioned species as Rhipicephalus microplus, Rhipicephalus sanguineus and Ixodes scapularis, and between sea lice are those of the Caligus and Lepeophtheirus genera.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Nucleic acid molecule comprising a nucleic acid sequence coding for a haemocyanin
InactiveUS7125556B1Sufficient amountSufficient amount and inexpensivelyPeptide/protein ingredientsGenetic material ingredientsInteinScreening method
A nucleic acid molecule or construct alone or with a promoter suitable for expression control is contemplated that codes for a haemocyanin, a haemocyanin domain or a fragment thereof with the immunological properties of at least one domain of haemocyanin, and comprises at least one intron sequence, as well as haemocyanin fusion proteins. The construct furthermore can comprise a nucleic acid sequence that codes for an antigen. Host cells are also contemplated that contain the nucleic acid molecule or construct and a recombinant expression product thereof. The invention furthermore relates to a pharmaceutical composition that comprises the expression product and antibodies obtainable by immunization of an animal therewith, as well as the use the antibodies in screening methods for the identification of tumors.
Owner:BIOSYN ARZEIMITTEL GMBH
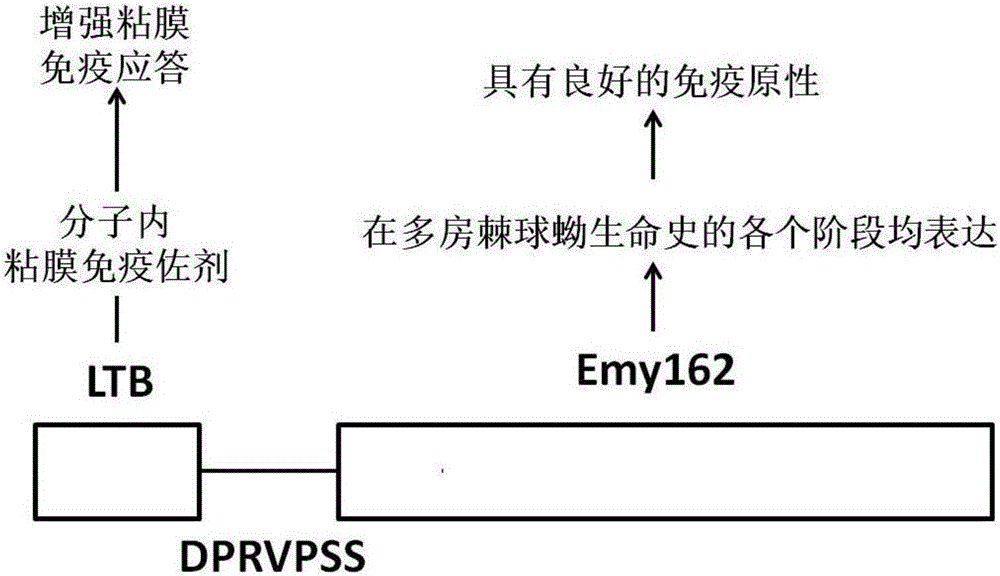
Design and preparation method and application of echinococcus multilocularis subunit vaccine LTB-Emy162
ActiveCN106581667AImprove securityIncrease productionBacteriaAntiparasitic agentsEscherichia coliDisease
The invention relates to a design and preparation method and an application of an echinococcus multilocularis subunit vaccine LTB-Emy162. The active component of the echinococcus multilocularis subunit vaccine LTB-Emy162 is a polypeptide, which is mainly composed of an echinococcus multilocularis antigen protein Emy162 amino acid sequence and a mucosal immunoadjuvant E. coli heat-labile enterotoxin B subunit (LTB) amino acid sequence. In the invention, the gene sequence of the echinococcus multilocularis subunit vaccine LTB-Emy162 is synthesized through gene synthesis technology, the gene sequence is then linked to an expression vector through dual enzyme-cut, the expression vector then is converted into Arctic Express to perform expression of fusion protein, after purification of the protein, the echinococcus multilocularis subunit vaccine LTB-Emy162 is produced. The echinococcus multilocularis subunit vaccine can induce body to generate T-cell and B-cell immunologic response of the echinococcus multilocularis and humoral immune response of a high-titer specific antibody, and can be applied in prevention and therapy of echinococcus multilocularis infection related diseases.
Owner:QINGHAI UNIVERSITY
Combined facilitator, antigen and DNA vaccine for preventing and treating autoimmune diseases
InactiveUS20150044244A1Allergen ingredientsWhole-cell/virus/DNA/RNA ingredientsAutoimmune conditionTransplant rejection
The present invention relates to treating and preventing symptoms of an allergy, asthma, an autoimmune disease, and transplant rejection using a combination vaccine containing a vaccine facilitator comprising a Na / K pump inhibitor, an antigen and a DNA encoding the antigen.
Owner:BEIJING ADVACCINE BIOTECH
Method for treating inflammation with an Ac-TMP-2 protein
Owner:JAMES COOK UNIVERSITY
Hypoallergenic polypeptides for the treatment of house dust mite allergy
The present invention relates to a polypeptide comprising the amino acid sequence as shown in SEQ ID NO:9 or 7. The invention further pertains to nucleic acids encoding the polypeptide, pharmaceutical compositions and vaccines.
Owner:BIOMAY AG
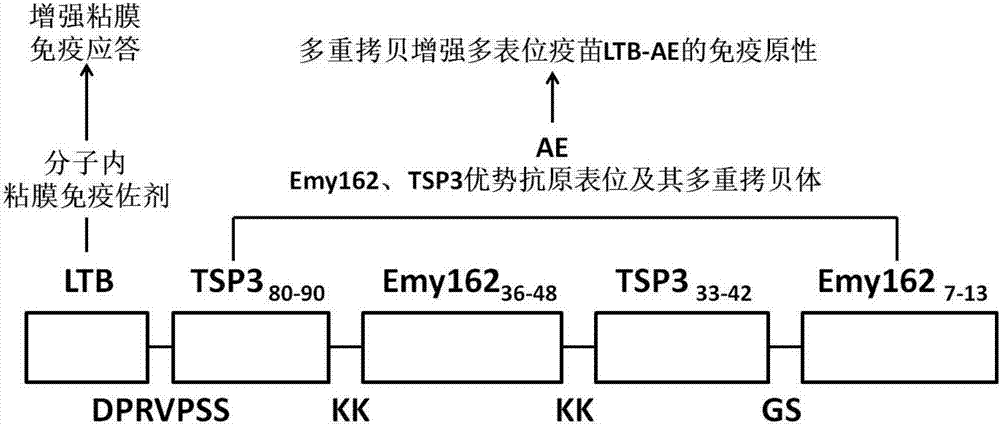
A design, preparing method and applications of an echinococcus multilocularis multi-epitope vaccine LTB-AE
The invention relates to a design, preparing method and applications of an echinococcus multilocularis multi-epitope vaccine LTB-AE. An active component of the multi-epitope vaccine LTB-AE is a polypeptide. The polypeptide mainly comprises an echinococcus multilocularis multi-epitope peptide AE, and a mucosal immune adjuvant that is an escherichia coli heat-labile enterotoxin B subunit (LTB). A gene sequence of the multi-epitope vaccine LTB-AE is synthesized mainly by a gene synthesis technique, and is connected to an expression vector through double digestion, then the expression vector is converted into arctic express to perform expression of a fusion protein, and after the protein is purified, the multi-epitope vaccine LTB-AE is obtained. The multi-epitope vaccine can induce a body to generate immune responses and high-titer specific antibody humoral immune responses to echinococcus multilocularis T cells and B cells, and can be used for preventing and treating echinococcus multilocularis infection related disease.
Owner:QINGHAI UNIVERSITY
Attenuated live vaccine for preventing toxoplasma gondii infection and application of attenuated live vaccine
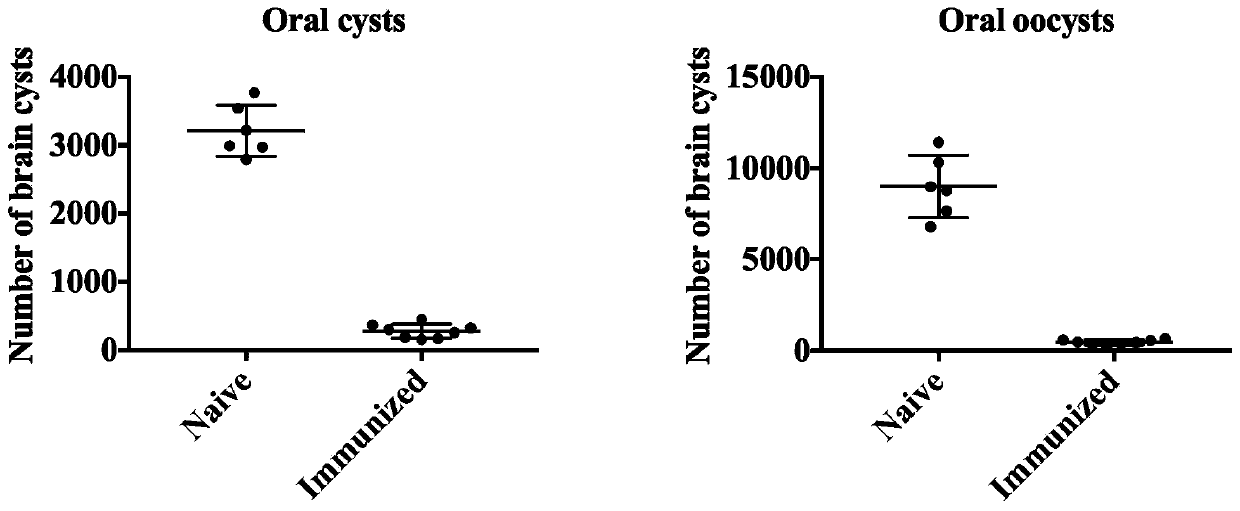
PendingCN110384797AImprove securityGood immune protectionAntiparasitic agentsInvertebrate antigen ingredientsAcute infectionAttenuated Live Vaccine
The invention discloses an attenuated live vaccine for preventing toxoplasma gondii infection and application of the attenuated live vaccine. The attenuated live vaccine is tachyzoite suspension, prepared by taking PBS as a solvent, of a toxoplasma gondii attenuated stain RH: delta gra17 delta npt 1. Toxoplasma gondii double gene knockout is conducted, the immune dosage form of the attenuated livevaccine is determined, the immune vaccination program and dosage of the attenuated live vaccine are verified, it is found that the attenuated live vaccine has a good immune protection effect on toxoplasma gondii acute infection, chronic infection and congenital infection, and toxoplasmosis can be effectively prevented.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
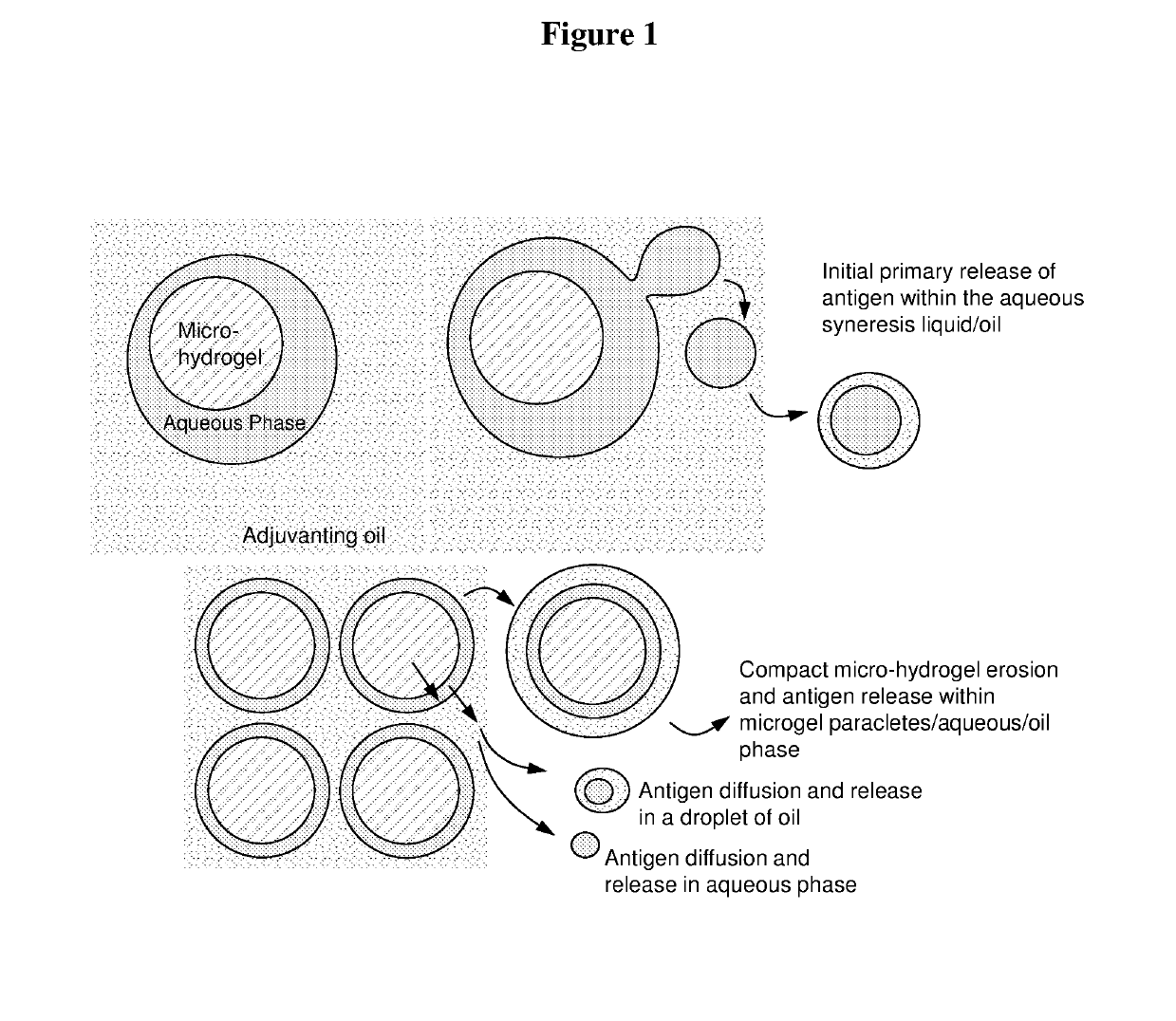
Injectable composition for delivery of a biologically active agent
ActiveUS20190125661A1Fast deliveryRapid and sustained deliveryPowder deliveryPeptide/protein ingredientsActive agentWater in oil emulsion
The invention relates to an injectable composition for rapid and sustained delivery of a biologically active agent and to uses of the injectable composition in the treatment or prevention of a condition in a subject. The injectable composition comprises a water-in-oil emulsion having an aqueous phase dispersed in an oil phase. The aqueous phase comprises a plurality of hydrogel particles and an aqueous liquid and a biologically active agent is contained in the hydrogel particles and in the aqueous liquid.
Owner:CAPSULAR TECH PTY LTD
Vaccination of companion animals to elicit a protective immune response against tick infestations and tick-borne pathogen transmission
ActiveUS9408896B2Reduce morbidityReduces and eliminates infestationImmunological disordersInvertebrate antigen ingredientsDiseaseVaccination
Compositions of either the aquaporin protein from the cattle tick, Rhipicephalus microplus, or a nucleic acid construct incorporating a nucleic acid sequence encoding this aquaporin protein, are effective for eliciting a protective immune response against other tick species in non-bovine animals. The R. microplus aquaporin protein is antigenic and can be administered as a protein vaccine, or in the alternative, the nucleic acid construct can be utilized as a DNA vaccine. Induction of the immune response significantly reduces or eliminates the infestation of treated, non-bovine animals with ticks other than the cattle tick, particularly the brown dog tick, Rhipicephalus sanguineus. Moreover, as ticks are vectors of a variety of pathogenic agents, the reduction in the incidence of tick infestation afforded by the vaccines may concurrently reduce the incidence of diseases caused by these pathogenic agents in susceptible animals.
Owner:LOUISIANA STATE UNIV AGRI CENT +1
Design and preparation method and application of novel fasciola hepatica multi-epitope vaccine
The invention provides a novel fasciola hepatica multi-epitope vaccine. An active ingredient of the novel fasciola hepatica multi-epitope vaccine is a polypeptide. The polypeptide is mainly formed by fasciola hepatica sphingolipid activated mucin-like protein-2 Th, a multicopy body of B-cell epitope and a mucosal immunologic adjuvant cholera toxin B subunit. An artificial gene is mainly synthesized through the gene synthesis technology and comprises Th of sphingolipid activated mucin-like protein-2 and a gene sequence of the multicopy body of the B-cell epitope, then the artificial gene is coupled with the gene sequence of the cholera toxin B subunit, and a fusion gene is formed. An escherichia coli prokaryotic expression system is utilized for expressing the fusion gene, and the fasciola hepatica multi-epitope vaccine is obtained after protein purification. The fasciola hepatica multi-epitope vaccine can induce an organism to generate sphingolipid activated protein T cell immune responses and high-titer specific antibody humoral immune responses, and can be used for preventing and treating fasciola hepatica infection related diseases.
Owner:QINGHAI UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com