Design and preparation method and application of echinococcus multilocularis subunit vaccine LTB-Emy162
A multilocular hydatid and subunit vaccine technology, applied in the field of biomedicine, can solve the problems of poor immune effect, large human damage, and high recurrence rate, and achieve strong immunogenicity, high safety, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Molecular structure design of multilocular Echinococcus subunit vaccine LTB-Emy162
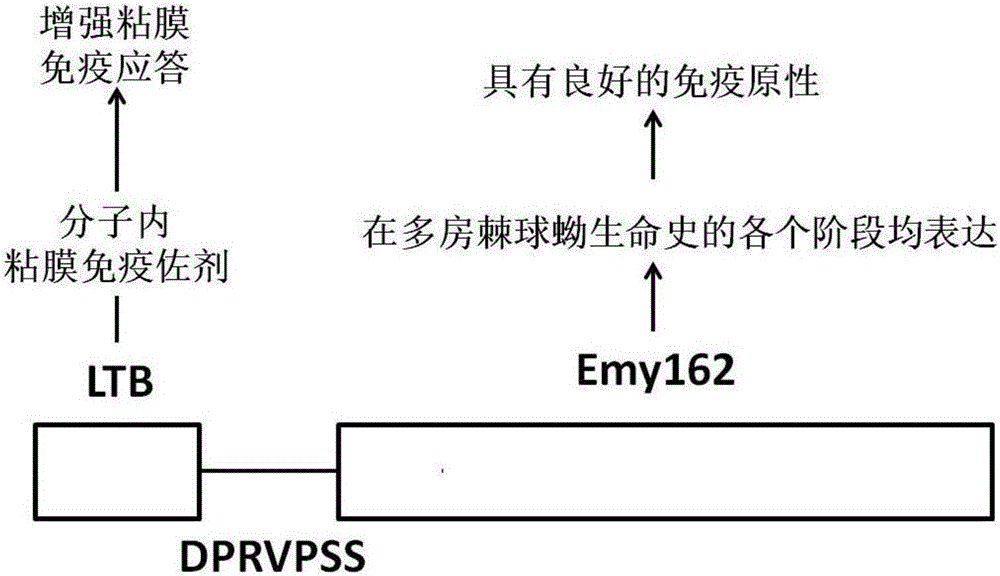
[0044] In the design of Echinococcus multilocularis subunit vaccine, the present invention selects the Emy162 antigen, and uses Escherichia coli heat-labile enterotoxin B subunit (LTB) as an intramolecular immune adjuvant, which is fused to the N-terminal of Emy162 to enhance the efficacy of Emy162. Immunogenicity.
[0045] Results: The molecular structure design characteristics and ideas of the multilocular Echinococcus subunit vaccine LTB-Emy162 are as follows: figure 1 shown.
Embodiment 2
[0046] Example 2: Construction of the recombinant expression vector pCzn1-LTB-Emy162 (containing the fusion gene LTB-Emy162)
[0047] The amino acid sequence of the previously designed multi-epitope peptide LTB-Emy162 was converted into the corresponding nucleotide sequence according to the codon preference principle of Escherichia coli, and the full-length splicing primer was designed based on the method of PAS (PCR-based Accurate Synthesis) , A protective base synthesis gene LTB-Emy162 was designed at both ends of the primers, and it was connected into the expression vector pCzn1 through the cloning sites Nde I and Xba I.
[0048] Results: The recombinant plasmid pCzn1-LTB-Emy162 to be detected was digested with Nde I and Xba I, reacted at 37°C for 2 hours, and detected by 1% agarose gel electrophoresis. The theoretical size of the gene LTB-Emy162 is consistent, as figure 2 shown. The vector construction map of the recombinant expression vector pCzn1-LTB-Emy162 is as foll...
Embodiment 3
[0049] Example 3: Prokaryotic expression of multi-epitope peptide fusion protein LTB-Emy162
[0050] The correct recombinant expression plasmid pCzn1-LTB-Emy162 was verified to be transformed into Escherichia coli Arctic Express strain. On the pre-prepared LB plate containing 50 μg / mL Amp, inoculate the loop-streaked genetically engineered strain pCzn1-LTB-Emy162 / Arctic Express, place it upside down in a 37°C incubator, and after cultivating overnight, pick a single colony and inoculate it on a plate containing In LB medium containing 50µg / mL Amp, culture overnight at 37°C, 220rpm. Inoculate the recombinant bacteria with 2% inoculum in LB medium containing 50 µg / mL Amp, 37°C, 220 rpm, cultivate until the OD600 of the bacteria is 0.6-0.8 (about 2 hours), add IPTG to make the final concentration reach 1mmol / L, 37 The expression was induced at 220 rpm for 4 hours at ℃, and the carrier strain pCzn1-LTB-Emy162 / Arctic Express induced without IPTG was used as a negative control.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com