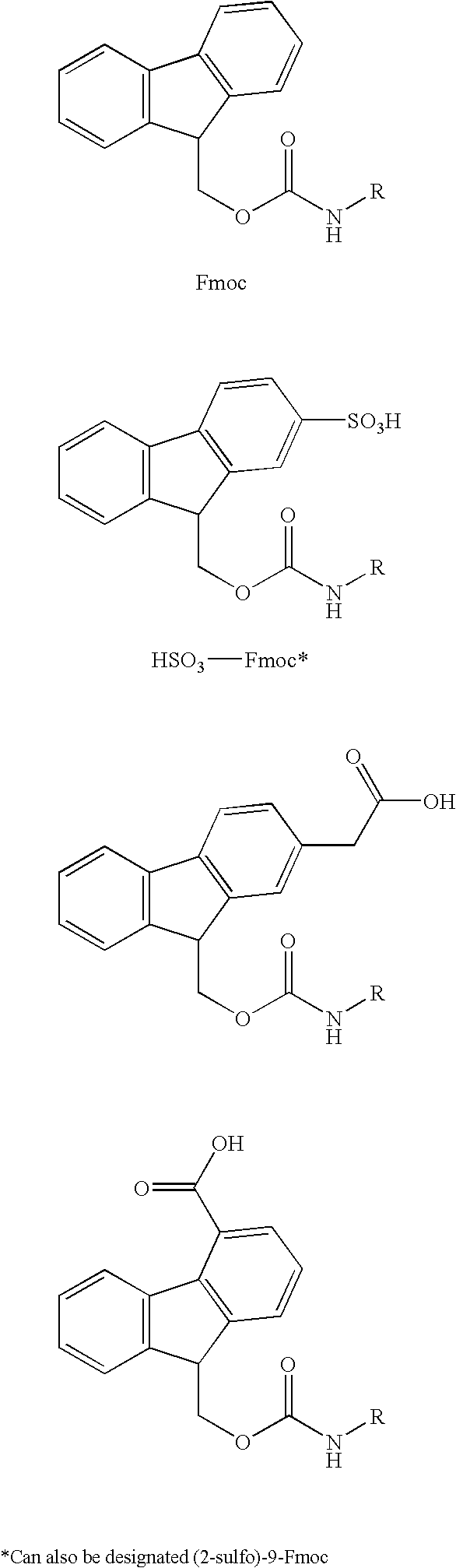
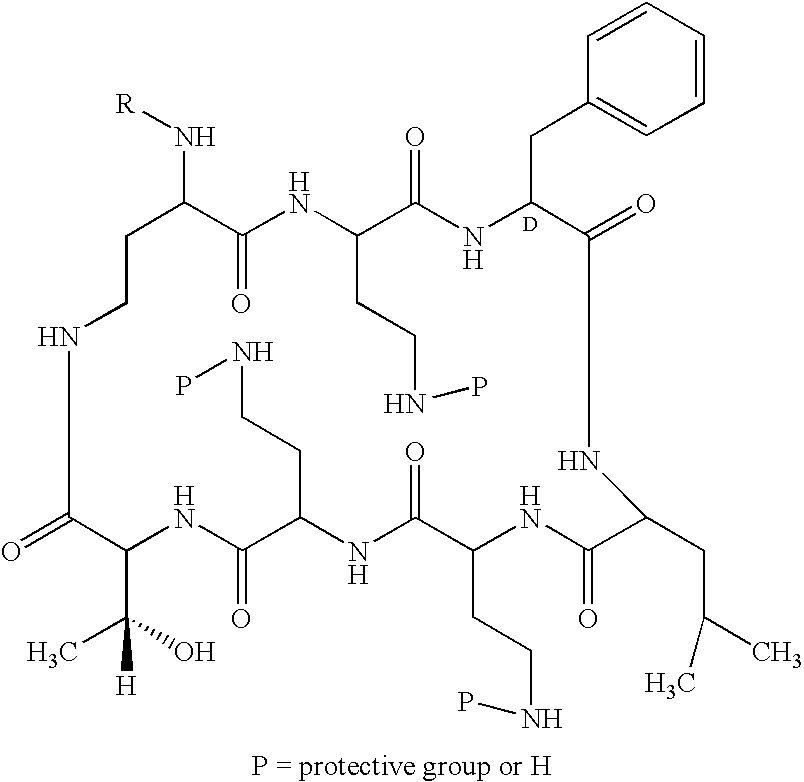
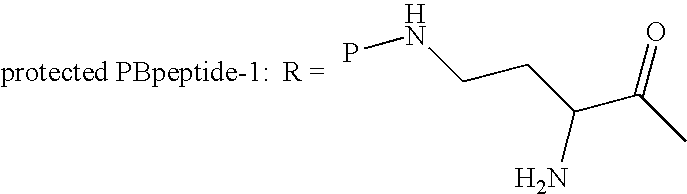
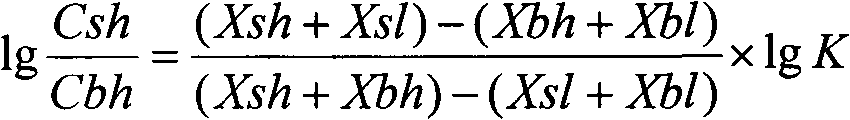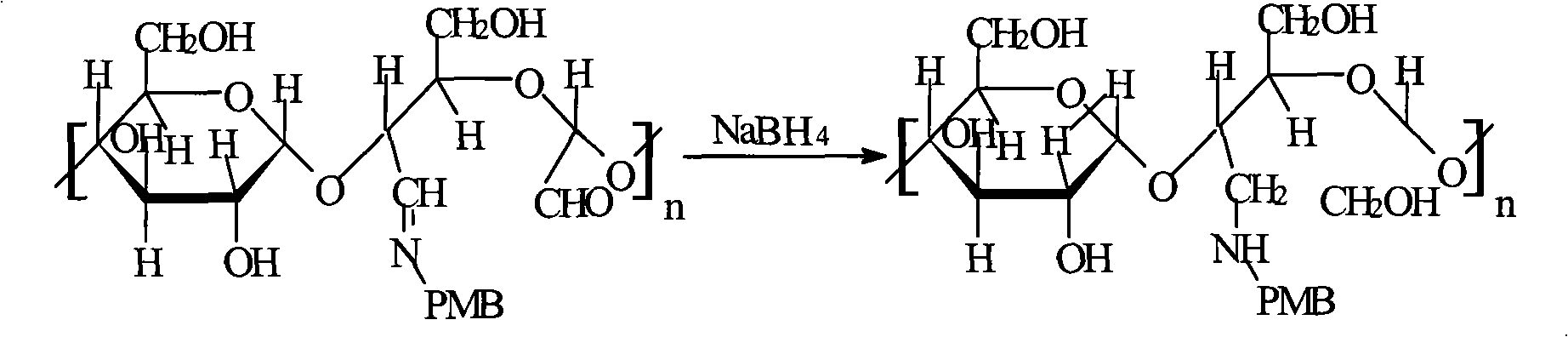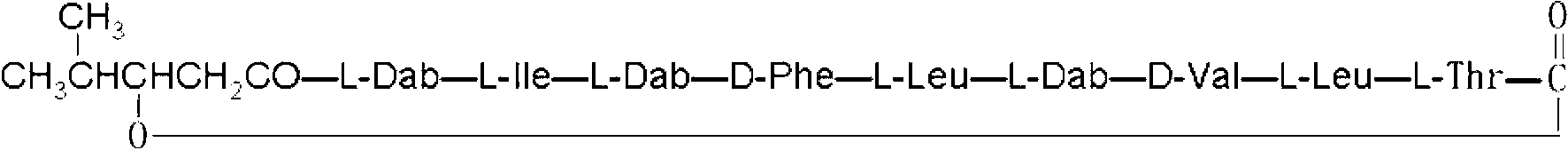Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
137 results about "B polymyxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
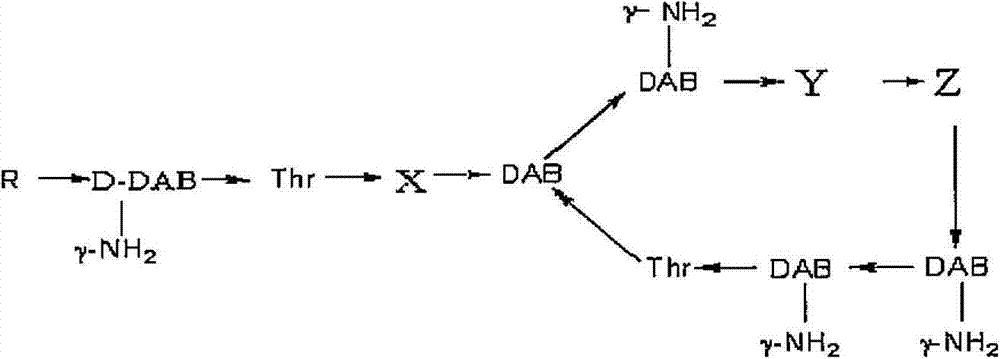
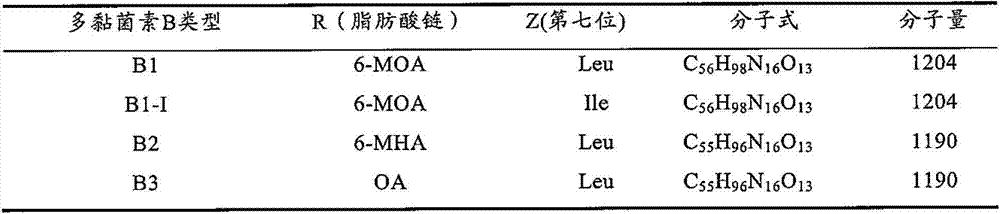
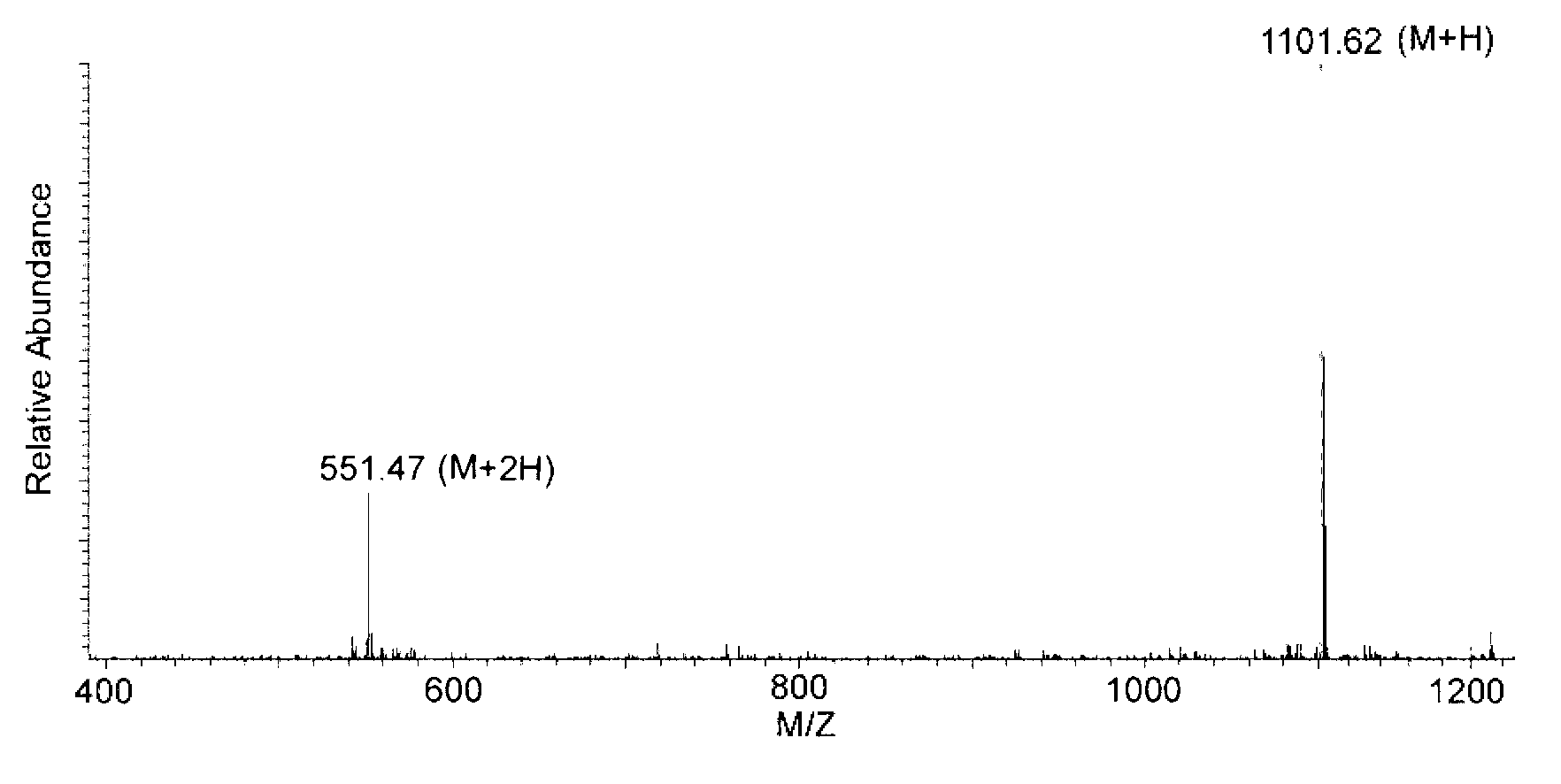
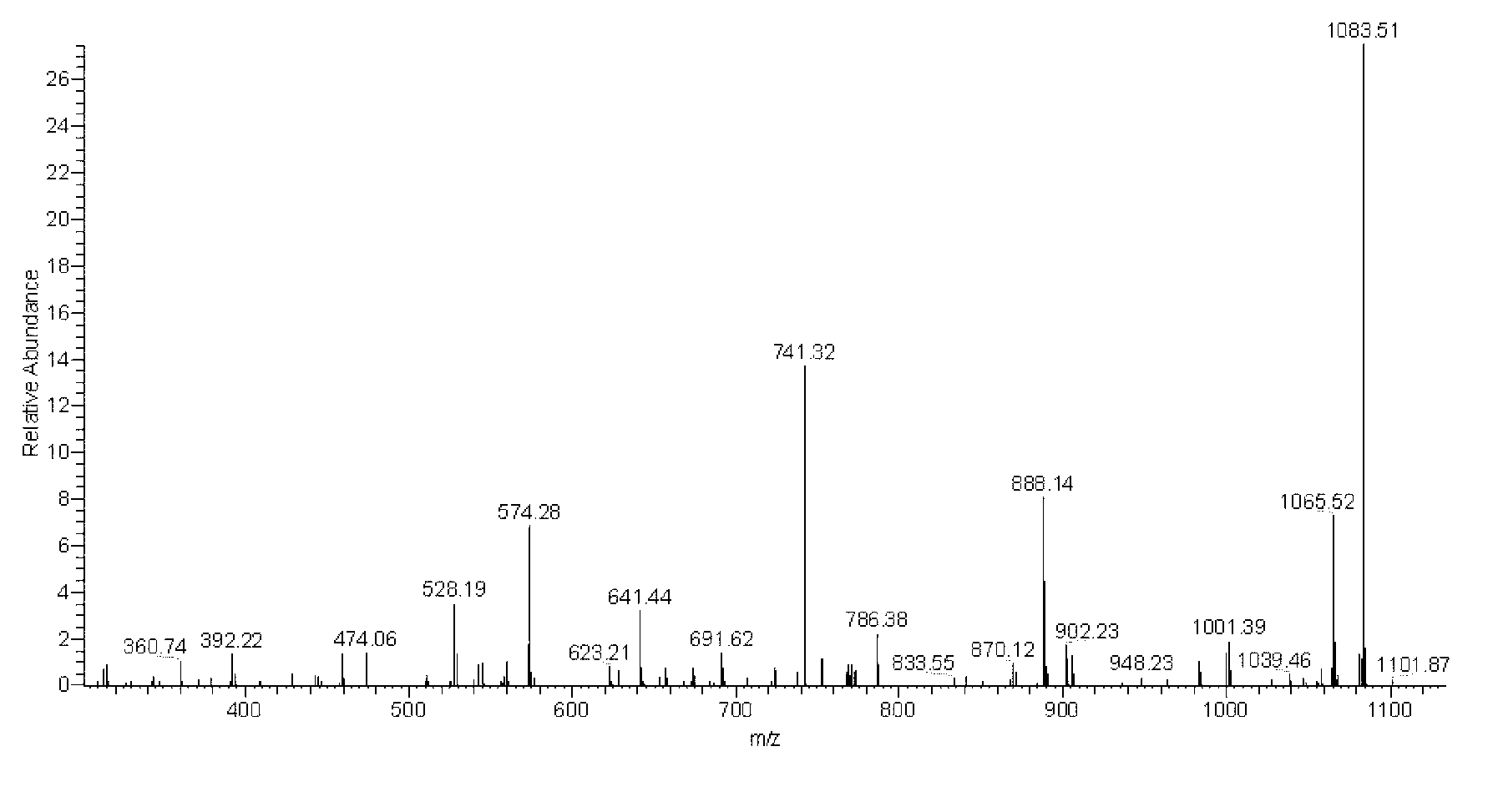
Polymyxin B is an antibiotic primarily used for resistant Gram-negative infections. It is derived from the bacterium Bacillus polymyxa. Polymyxin B is composed of a number of related compounds (see "Mixture composition").
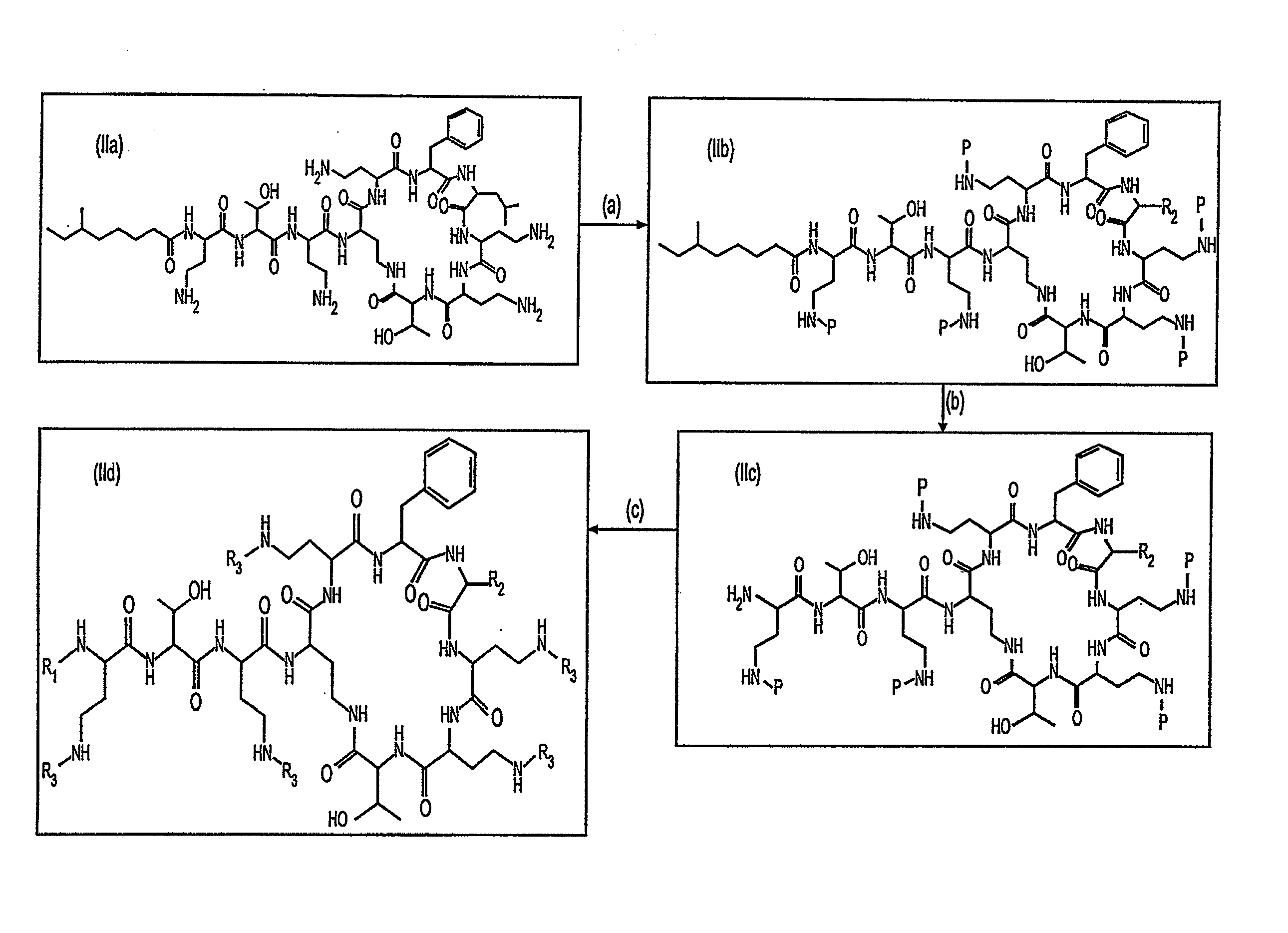
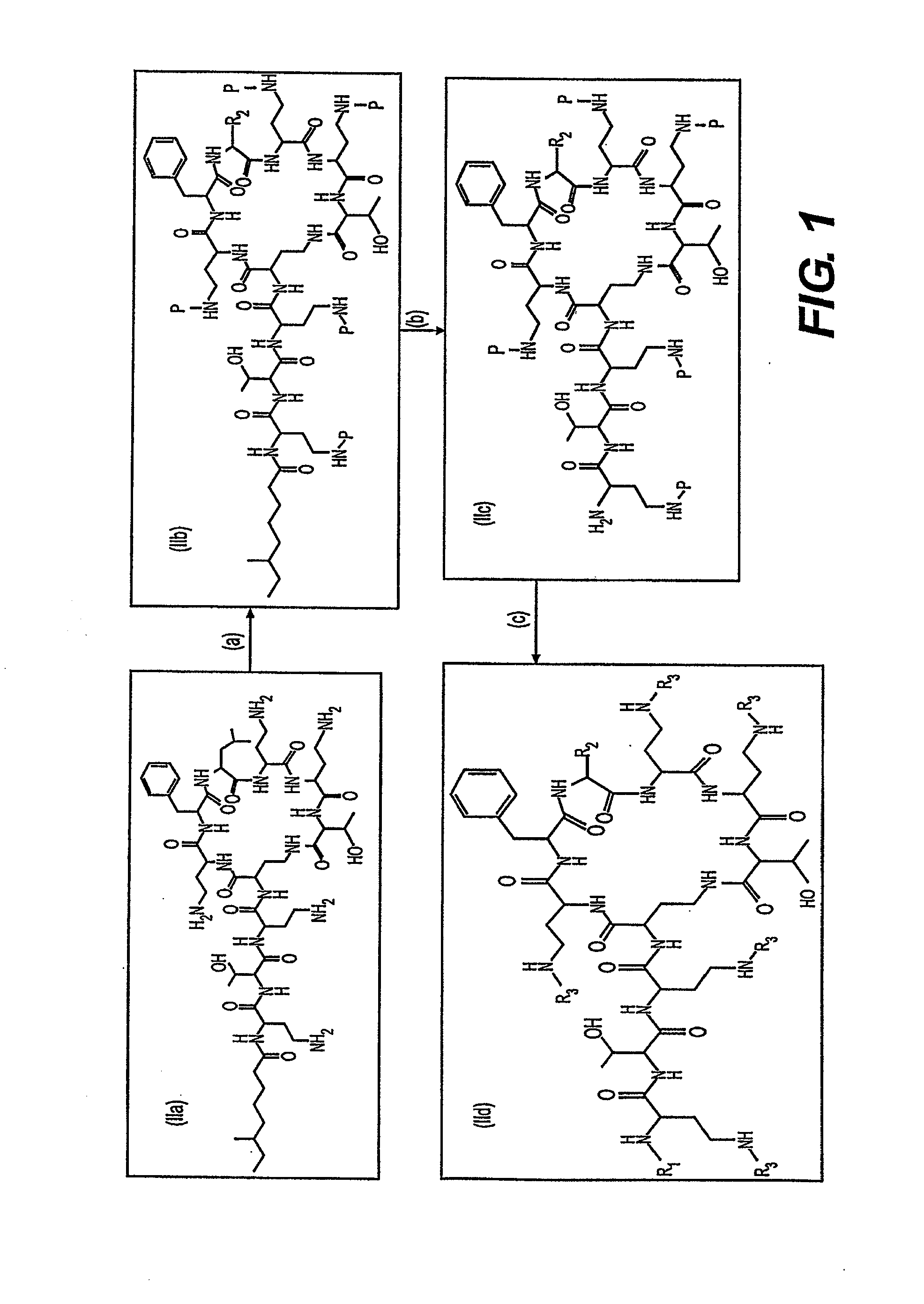
Peptide antibiotics and peptide intermediates for their prepartion
UndeterminedUS20060004185A1Potent activityReadily in aqueous solutionAntibacterial agentsPeptide/protein ingredientsAntibacterial activityAntibiotic Y
Novel protected cyclopeptide intermediates are prepared from polymyxin B are used to synthesize new peptide antibiotics. Intermediates are readily derivatized and deprotected to provide new families of antibiotics, which have potent anti-bacterial activity against gram-negative bacteria; but also are useful and potent against gram-positive bacteria.
Owner:BIOSOURCE PHARM INC
Endotoxin absorbing agent for blood perfusion and its preparation method
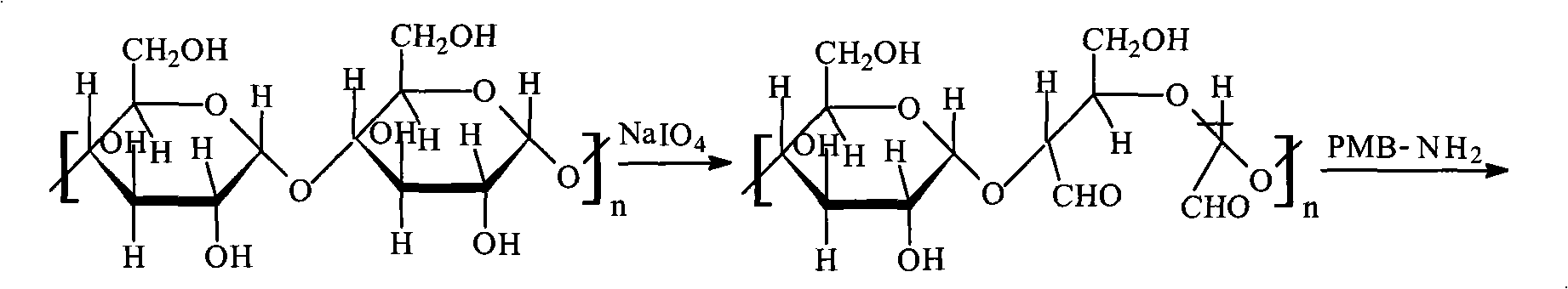
An endotoxin adsorbent used for blood perfusion is prepared from agat through adding the aqueous solution to the liquid mixture of toluene and CCl4 to obtain spherical agar, activating by epoxy chloropropane under alkaline condition, reacting on diamine and then on glutaraldehyde to obtain high-strength agar beads used as the carrier of adsorbent, reacting on the amino contained ligand (polymyxin B), and reducing by NaBH4. Its advantages are high compatibility to blood and high effect on absorbing endotoxin from blood.
Owner:NANKAI UNIV
Antibiotic compositions for the treatment of gram negative infections
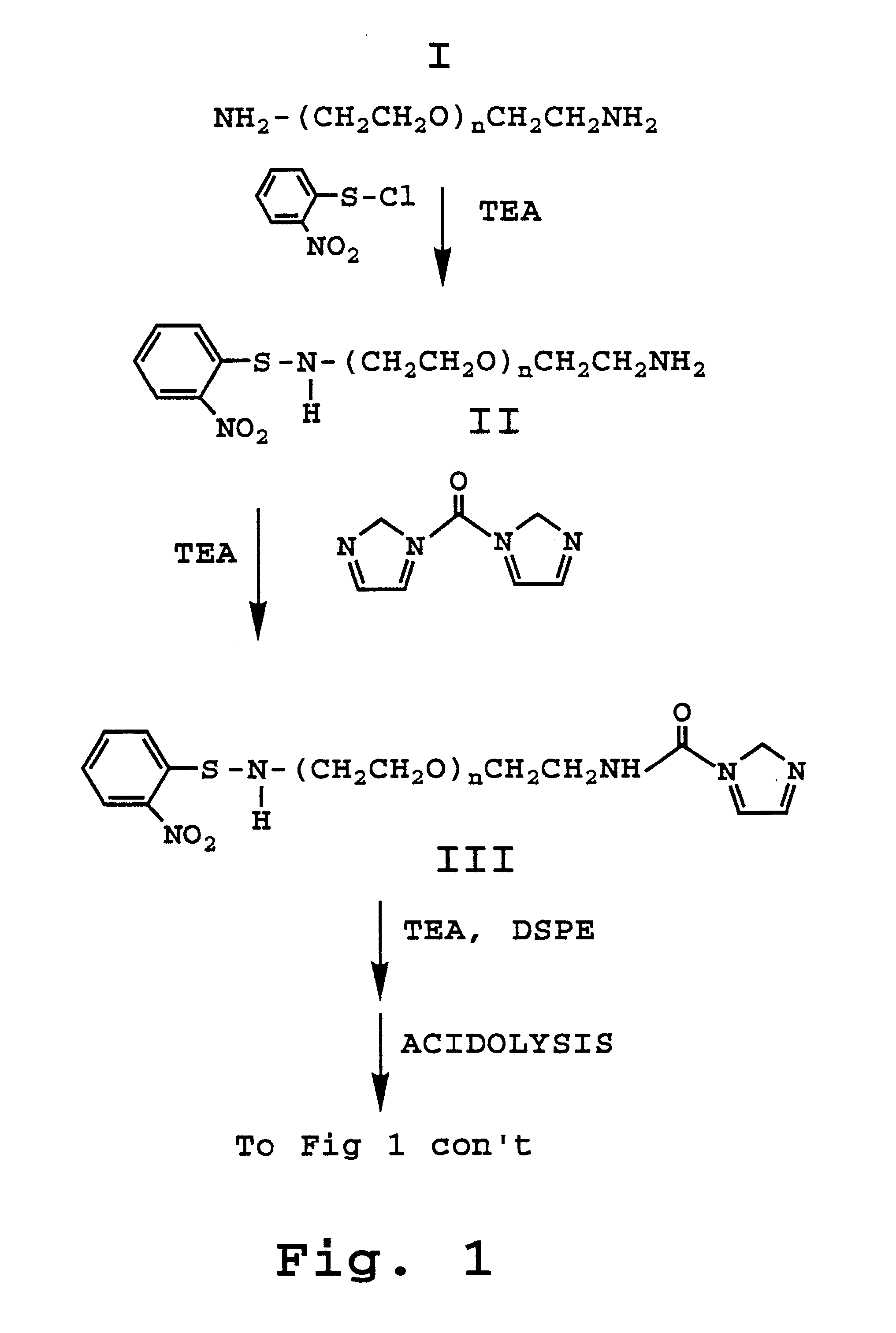
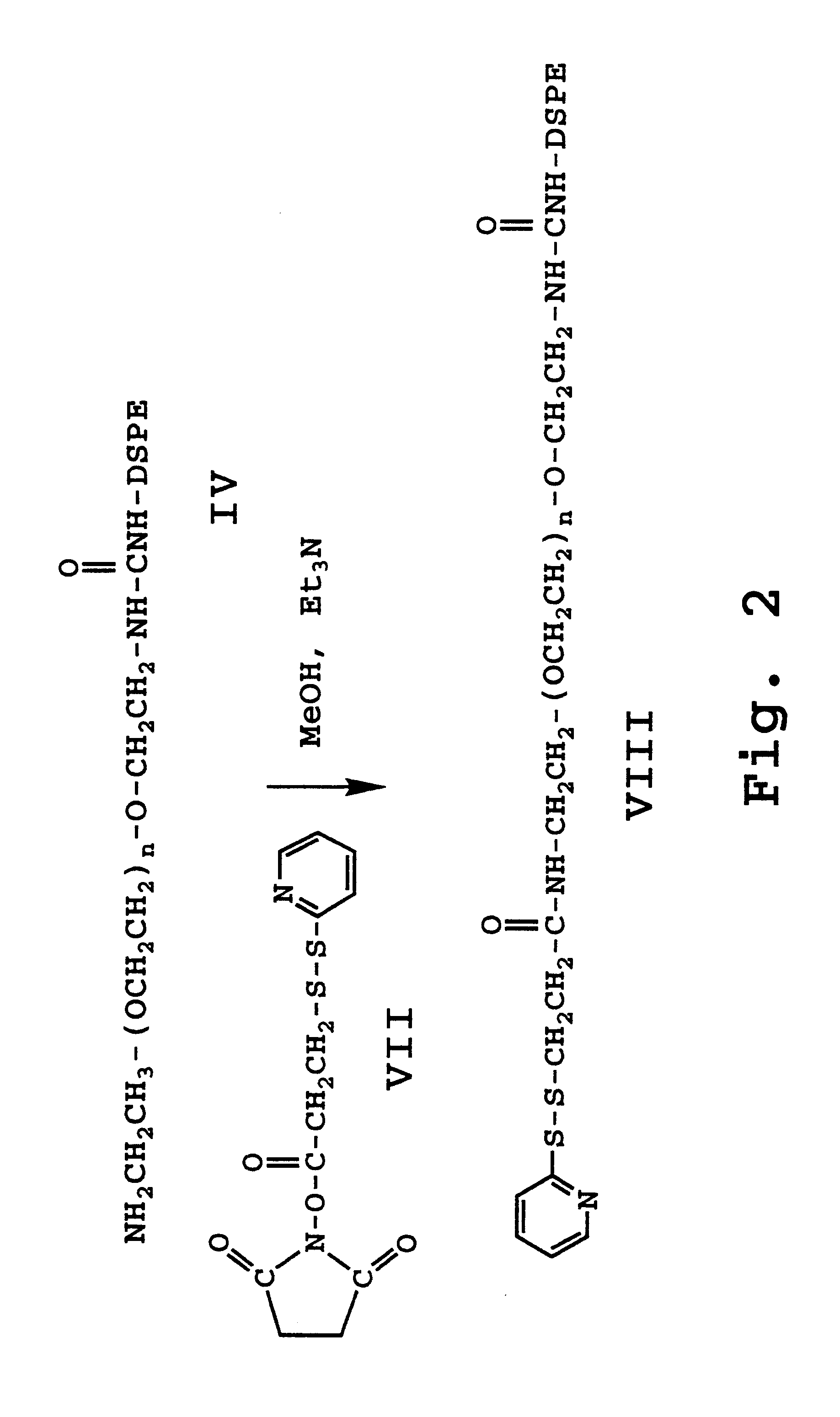
Provided herein are novel compounds and novel protected compounds that can be derived from polymyxin, including, e.g., polymyxin B1 and [Il7] polymyxin B1. The novel compounds have antibacterial properties against a diverse range of gram negative bacteria and reduced toxicity compared to polymyxins such as polymyxin B. Also provided are antibacterial pharmaceutical compositions containing the novel compounds and novel protected compounds, as well as methods for preparing the antibacterial compounds and protected compounds.
Owner:BIOSOURCE PHARM INC
Preparation method of endotoxin absorbent for blood perfusion
InactiveCN1864755AReduce non-specific adsorptionAvoid instabilityOther blood circulation devicesHaemofiltrationEndotoxin removalBiocompatibility Testing
The invention relates to an endotoxin adsorbent, which in detail relates to a method of preparing endotoxin adsorbent for blood perfusion. The method employs spherical agarose gel with good biocompatibility as base material, employs epichlorohydrin, hexamethylene diamine, 1, 1'-carbonyldiimidazole as activating agent, and bonds polymyxin B for endotoxin removal. The invention is characterized in that it overcomes the toxicity of cyanogen bromide and instability of aglycone, improves the safty and reliability of operation, reducesnon-specific adsorption through bonding polymyxin B with 1, 1'-carbonyldiimidazole, and improves biocompatibility of adsorbent and suits for treating endotoxemia. The invention is also characterized by simple experimental procedure and high clearance for endotoxin.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
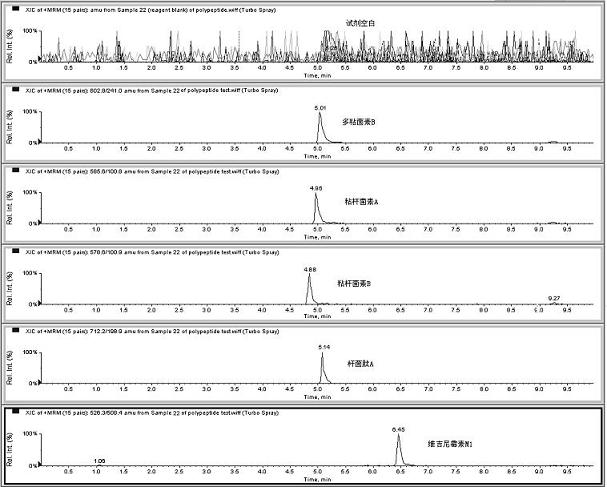
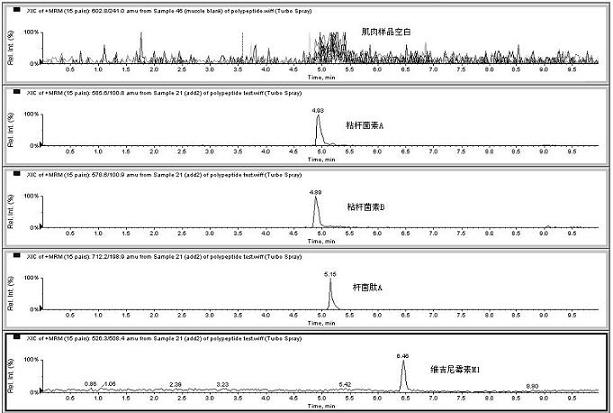
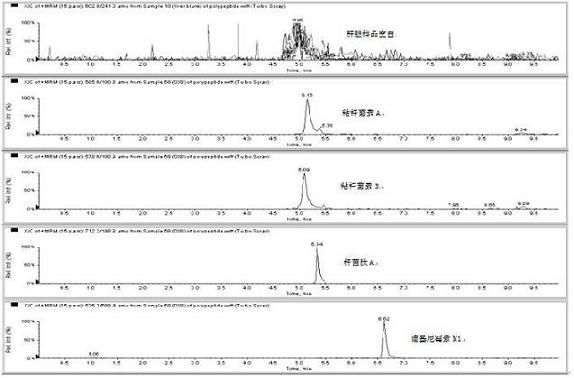
Method for detecting residual quantity of multiple polypeptidepolypeptide veterinary drugs in animal-derived food
InactiveCN102236005AAchieving Simultaneous DetectionReduce dissolutionComponent separationVirginiamycinVeterinary Drugs
The invention relates to the fields of analytical chemistry and food safety, particular to a method for detecting the residual quantity of multiple polypeptide veterinary drugs in animal-derived foods. The method comprises the following steps of: processing a sample with a TCA(trichloroacetic acid) and acetonitrile system for depositing proteins; extracting with a carbinol and 0.1% formic acid aqueous solution system; purifying with a Oasis HLB solid phase extraction column; performing gradient elution with an Eclipse XDB-C18 analytical column in the presence of an acetonitrile and 0.1% formic acid aqueous solution used as a mobile phase; and then performing electrospray and positive ion scanning mode separation for finally detecting four polypeptides. The limits of the four polypeptides, namely colistin, bacitracin A, polymyxin B and virginiamycin M, are 25 micrograms / kilogram, 100 micrograms / kilogram, 250 micrograms / kilogram and 120 micrograms / kilogram respectively; the recovery rates of colistin, bacitracin A, polymyxin B and virginiamycin M are 74.9-88.1%, 76.2-89.0%, 76.6-81.2% and 77.3-86.9% respectively; and the coefficients of variation (CV%) of colistin, bacitracin A, polymyxin B and virginiamycin M are 5.7-15.1%, 7.2-15.7%, 6.0-8.0% and 9.5-18.6% respectively. The detection limit, the recovery rate, the accuracy and other technical indexes all meet related detection requirements at home and aboard.
Owner:林维宣
Preparing method of high-purity polymyxin B
InactiveCN103130876AImprove product qualityHigh purityPolymyxinsPeptide preparation methodsFreeze-dryingPhosphate
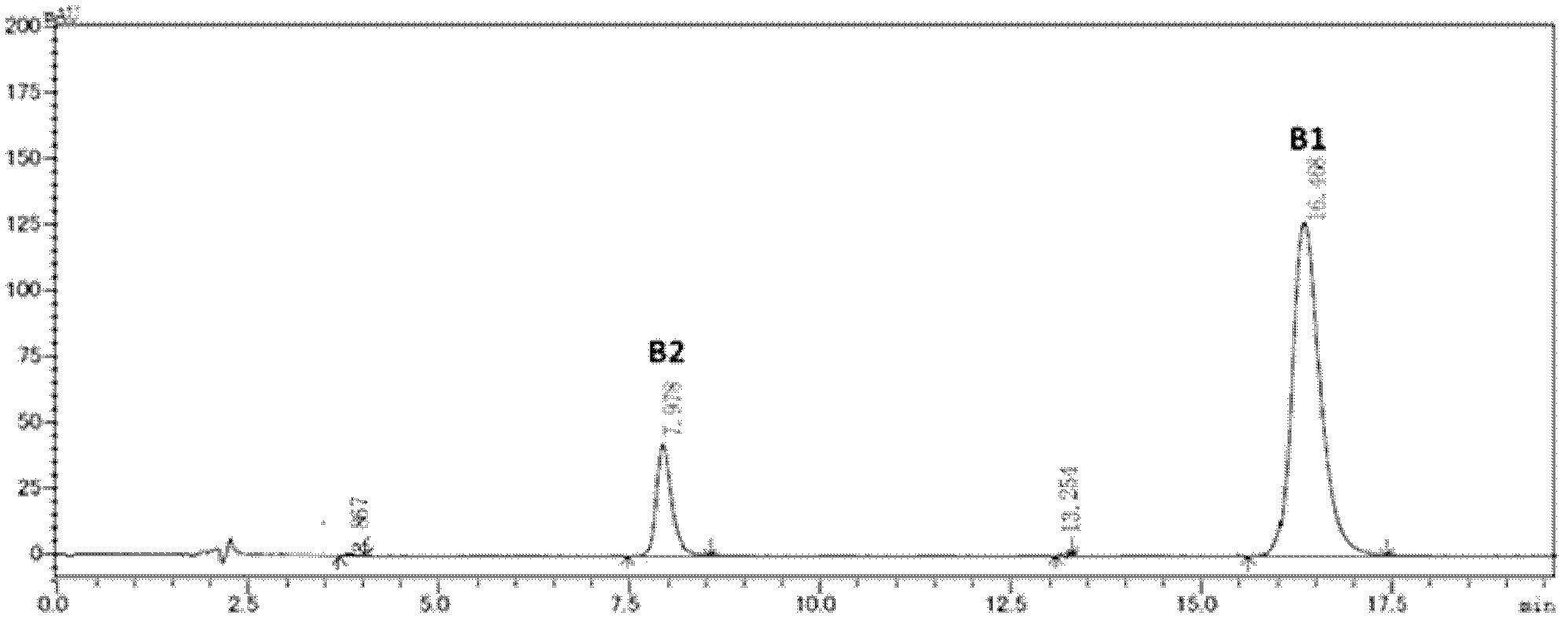
The invention discloses a preparing method of high-purity polymyxin B. The preparing method comprises the steps of preparation of column-loading solution; injecting the column-loading solution into a high performance liquid chromatography preparative column, wherein sodium sulfate - phosphate buffer / organic solvent is used as mobile phase, collecting target components, removing impurities, especially B1 - 1 and B3, and determining collected solution by means of high performance liquid chromatography (HPLC), wherein chromatographic purity of the target components B1 or B2 or mixture of the B1 and the B2 is more than 99.5%; and purification, wherein vacuum concentration is carried out on the collected target components so that the organic solvent is removed, organic solvent desalination concentration is carried out on balance phase, concentrated solution is spray-dried or freeze-dried to produce the polymyxin B with purity of more than 99.5%. The preparing method of the high-purity polymyxin B has the advantages of being high in purity, high in yield, simple in process, and capable of injecting sample solution continuously. Quality of products is far better than the medical standard regulated by the European Pharmacopoeia (EP) and the United States Pharmacopeia (USP). One-time investment of equipment is slightly high, while operation cost is not high, and therefore the preparing method of the high-purity polymyxin B is suitable for industrial production.
Owner:HUBEI RUIHAO ANKE MEDICINE TECH DEV CO LTD
Enhanced circulation effector composition and method
InactiveUS6586002B2Stimulate immune responseBiocideOrganic active ingredientsCrystallographySurface layer
A liposome composition comprising small, surface-bound effector molecules is disclosed. The liposomes have a surface layer of hydrophilic polymer chains, for enhanced circulation time in the bloodstream. The effector molecules are attached to the distal ends of the polymer chains. In one embodiment, the effector is polymyxin B, for treatment of septic shock.
Owner:ALZA CORP
Polypeptin and preparation and application thereof
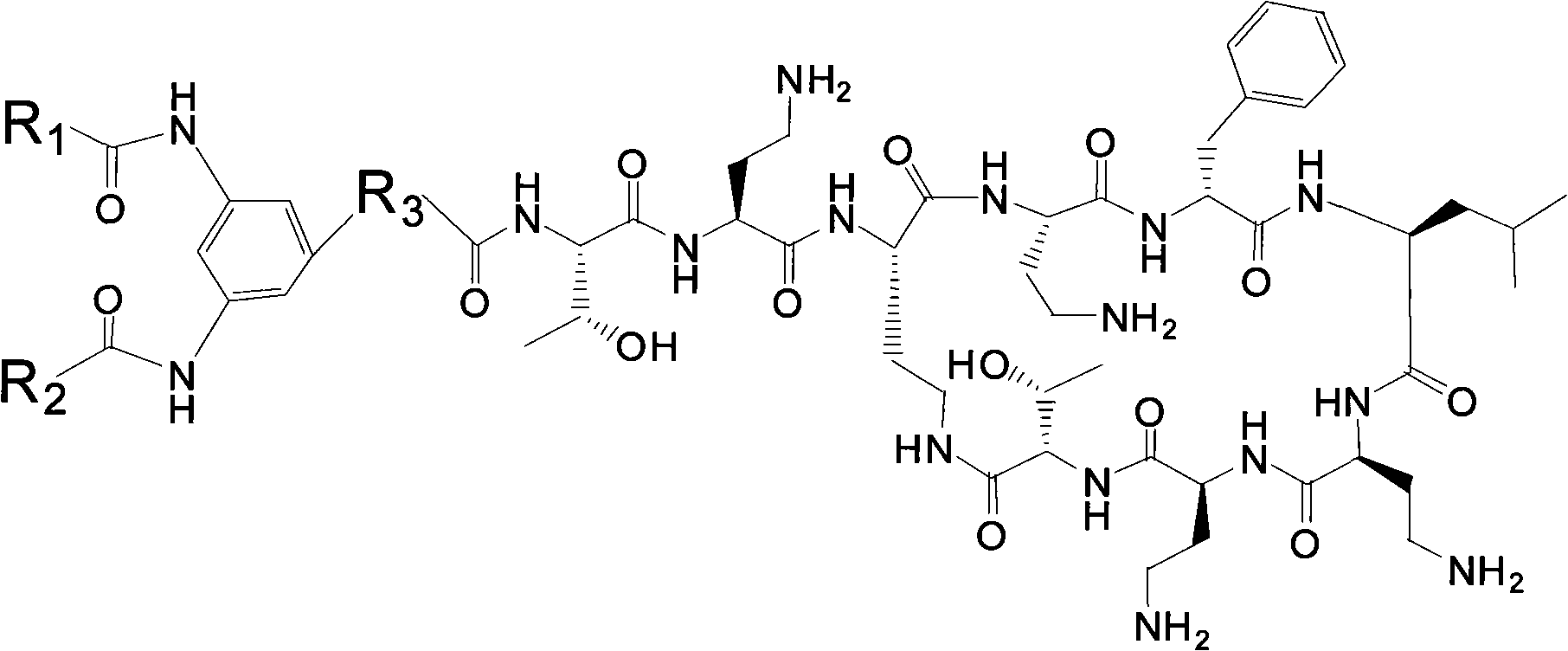
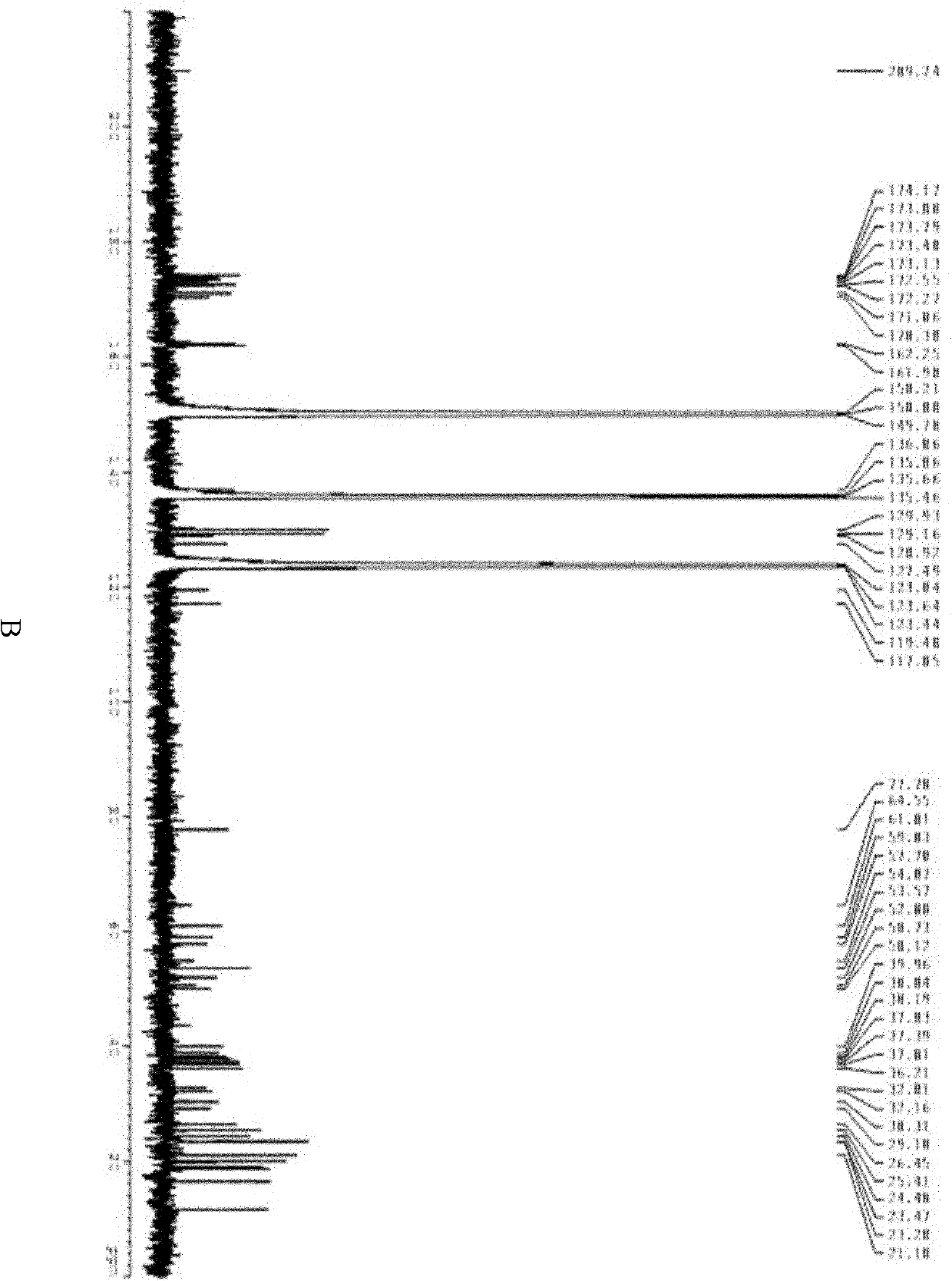
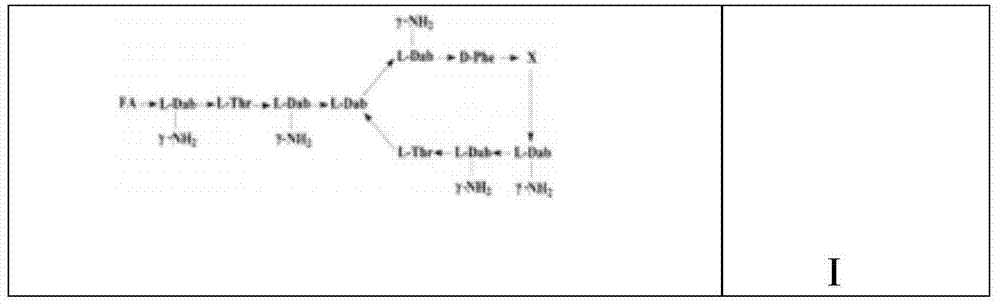
InactiveCN101962401ALow toxicityImprove securityBiocideMicroorganism based processesPolymyxin BSheath blight
The invention provides novel polypeptin having potential value of biological control and medical applications, and a preparation method and application thereof. The structure of the polypeptin is shown as a formula (I). The invention has the advantages of providing the polypeptin which has the toxicity lower than that of polymyxin B and has the potential value of biological control and medical applications, and the preparation method and the application thereof. In vitro experiments show that the compound has the obvious effect of inhibiting all the tested gram-positive bacterium, gram-negative bacterium and fungi. Disk-culturing experiments show that the compound has certain effect of preventing and treating various fungal plant diseases and particularly has obvious effects of preventing and treating rice sheath blight.
Owner:ZHEJIANG UNIV +1
Polymyxin derivative and preparation method thereof
InactiveCN101851270AHigh antibacterial activityHuman toxicity is smallAntibacterial agentsPeptide preparation methodsPolymixin EGram
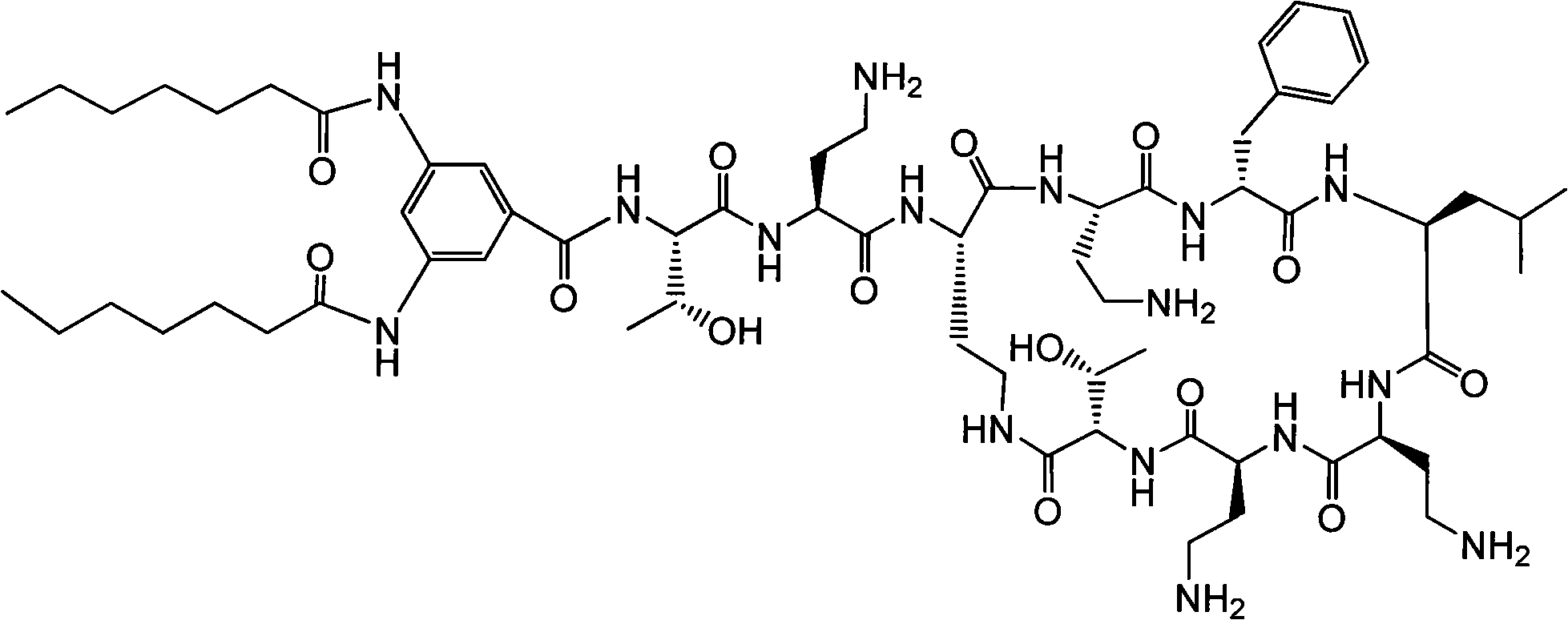
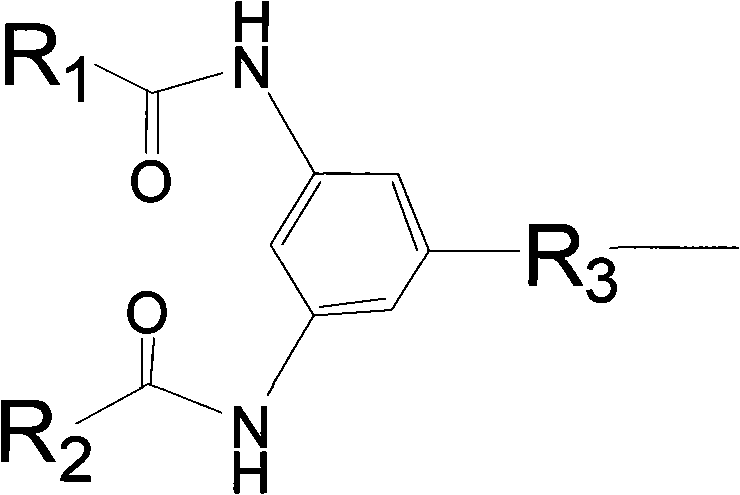
The invention discloses a polymyxin derivative and a preparation method thereof. Since zymohydrolysis, fatty acid chain on the end N of polymyxin B and one diaminobutyric acid residue are removed, a 3, 4-oxamido-benzoyl group is introduced through a chemical decoration technology or a benzene ring group with two fatty acid chains is introduced, so the polymyxin derivative is obtained. The derivative can be used for the clinical treatment of infection of Gram-negative multidrug resistance bacteria.
Owner:梁浩 +1
Culture medium used for separating and screening lactic acid bacteria, preparation method and application thereof
The invention provides a culture medium used for separating and screening lactic acid bacteria. The formula of the culture medium comprises the following components: relative to the dosage of 1000ml of a solvent, 5.0-15.0g of tryptone, 5.0-15.0g of beef extract, 2.0-10.0g of yeast extract, 1.0-3.0g of diammonium citrate, 15.0-25.0g of glucose, 0.2-2.0 mL of Tween-80, 2.0-8.0g of sodium acetate, 15.0-20.0g of agar, 2.0-30.0g of calcium carbonate, a compound acid-base indicator containing 0.01-0.5g of methyl red and 0.01-0.7g of bromothymol blue, 0.5-5.0g of ascorbic acid, 0.1-0.5g of L-cysteine HCl, and a selective antibiotic composed of 250000-500000 IU of polymyxin B and 0.01-0.05g of nalidixic acid, wherein the pH value of the culture medium is 6.8; and the solvent is composed of a primary solvent and a secondary solvent, the primary solvent is distilled water or aged seawater, and the secondary solvent is saline solution. The culture medium has the characteristics of being high in accuracy, obvious in authentication phenomenon and the like during separation and screening for lactic acid bacteria.
Owner:江门市澳保生物科技有限公司
Sterile cultivation method for enteromorpha
InactiveCN103609425AUseful for laboratory researchImprove accuracyClimate change adaptationCultivating equipmentsPenicillinNeomycin
The invention relates to a sterile cultivation method for algae, in particular to a sterile cultivation method for enteromorpha. Firstly, enteromorpha seedlings are washed 2-3 times, and the surfaces of the enteromorpha seedlings are absorbed dry; secondly, three antibiotics including the penicillin G, the neomycin and the polymyxin B are added into a VSE nutrient solution to be prepared into an antibiotic nutrition solution; thirdly, the enteromorpha seedlings are placed in the antibiotic nutrition solution, the cultivation temperature ranges from 18 DEG C to 25 DEG C, the illumination intensity ranges from 130mumol / m<2>*s, the photoperiod is 12L:12D, the enteromorpha seedlings are cultivated in an inflation mode, and the antibiotic nutrition solution is replaced every 3-6 days until the enteromorpha seedlings grow into mature algae. The sterile cultivation is carried out on the enteromorpha collected in the field in a laboratory, the enteromorpha is tested and has no infectious microbe growth, and accordingly the accuracy of the later experiment results of the enteromorpha is greatly improved. The sterile cultivation method is easy to operate, the enteromorpha cultivation system does not need to be changed, raw materials are easy to obtain, price is low, and cost is low.
Owner:SHANGHAI OCEAN UNIV
Method for synthesizing polymyxin B by fermentation method
InactiveCN101235407ARaise the fermentation unitMicroorganism based processesFermentationNitrogen sourcePolymyxin B
Owner:SHANGHAI DUOMIRUI BIOLOGICAL TECH CO LTD +1
Method for extracting polymyxin B and E from fermentation technique culture
InactiveCN101974075AHigh yieldProduction cost caused by high yield and lowPolymyxinsPeptide preparation methodsSeparation technologySolvent
The invention relates to a method for extracting polymyxin B and E from a fermentation technique culture, which comprises the following steps of: adjusting the pH value of the fermentation technique culture to 10.0 to 14.0 by utilizing an alkaline substance under the temperature of 0 to 25 DEG C and then stirring for 30 min; collecting fungus dregs by utilizing a solid-liquid separation technique to obtain a polymyxin B and E free alkali precipitate; adjusting the pH value of the free alkali precipitate obtained by the solid-liquid separation technique to 1.0 to 6.0 by using an acid substance or solution; after the polymyxin B and E free alkali precipitate is dissolved, carrying out solid-liquid separation to obtain the concentrated solution of polymyxin B and E salts; removing inorganic salts by using a nanofiltration technology; and finally carrying out spray drying to obtain the finished product. The method is characterized by extracting the polymyxin B and E from fermentation liquor in a convenient and high-efficiency mode, solves the problem of high production cost because of high labor strength, long working procedure and low extraction yield in the production process of other extraction methods, and has the advantages of no use of solvents, low cost, environment protection, high yield and low characteristic requirements on the fermentation liquor and equipment.
Owner:SHANDONG LUKANG PHARMA
Legionnella selective separation medium
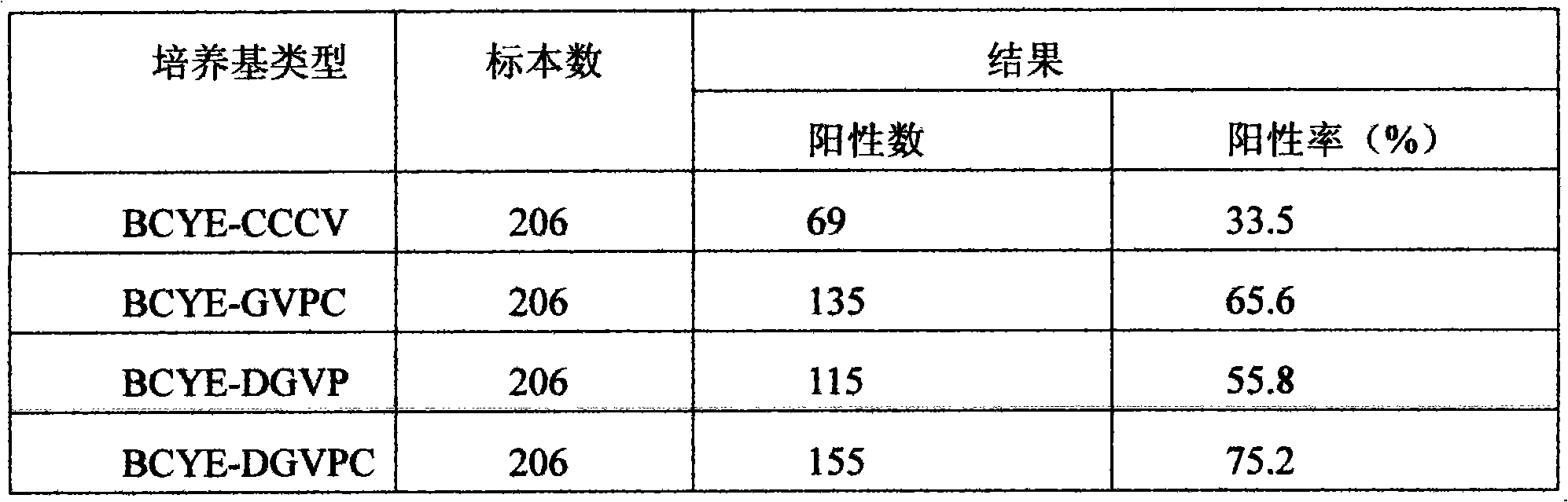
ActiveCN101643713AImprove the positive rate of isolation and cultureGrowth inhibitionBacteriaMicrobiological testing/measurementAntibiotic YPolymyxin B
The invention discloses a legionnella selective separation medium. Dyes such as bromothymol blue and bromocresol purple are added to the basic formulation of the basic medium of legionnella BCYE alphaand legionnella selective antibiotics of glycin, vancocin, polymyxin B and actinone are also added. The added dyes produce pigment in the growth process of legionnella; the shape and the color of a colony are beneficial to identifying and distinguishing a target bacterium of the legionnella; the concentrations of the selective antibiotics are added and regulated, the inhibiting capability to non-legionnella and microbial class is enhanced and the inhibiting performance for the legionnella is reduced, thereby enhancing the positive rate for the separation culture of the legionnella; the detection cost is reduced and the legionnella selective separation medium is economic and practical and is convenient for popularization and application for clinical and health and epidemic prevention departments.
Owner:贵州金域医学检验中心有限公司
Method for separation purification of polymyxin B1 from polymyxin B mixed component
InactiveCN103923190AEasy to operateEasy industrial scale-upPolymyxinsPeptide preparation methodsOrganic solventElution
The present invention provides a method for separation purification of polymyxin B1 from a polymyxin B mixed component. The method comprises: (a) loading a chromatography medium of a macroporous absorption resin to obtain a chromatography column; and (b) adding a polymyxin B solution, and adopting an organic solvent-containing acid solution as an eluent to sequentially elute various components of the polymyxin B so as to obtain the polymyxin B1 solution after elution. According to the method, the operation is simple, the adopted separation medium has characteristics of stable chemical characteristic and high re-use rate, industrial scale-up is easily achieved, and the peak purity of the obtained polymyxin B1 can be more than 98%.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Method for extracting polymyxin B from fermentation broth
InactiveCN103130875AAvoid infectionLower resistanceAntibacterial agentsPolymyxinsTime-ConsumingChemistry
The invention discloses a method for extracting polymyxin B from a fermentation broth of the polymyxin B. The method for extracting the polymyxin B from the fermentation broth of the polymyxin B comprises the following steps: a, conducting solid-liquid separation on the fermentation broth of the polymyxin B and obtaining a filtrate; b, extracting the filtrate with a polar organic solvent, and extracting an extract liquor reversely with water when the extract liquor is at the potential hydrogen (pH) value of 1.0-2.0; c, adding the reversal extract liquor which is obtained from the step b into inorganic salt, wherein the volume of the reversed extract liquor of the inorganic salt is 3-8% (w / v), and adjusting the pH value to be 9.0-10.0 so that the polymyxin B is obtained after precipitation and sediment. Preferably, the method for extracting the polymyxin B from the fermentation broth further comprises a step of d: reacting the polymyxin B and sulfuric acid to form salt, and dissolving and precipitating, thereby obtaining the polymyxin B sulfate. The polymyxin B or the sulfate prepared by means of the method is higher in yield and purity, the purity of a product is above 85%, the tilter is above 8100IU / g, and the yield is above 60%. The method for extracting polymyxin B from the fermentation broth has the advantages of being easy and convenient to operate, short in time consuming, low in requirement of equipment, and capable of recycling the organic solvent and being used for large-scale industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Method for preparing endotoxin adsorbing agent
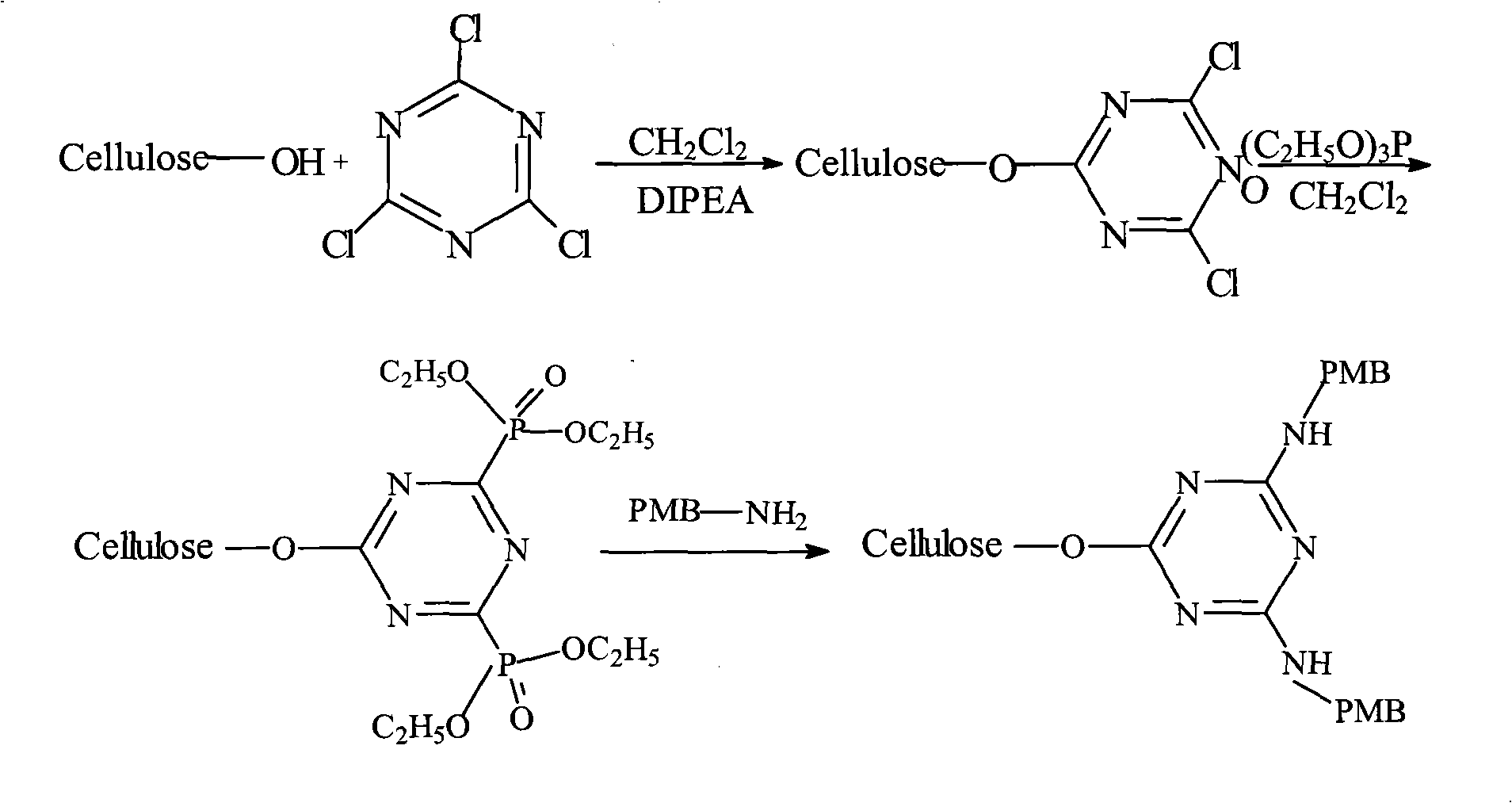
The invention relates to a preparation method of an endotoxin adsorbent. The spherical porous cellulose is taken as a carrier; the spherical porous cellulose is activated by triazine trichloride, coupled with triethyl phosphate, then immobilized polymyxin B, thus the endotoxin adsorbent is obtained. The endotoxin adsorbent obtained by the method has stable performance and good adsorption property.
Owner:JAFRON BIOMEDICAL
Endotoxin absorbent and preparation thereof
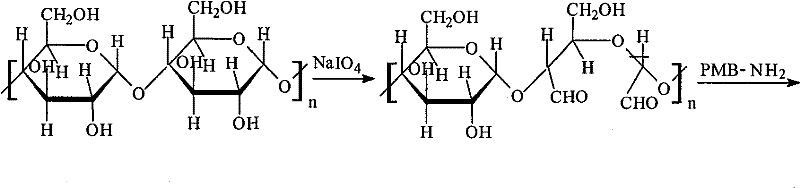
The present invention relates to an endotoxin adsorbent which is composed by taking spherical porous cellulose as a carrier and immobilizing effective amount of polymyxin B in the endotoxin adsorbent for blood perfusion as a ligand. The preparation method of the endotoxin adsorbent includes that the spherical porous cellulose is taken as the carrier, the spherical porous cellulose first reacts with sodium periodate and is bonded with the effective amount of polymyxin B, and the endotoxin adsorbent is obtained after reduction by sodium borohydride. The endotoxin adsorbent has the advantages of stable performance, large supported quantity of polymyxin B, good adsorption effect, etc.
Owner:JAFRON BIOMEDICAL
Broad spectrum polypeptin and application thereof
InactiveCN102030819AEnhanced inhibitory effectGood control effectBiocidePeptide/protein ingredientsPolymyxin BPlant disease
The invention provides novel polypeptin with biological prevention and control and medical application potential values, and application thereof. The polypeptin has a structure shown in a formula (I). The invention has the main advantage of providing the polypeptin with toxicity lower than that of polymyxin B and biological prevention and control and medical application values, and a preparation method and application thereof. In-vitro experiments show that the compound has a remarkable inhibiting effect on gram-positive bacteria, gram-negative bacteria and fungi; and in-plate cultivation experiments show that the compound has certain prevention and control effects on a plurality of fungal plant diseases and particularly has the most remarkable prevention and control effects on rhizoctonia solani.
Owner:ZHEJIANG UNIV +1
Selective coloration culture medium of clostridium perfringens
InactiveCN101659980ARapid cultivationRapid identificationMicrobiological testing/measurementMicroorganism based processesBacteroidesSulfite salt
The invention relates to a selective coloration culture medium for fast culturing and identifying clostridium perfringens. The selective coloration culture medium is characterized in that the preparation of 1000ml of the culture medium requires 12.0g-20.0g of casease hydrolysate, 6.0g-12.0g of yeast extract powder, 0.20g-0.80g of sodium sulfite, 0.005g-0.015g of polymyxin B, 0.05g-0.20g of sulfadiazine, 0.20g-0.80g of ferric citrate, 4-12 units of Y.S.N. and 10.0g-18.0g of agar powder and also requires the final addition of distilled water till the volume of 1000ml is achieved. The selective coloration culture medium has the advantages that the culturing time is obviously reduced when being compared with a conventional method and other bacteria with the same culturing characteristics are inhibited and do not grow so that the effect of fast clostridium perfringens culturing and identification can be realized.
Owner:CHENGDU RICH SCI IND
Antibacterial drug composition for treating Gram-negative bacterial infections
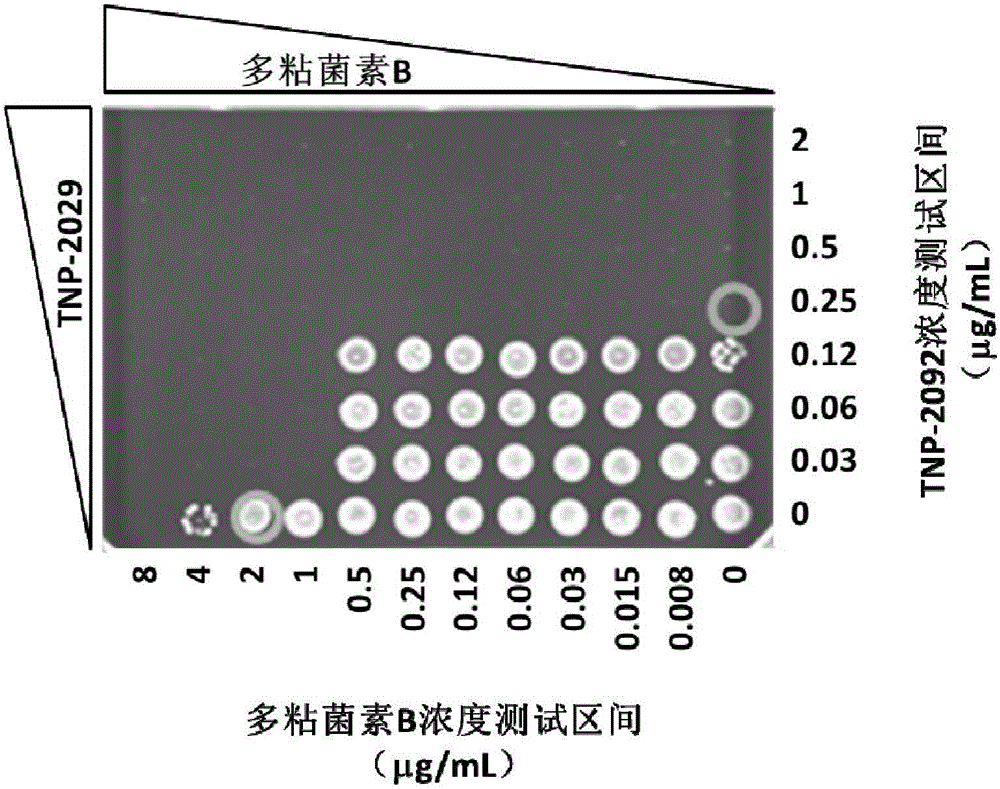
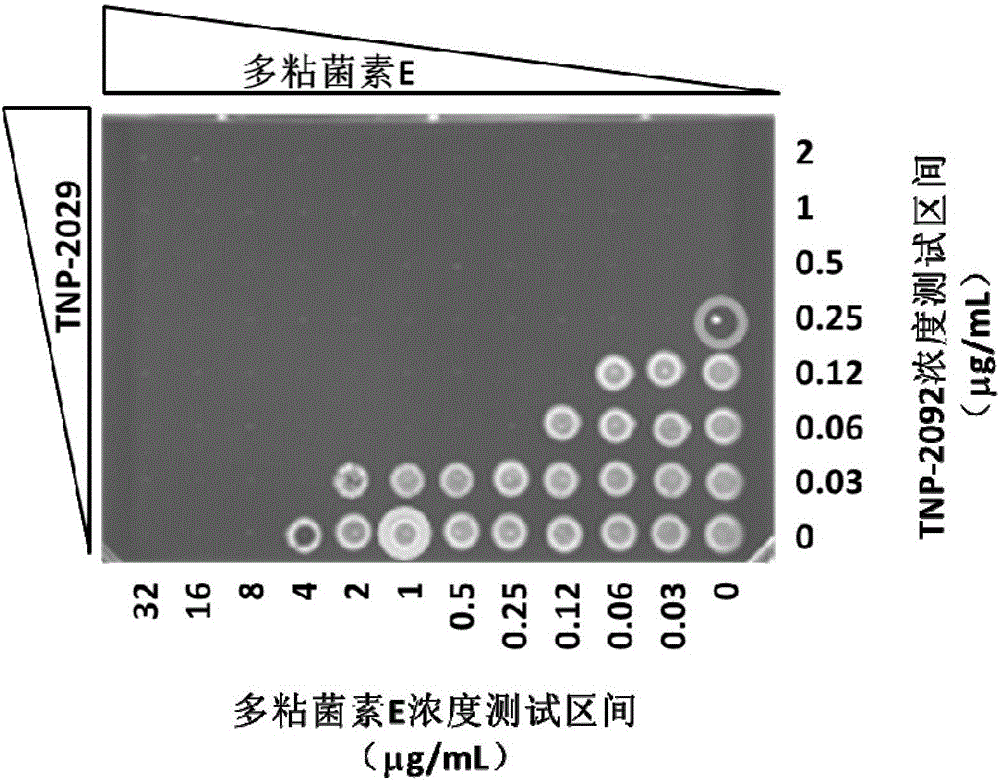
InactiveCN105879009AHigh antibacterial activityAntibacterial agentsOrganic active ingredientsAntimicrobial drugPolymixin E
The invention discloses an antibacterial drug composition for treating Gram-negative bacterial infections. The composition is prepared from TNP-2092 and a cell membrane penetrant, wherein the cell membrane penetrant is polymyxin B or polymyxin E. According to the antibacterial drug composition for treating Gram-negative bacterial infections, the Gram-negative bacterial infections are treated through the combined use of TNP-2092 and the cell membrane penetrant, the antibacterial activity of the composition is enhanced compared with the use of TNP-2092 or the cell membrane penetrant alone, and the composition has an antibacterial synergistic effect and can be used for treating the Gram-negative bacterial infections, including drug-resistance bacterial infections.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
Sulfate polymyxin B crystal and preparation method thereof
The invention provides a sulfate polymyxin B crystal and a preparation method thereof. The preparation method comprises the following steps: firstly, adsorbing polymyxin B fermented liquid with resin,and adding a sulfate aqueous solution and / or an acid ethanol aqueous solution for desorption to obtain a desorption solution; secondly, adjusting a pH value of the desorption solution obtained in thefirst step to 5.0 to 7.0; and then concentrating until a solid is precipitated to obtain a sulfate polymyxin B saturated solution; and thirdly, in a stirring state, dropwise adding an organic solventinto the sulfate polymyxin B saturated solution obtained in the second step or dropwise adding the sulfate polymyxin B saturated solution obtained in the second step into the organic solvent so as toseparate out the crystal, filtering and drying a filter cake, thus obtaining the sulfate polymyxin B crystal. The solvent used in the preparation method disclosed by the invention is low in cost andeasy to obtain; the preparation method disclosed by the invention is suitable for large-scale industrial production; and the prepared sulfate polymyxin B crystal product has the advantages of higher purity and clarity and low moisture absorption.
Owner:HUBEI RUIHAO ANKE MEDICINE TECH DEV CO LTD
Method for preparing endotoxin adsorbing agent
Owner:JAFRON BIOMEDICAL
Polymyxin B production fermentation culture medium, preparation method and uses thereof
ActiveCN104774892AImprove fermentation yieldReduce viscosityMicroorganism based processesFermentationInorganic saltsNitrogen source
The present invention discloses a polymyxin B production fermentation culture medium, a preparation method and uses thereof, wherein the culture medium comprises, by weight, 0.1-1.5 w / w% of an organic nitrogen source, 5.0-15.0 w / w% of whole wheat powder, and 0.3-1.2 w / w% of an inorganic salt.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Endotoxin absorbent and preparation thereof
The invention relates to an endotoxin adsorbent which is composed by taking spherical porous cellulose as a carrier and immobilizing effective amount of polymyxin B as a ligand. The preparation method of the endotoxin adsorbent includes that the spherical porous cellulose is taken as the carrier, the spherical porous cellulose first reacts with sodium periodate and is bonded with the effective amount of polymyxin B, and the endotoxin adsorbent is obtained after reduction by sodium borohydride. The endotoxin adsorbent has the advantages of stable performance, large supported quantity of polymyxin B, good adsorption effect, etc.
Owner:JAFRON BIOMEDICAL
Application of pithecellobium clypearia extracts to preparation of multi-drug resistant acinetobacter baumannii medicine
ActiveCN105816511AReduce dosageAddressing drug resistanceAntibacterial agentsPlant ingredientsAntibiotic sensitivityAntibiotic Y
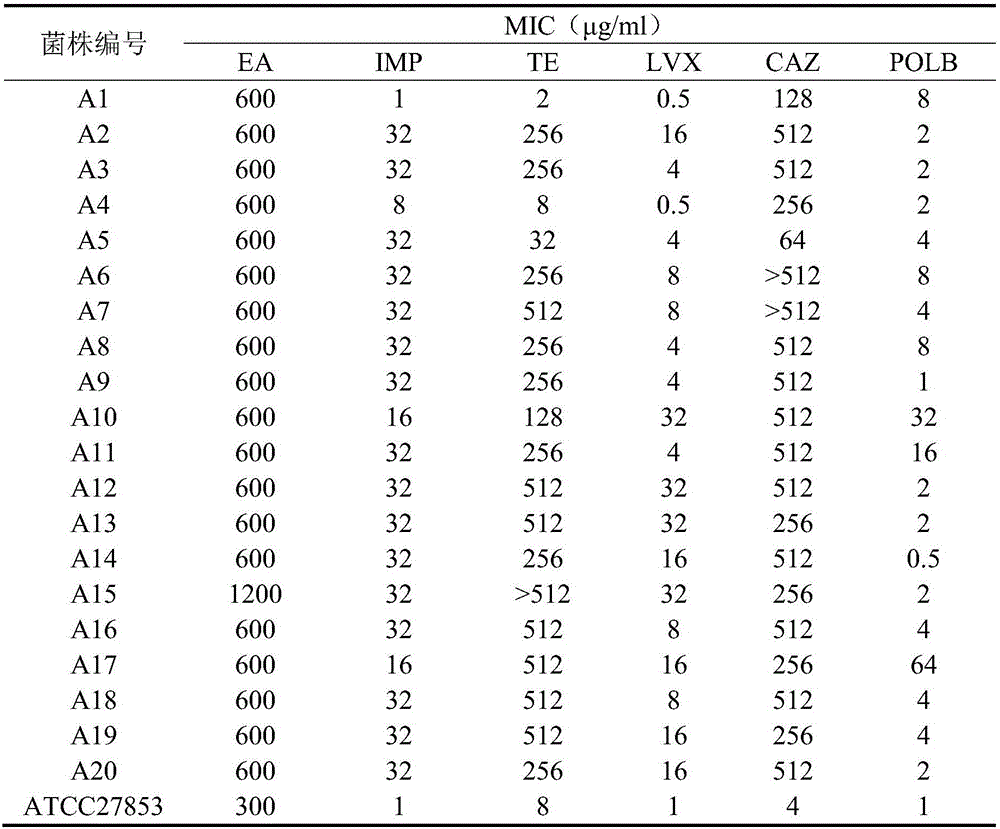
The invention discloses application of pithecellobium clypearia extracts to preparation of multi-drug resistant acinetobacter baumannii medicine. The pithecellobium clypearia extracts are prepared through the method includes the steps that pithecellobium clypearia coarse powder is extracted with water or an ethyl alcohol solution, the obtained extraction liquid is extracted with ethyl acetate, and the obtained extracts are target products. The antibacterial function of the pithecellobium clypearia extracts to multi-drug resistant acinetobacter baumannii and the sensitivity enhancing function of the pithecellobium clypearia extracts to similar antibiotics are disclosed for the first time. Tests prove that the pithecellobium clypearia extracts and imipenem or tetracycline or polymyxin B or ceftazidime or levofloxacin take effect together, and the use amount of antibiotics can be reduced by 50-87% compared with the mode that only the pithecellobium clypearia extracts are used. The pithecellobium clypearia extracts can serve as natural multi-drug resistant acinetobacter baumannii medicine or an antibiotic sensitivity-enhancing agent, and is applied to treatment of diseases caused by acinetobacter baumannii. A new means and alternative medicine are provided for solving the drug resistance problem of similar antibiotics.
Owner:HUACHENG PHARMA FACTORY GAUNGZHOU
Factor c for treating gram-negative bacterial infection
InactiveUS20080085865A1Strong antibacterial effectSuppress LPS-induced cytokinesAntibacterial agentsBiocideEndotoxin removalClearance rate
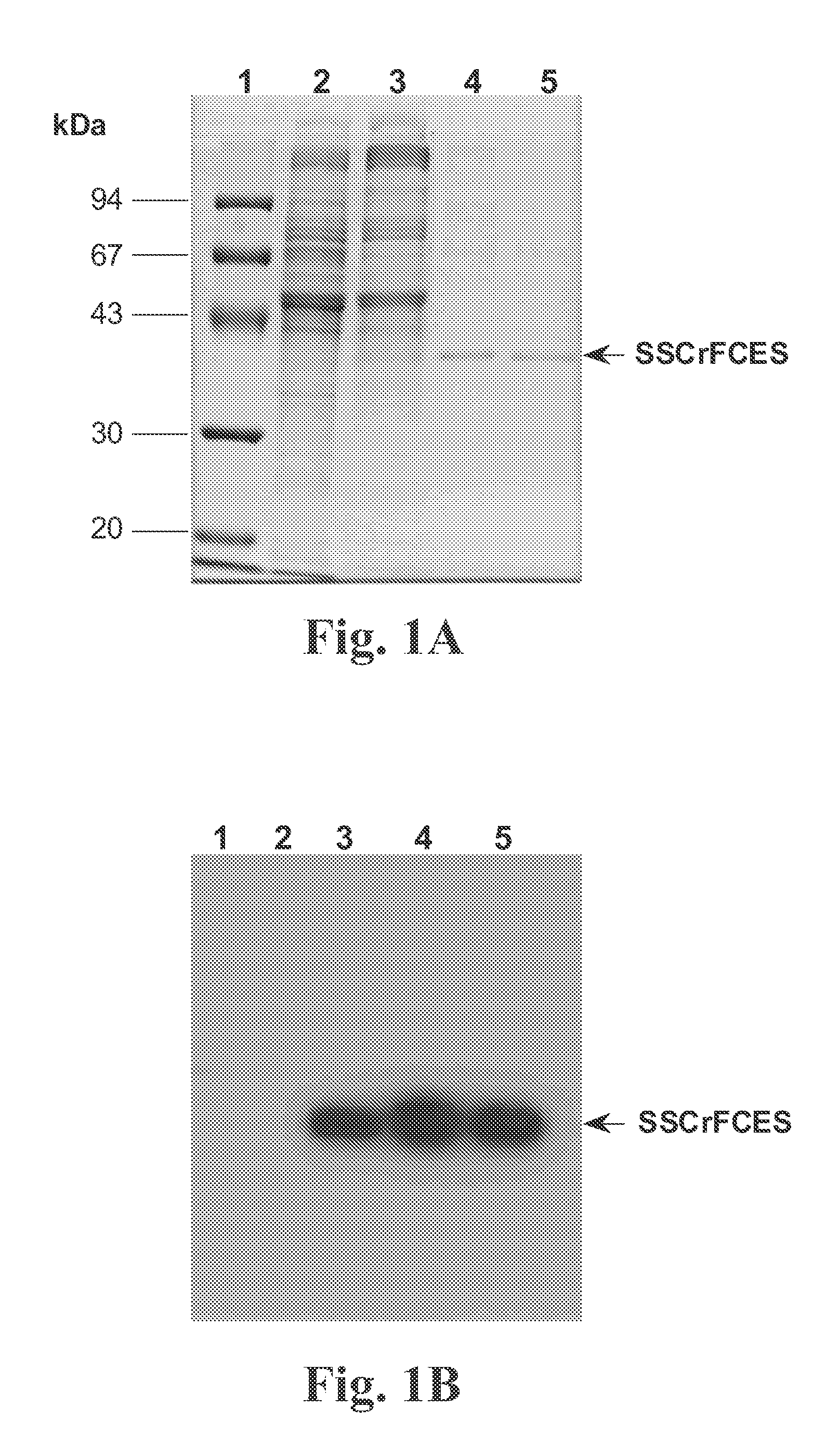
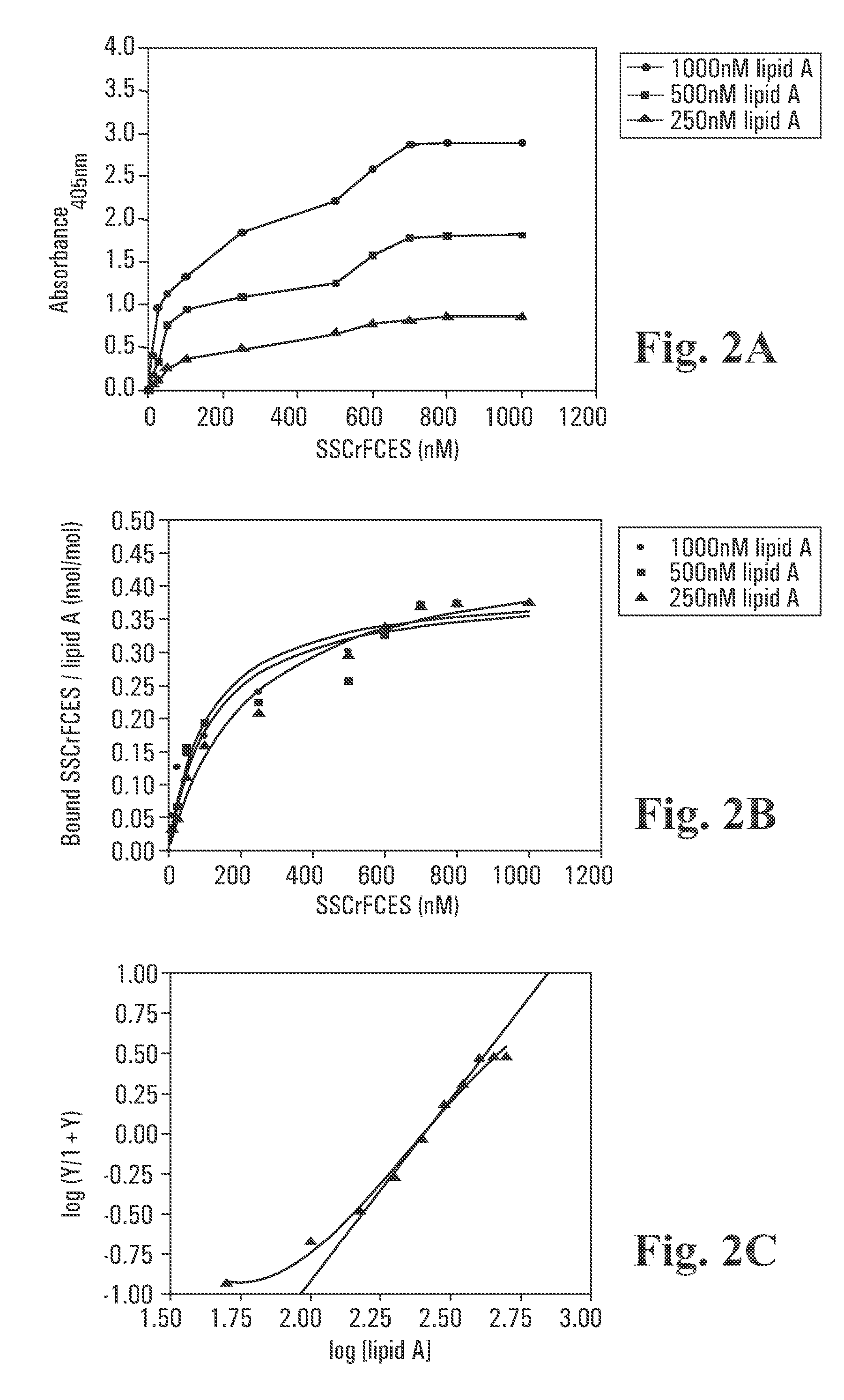
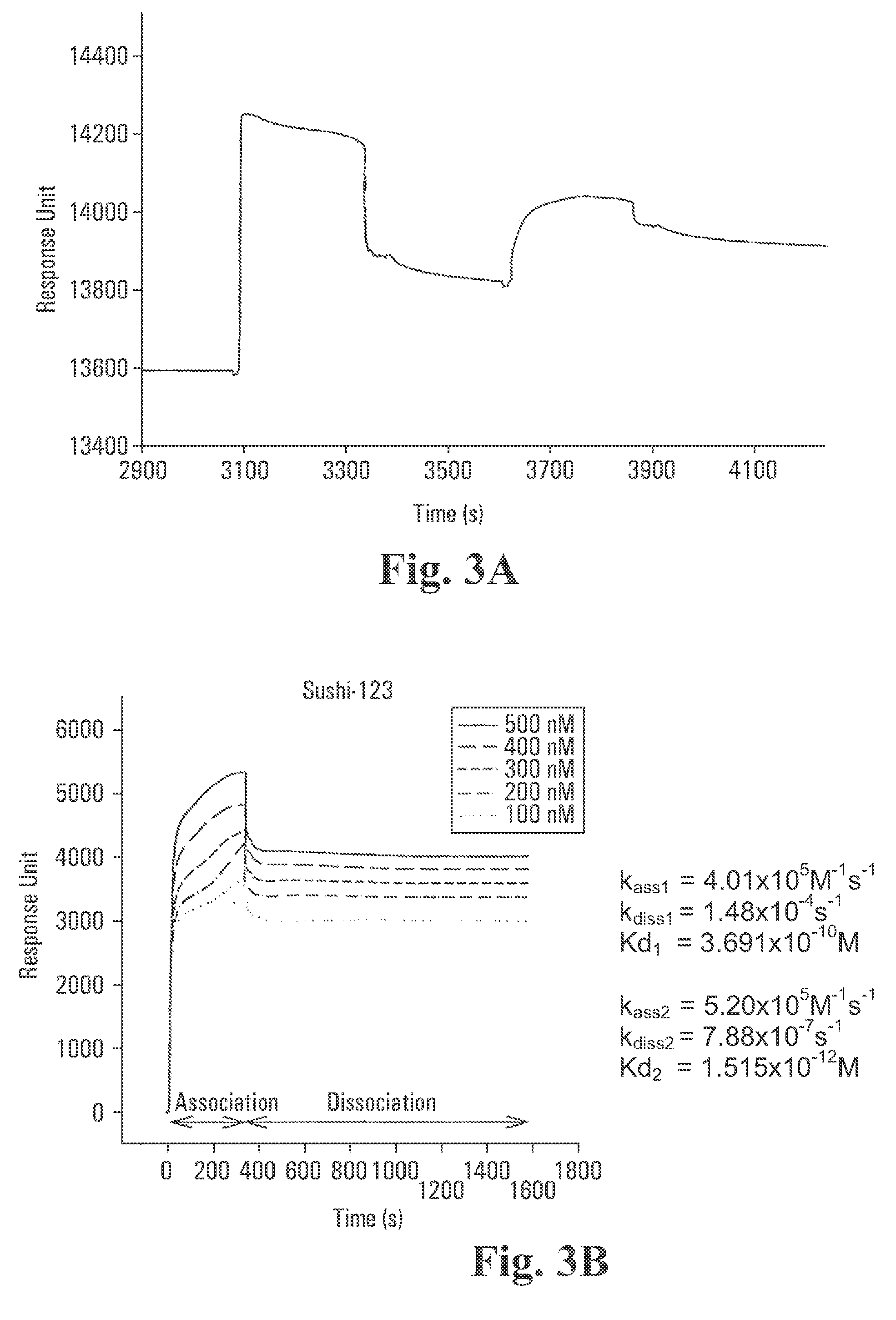
Recombinant fragments of Factor C are disclosed. These proteins and peptides show great potency in recognizing, binding to, neutralizing and removing endotoxin. These molecules can thus be used for anti-microbial, anti-endotoxin, and anti-sepsis therapy. SSCrFCES is a 38 kDa protein representing the LPS-binding domain of Factor C. The ability of SSCrFCES to bind lipid A was analyzed using an ELISA-based assay as well as surface plasmon resonance. Surface plasmon resonance similarly carried out for SSCrFC-sushi-1,2,3-GFP, SSCrFC-sushi-1GFP, and SSCrFC-sushi-3GFP confirmed their superior affinity for endotoxin. The 50% endotoxin-neutralizing concentration of SSCrFCES against 200 EU of endotoxin is 0.069 μM, suggesting that SSCrFCES is an effective inhibitor of LAL coagulation cascade. Although partially attenuated by human serum, as low as 1 μM of SSCrFCES inhibits the LPS-induced secretion of hTNF-α and hIL-8 by THP-1 and human pheripheral blood mononuclear cells with a potency more superior than polymyxin B. SSCrFCES is non-cytotoxic, with a clearance rate of 4.7 ml / minute. The LD90 of SSCrFCES for LPS lethality in mice is achieved at 2 μM. These results demonstrate the endotoxin-neutralizing capability of SSCrFCES in vitro and in vivo, as well as its potential for use in the treatment of endotoxin-induced septic shock. Also embodied in this application is the use of the sushi peptides and their mutant derivatives as potent antimicrobials.
Owner:NAT UNIV OF SINGAPORE
Cyclic lipopeptide antibiotic and preparation and application thereof
InactiveCN102911257AEnhanced inhibitory effectHighly toxicAntibacterial agentsBacteriaAntibiotic YPolymyxin B
The invention relates to a novel cyclic lipopeptide antibiotic (cyclic nonapeptide avermectin) and preparation and application thereof, belonging to the technical field of biomedicine. The fermentation medium is inoculated with paenibacillus, the paenibacillus is cultured at 28-35 DEG C for 24-96 hours, the fermentation broth is centrifuged to take the supernatant, the supernatant is adsorbed by a macroporous adsorption resin, and separation and purification are performed further to obtain the cyclic lipopeptide antibiotic which can be used for preparing the bacterial-infection resisting medicament. The invention provides the cyclic nonapeptide avermectin with higher toxicity than polymyxin B and higher medical application value and the preparation and application of the cyclic nonapeptide avermectin. The experiments in vitro prove that the compound can inhibit all the tested Gram-positive and negative pathogens including the multiple and super medicament resisting bacteria significantly, and the in-vivo experiments prove that the compound can prevent and treat the mouse septicemia caused by theextended-spectrum medicament resisting pseudomonas aeruginosa significantly.
Owner:WUXI NO 4 PEOPLES HOSPITAL
Endotoxin absorbing agent for blood perfusion and its preparation method
An endotoxin adsorbent used for blood perfusion is prepared from agat through adding the aqueous solution to the liquid mixture of toluene and CCl4 to obtain spherical agar, activating by epoxy chloropropane under alkaline condition, reacting on diamine and then on glutaraldehyde to obtain high-strength agar beads used as the carrier of adsorbent, reacting on the amino contained ligand (polymyxin B), and reducing by NaBH4. Its advantages are high compatibility to blood and high effect on absorbing endotoxin from blood.
Owner:NANKAI UNIV
Preparation method of water-soluble alginate for in-vivo implantation
ActiveCN110804108AHigh puritySuitable for industrial mass productionPhysisorptionPharmaceutical Aids
The invention relates to the technical field of natural polysaccharides for in-vivo implantation, in particular to a preparation method of water-soluble alginate for in-vivo implantation. The preparation method comprises the following steps of: taking commercially available food-grade or pharmaceutical adjuvant-grade water-soluble alginate as a raw material, performing microporous filtration and multistage activated carbon adsorption, and then acidifying to form gel; eluting the gel with an alkaline solution for redissolving the gel, and adsorbing the gel with polymyxin B agarose; and separating out alginate with an organic solution in a sterile environment, and vacuum drying to obtain sodium alginate for in- vivo implantation. According to the invention, the synergistic effects of physical adsorption, affinity adsorption and chemical chelation are utilized to remove impurities such as foreign proteins, endotoxin and the like, impurity ions are not introduced in the preparation process, adverse effects of ion residues on product quality are avoided, and the obtained alginate has high purity and can be implanted in vivo; a folded membrane filter element and activated carbon adsorption are used to complete microporous filtration of high viscosity liquid; a two-fluid nozzle is adopted for dispersion granulation, and the method is suitable for industrial batch production.
Owner:QINGDAO BRIGHT MOON ALGINATE TISSUE ENG MATERIAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com