Endotoxin absorbing agent for blood perfusion and its preparation method
A hemoperfusion and adsorbent technology, which is applied in the field of biomedical materials, can solve problems such as ineffectiveness, unsuitability for promotion, and high cost, and achieve the effects of reducing treatment costs, good blood compatibility, and high scavenging capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1-1 Preparation of spherical agar gel carrier
[0032] Place a 500ml three-necked flask in a 60°C water bath, add 100ml of toluene, 50ml of carbon tetrachloride, and 0.5ml of Tween 80 into the bottle, and stir evenly.
[0033] Weigh 4 grams of agar powder and add 30ml of distilled water, heat and melt, pour the melted agar into the toluene-carbon tetrachloride system, stir, and disperse the agar solution into droplets of suitable size in the organic phase. Cool to room temperature, pour out the organic solvent in the upper layer, screen the agar balls of 0.45-1.0mm, wash with water, transfer to the Erlenmeyer flask, and store in a wet state at 4°C.
[0034] 1-2 Epichlorohydrin activation
[0035] Take 20.0 grams of agar balls, add 29.6 milliliters of 2M sodium hydroxide, then add 13.3 milliliters of epichlorohydrin, react at 40 ° C for 2 hours, wash with water until neutral, and thoroughly wash off the remaining epichlorohydrin, Epoxy-activated gel balls were obtained...
Embodiment 2
[0058] Effect of glutaraldehyde cross-linking on the properties of agar ball carrier
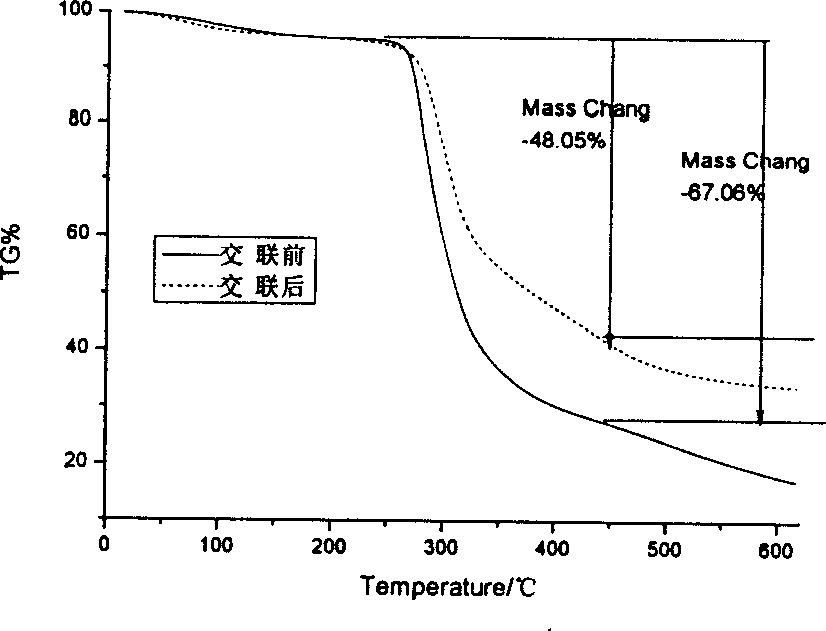
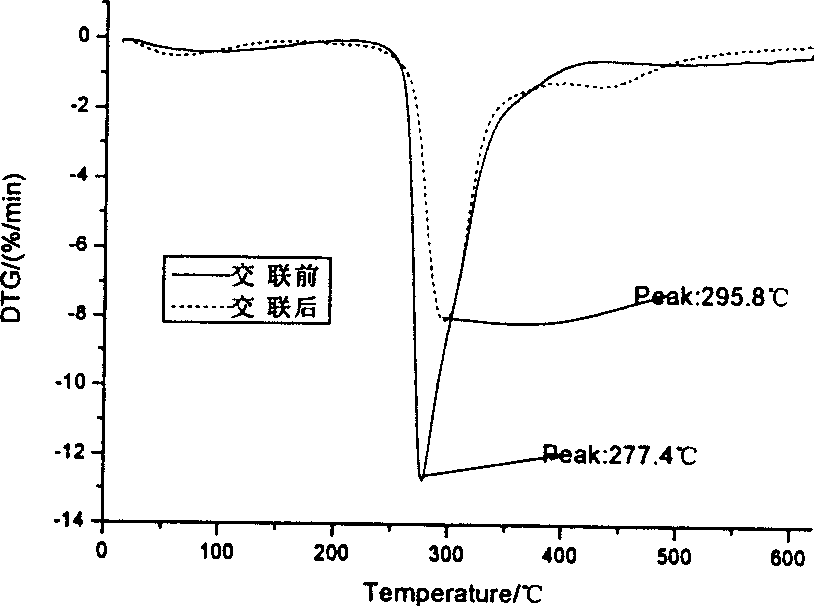
[0059] 2-1 Take 50 mg of agar balls from 1-1 before glutaraldehyde cross-linking and 1-4 after glutaraldehyde cross-linking respectively, wash them, dry them in vacuum and grind them into pieces. The TG209 thermogravimetric analyzer was used to measure the thermogravimetric curve of the carrier ball, the heating rate was 14.0°C / min, and the temperature rising range was 20-620°C. After crosslinking, the peak value of thermal weight loss increased from 277.4°C to 295.8°C, which indicated that the thermal stability of the agar balls was improved after crosslinking (see figure 1 ,2). figure 1 . Thermogravimetric curves of agar ball carrier before and after crosslinking. figure 2 .Derivative thermogravimetric curve of agar ball carrier before and after crosslinking.
[0060] Thermogravimetric method is one of the most important methods to evaluate the thermal stability of polymer materials. ...
Embodiment 3
[0063] blood compatibility test
[0064] Take 2 ml of the adsorbent prepared in Example 1, soak it in physiological saline for 12 hours, put it into the perfusion column, inject 5 ml of rabbit whole blood mixed with anticoagulant with a syringe, and perfuse it at a flow rate of 15 ml / min for 2 hours. At the same time, an empty perfusion column was used as a control column. The changes of blood components before and after perfusion were measured by MEK-6318K hematology analyzer. The results showed that before and after perfusion, various blood components did not change much, and the decreasing percentages were all within 5%, which indicated that this series of adsorbents had good blood compatibility and could be used for whole blood perfusion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com