Culture medium used for separating and screening lactic acid bacteria, preparation method and application thereof
A technology for separation, screening and culture medium, which is applied in the field of culture medium for separation and screening of lactic acid bacteria, which can solve the problems of inconspicuous identification of lactic acid bacteria, increased labor load of lactic acid bacteria, and reduced separation and screening efficiency, so as to enhance the inhibition and accuracy of the growth of miscellaneous bacteria High, improve the effect of screening efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
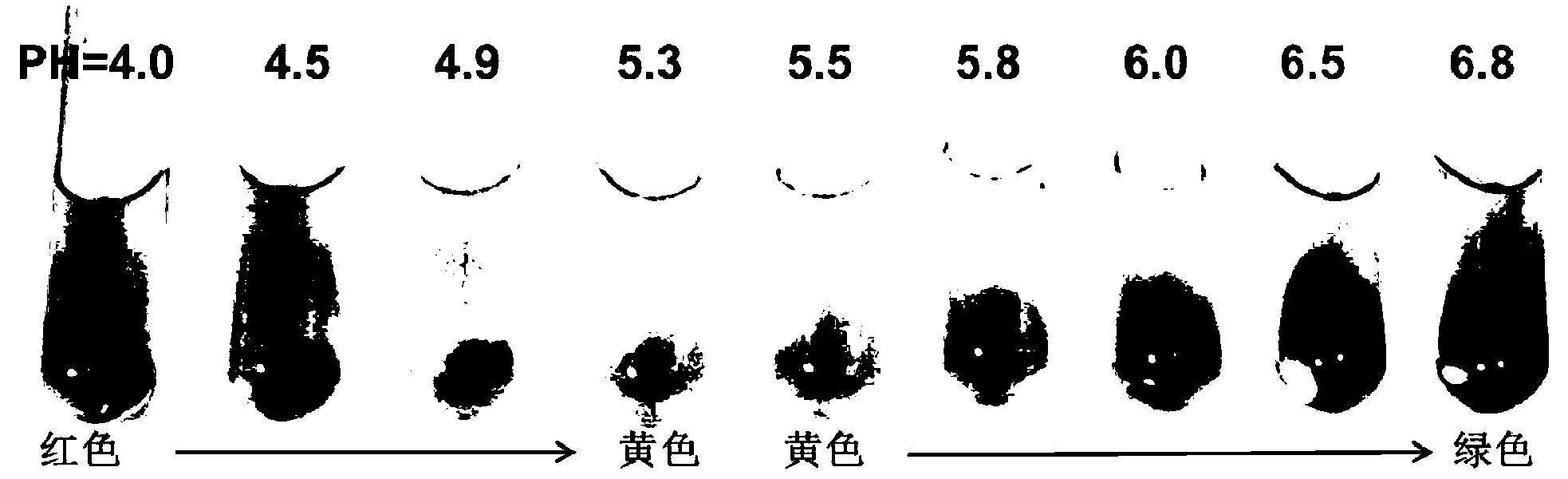
[0027] The medium formula used in this embodiment to separate and screen lactic acid bacteria is as follows :Tryptone 10.0g, beef extract 10.0g, yeast extract 5.0g, diammonium citrate 2.0g, glucose 20.0g, Tween-801.0mL, sodium acetate 5.0g, agar 18.0g, calcium carbonate 10.0g, containing formazan Compound acid-base indicator of 0.05g base red and 0.07g bromothymol blue, polymyxin B250000IU, nalidixic acid 0.02g, ascorbic acid 1.0g, L-cysteine-HCl 0.2g, salt solution 100mL , Chenhai 900mL, pH6.8.
[0028] In the medium formula of this embodiment, the formula of the salt solution is: 10.0 g of dipotassium hydrogen phosphate, 10.0 g of potassium dihydrogen phosphate, 2.0 g of magnesium sulfate heptahydrate, 0.5 g of manganese sulfate, 2 g of calcium chloride, and 1000 mL of aged sea water.
[0029] The compound acid-base indicator can be prepared by dissolving methyl red and bromothymol blue in an appropriate amount of ethanol, for example, dissolving 5.0g of methyl red and 7.0g of b...
Embodiment 2
[0041] The medium formula used in this embodiment to separate and screen lactic acid bacteria is as follows :Tryptone 5.0g, beef extract 15.0g, yeast extract 2.0g, diammonium citrate 1.0g, glucose 25.0g, Tween-802.0mL, sodium acetate 5.0g, agar 18.0g, calcium carbonate 10.0g, containing formazan Compound acid-base indicator of base red 0.01g and bromothymol blue 0.01g, polymyxin B500000IU, nalidixic acid 0.02g, ascorbic acid 5.0g, L-cysteine-HCl 0.5g, salt solution 100mL , Chenhai 900mL, pH6.8.
[0042] In the medium formula of this embodiment, the formula of the salt solution is: 10.0 g of dipotassium hydrogen phosphate, 10.0 g of potassium dihydrogen phosphate, 2.0 g of magnesium sulfate heptahydrate, 0.5 g of manganese sulfate, 2 g of calcium chloride, and 1000 mL of aged sea water.
[0043] In the medium formula of this embodiment, the aged seawater is taken from seawater which is far away from the land and is less polluted, and is placed in the dark place for several weeks.
...
Embodiment 3
[0051] The medium formula used in this embodiment to separate and screen lactic acid bacteria is as follows :Tryptone 10.0g, beef extract 10.0g, yeast extract 5.0g, diammonium citrate 2.0g, glucose 20.0g, Tween-801.0mL, sodium acetate 5.0g, agar 18.0g, calcium carbonate 4.0g, containing formazan Compound acid-base indicator of base red 0.5g and bromothymol blue 0.7g, polymyxin B500000IU, nalidixic acid 0.05g, ascorbic acid 1.0g, L-cysteine-HCl 0.1g, salt solution 50mL 950mL of distilled water, pH 6.8.
[0052] The formula of the salt solution in the medium formula of this embodiment is: 10.0 g of dipotassium hydrogen phosphate, 10.0 g of potassium dihydrogen phosphate, 2.0 g of magnesium sulfate heptahydrate, 0.5 g of manganese sulfate, 2 g of calcium chloride, and 1000 mL of distilled water.
[0053] The medium is prepared as follows : It is the same as embodiment 1, and will not be repeated.
[0054] Separation and screening : Collect shells, pufferfish, Macrobrachium rosenber...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com