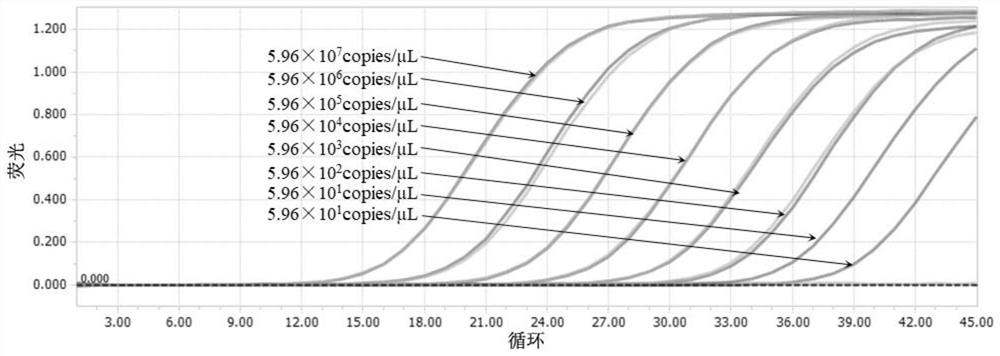
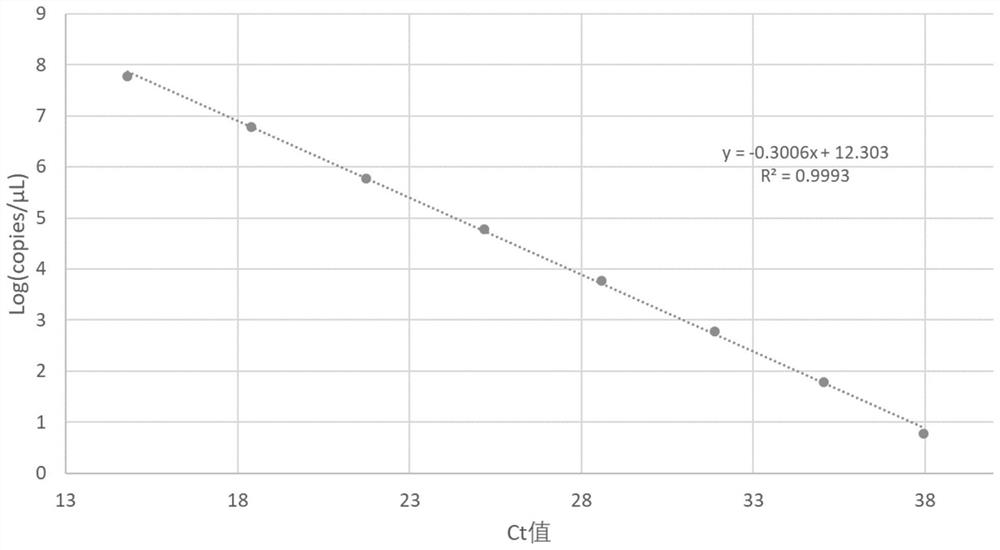
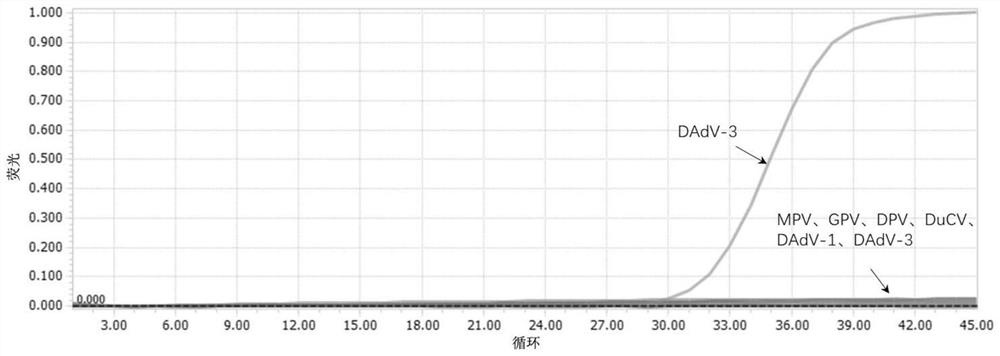
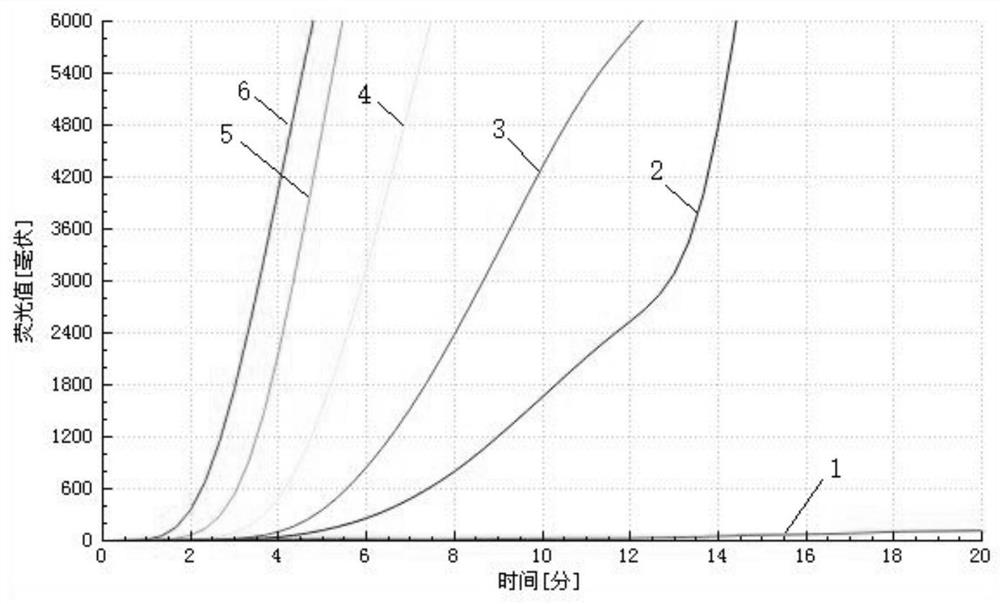
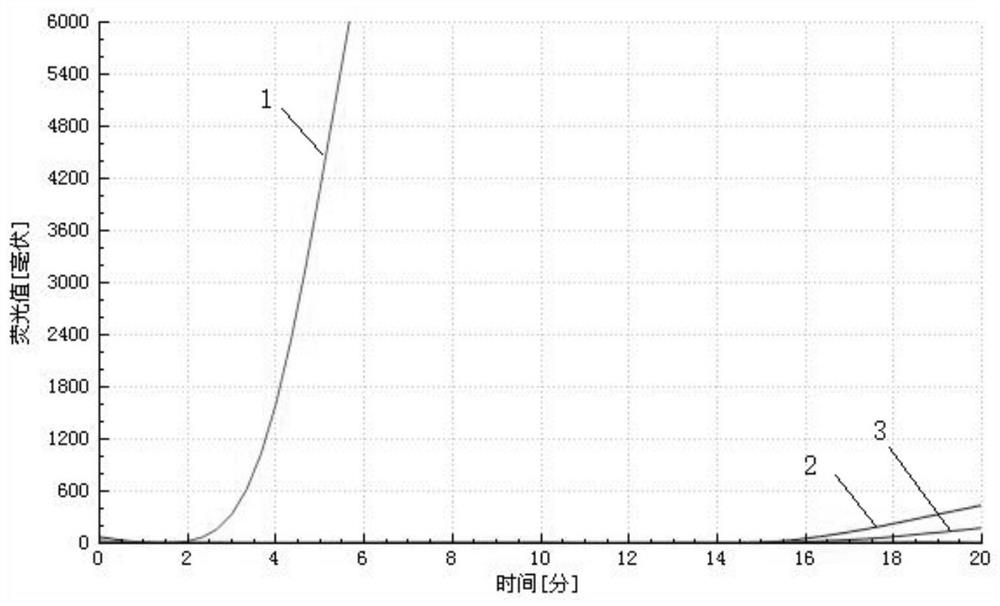
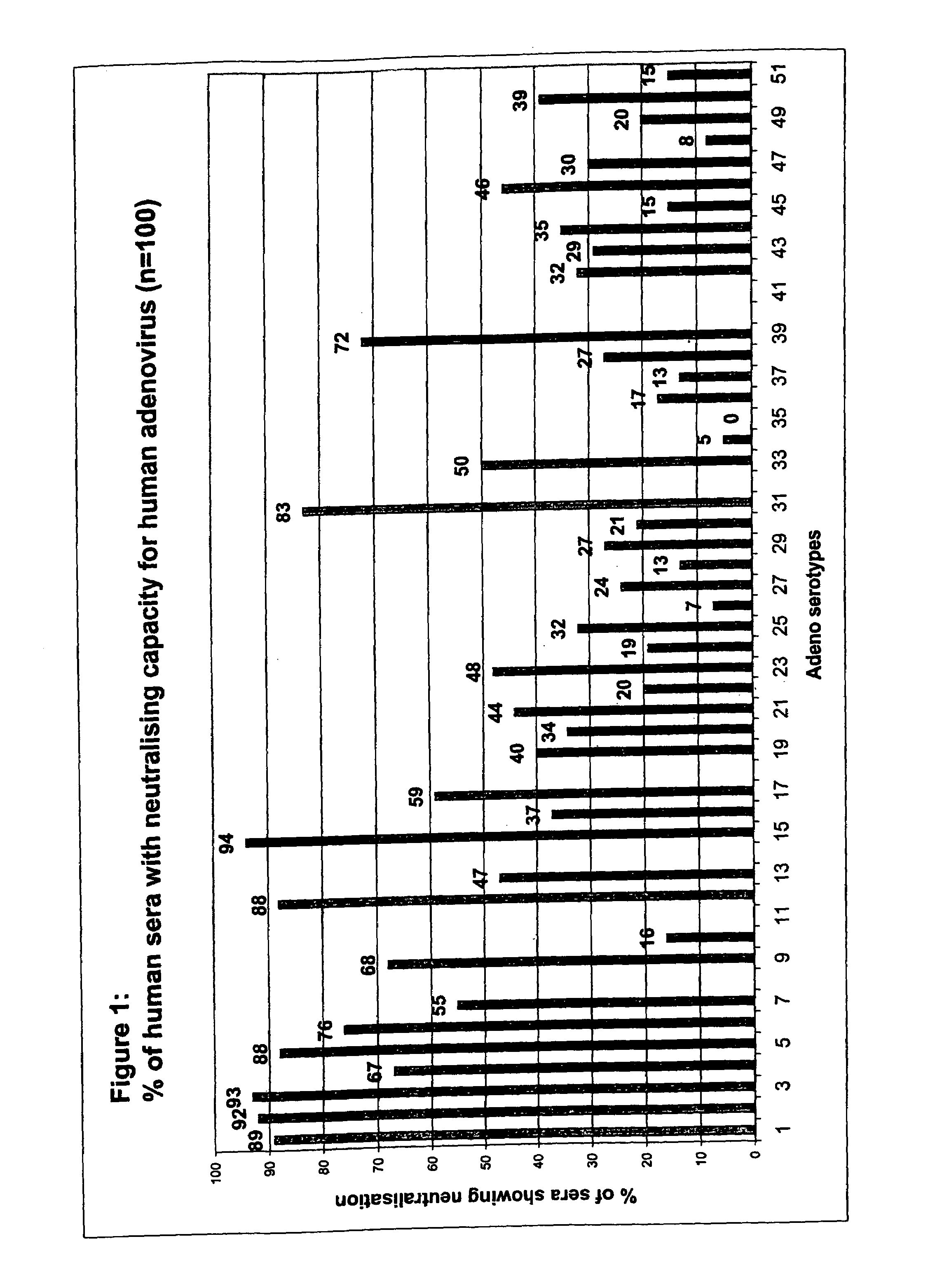
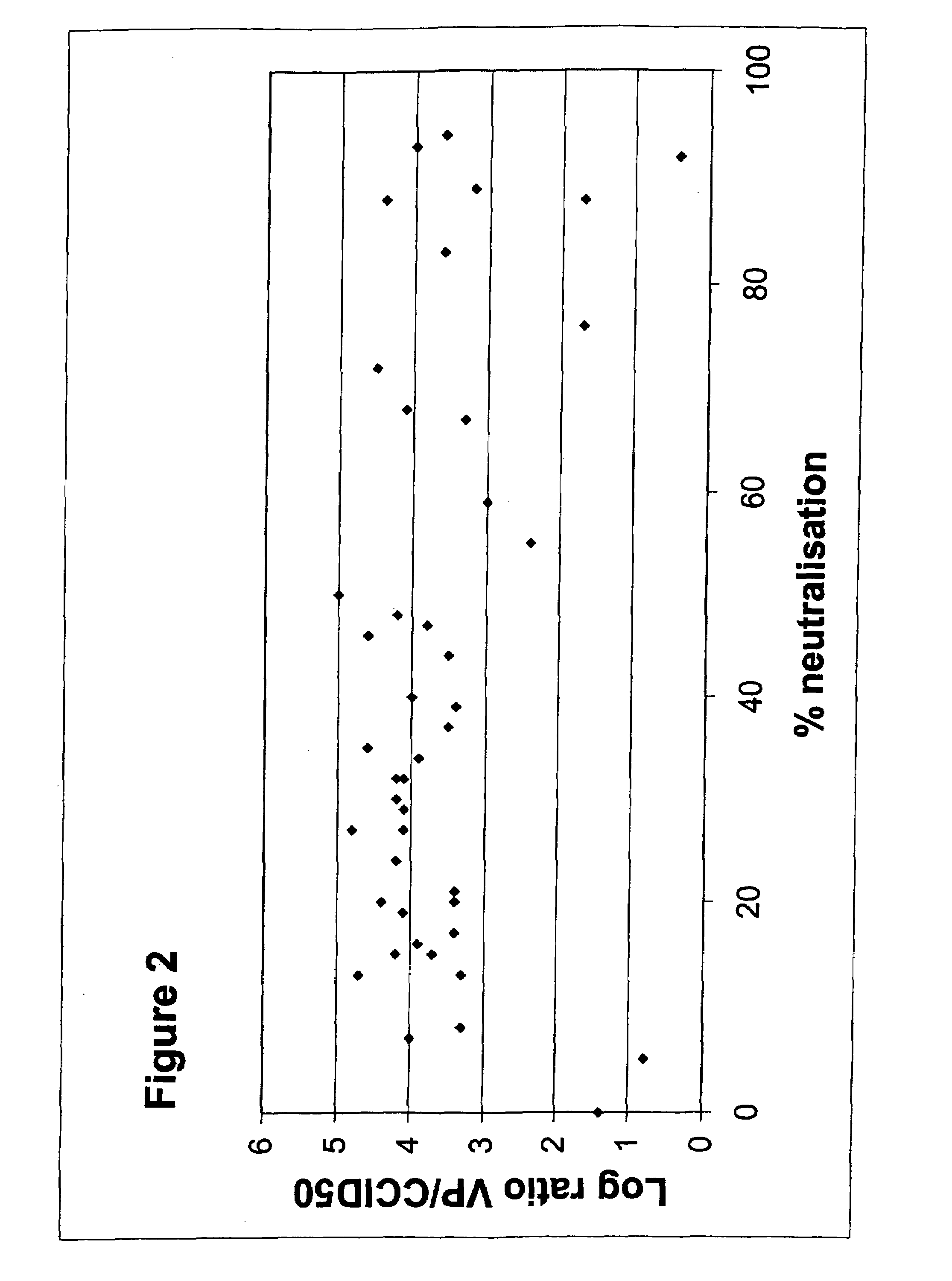
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Adenovirus typing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
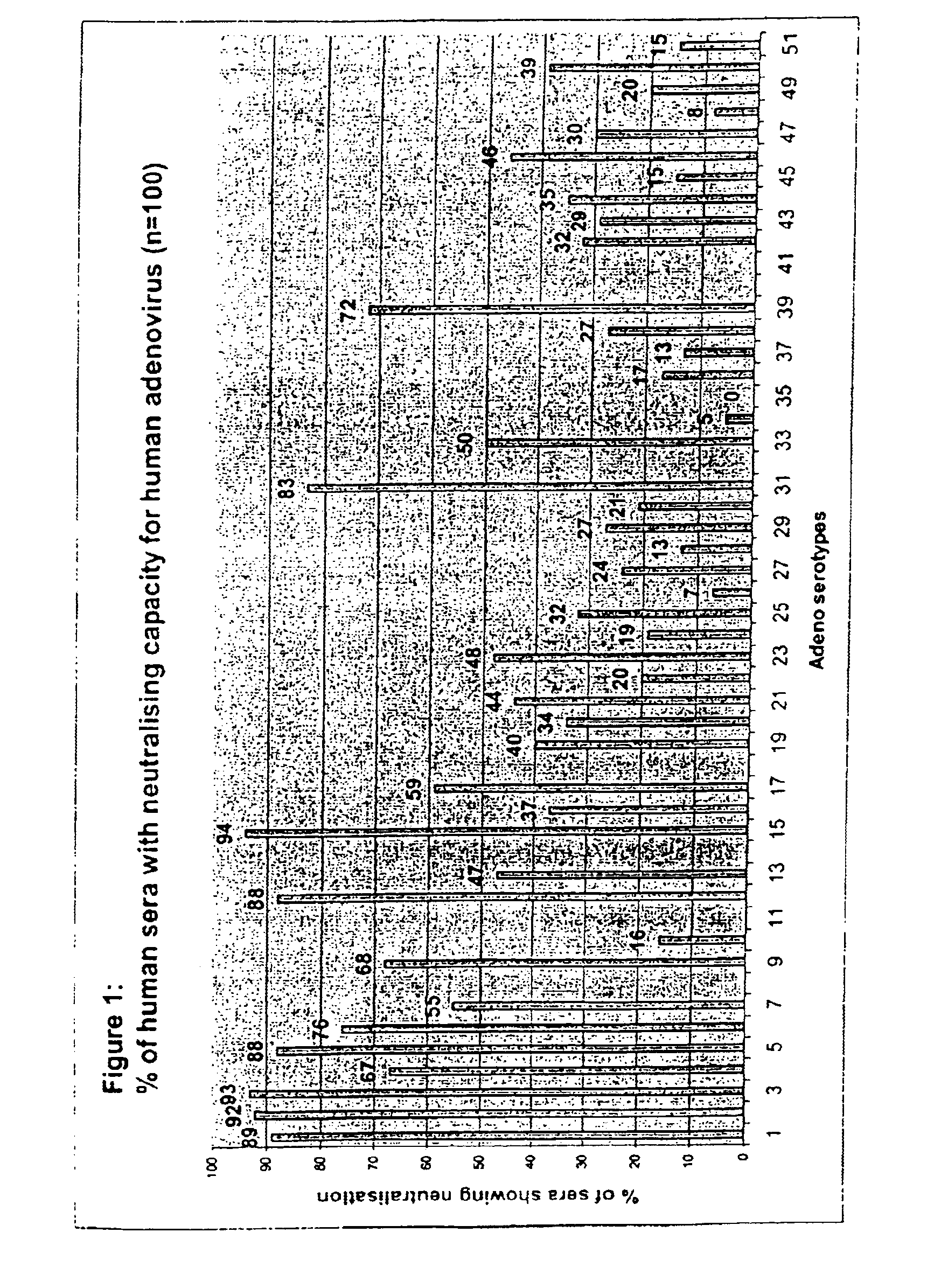
Adenovirus infections can be identified using antigen detection, polymerase chain reaction (PCR), virus isolation, and serology. Adenovirus typing is usually done by molecular methods. Even if a person has adenovirus infection, it does not necessarily mean it is causing the person’s particular illness.
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Viral vectors for gene therapy
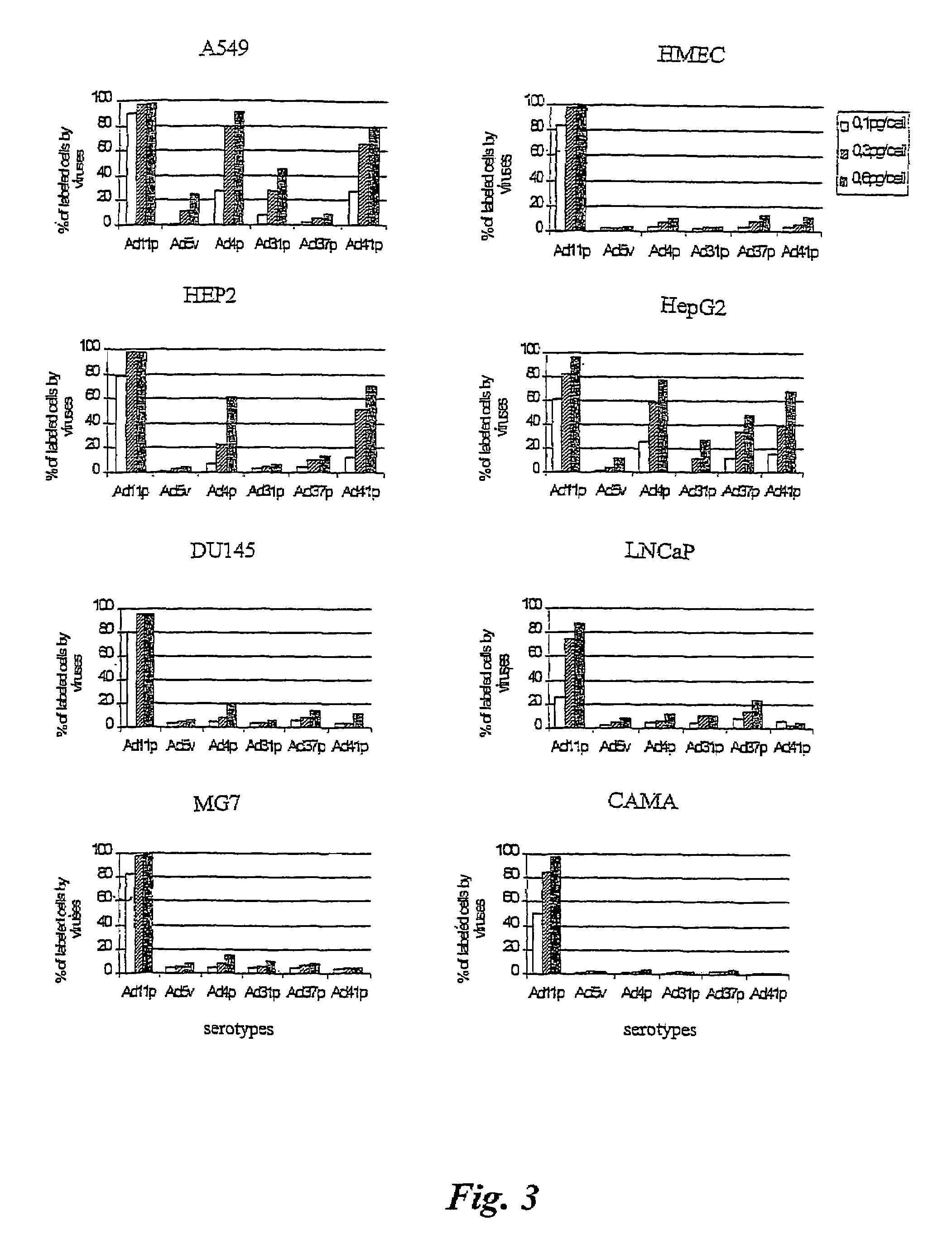
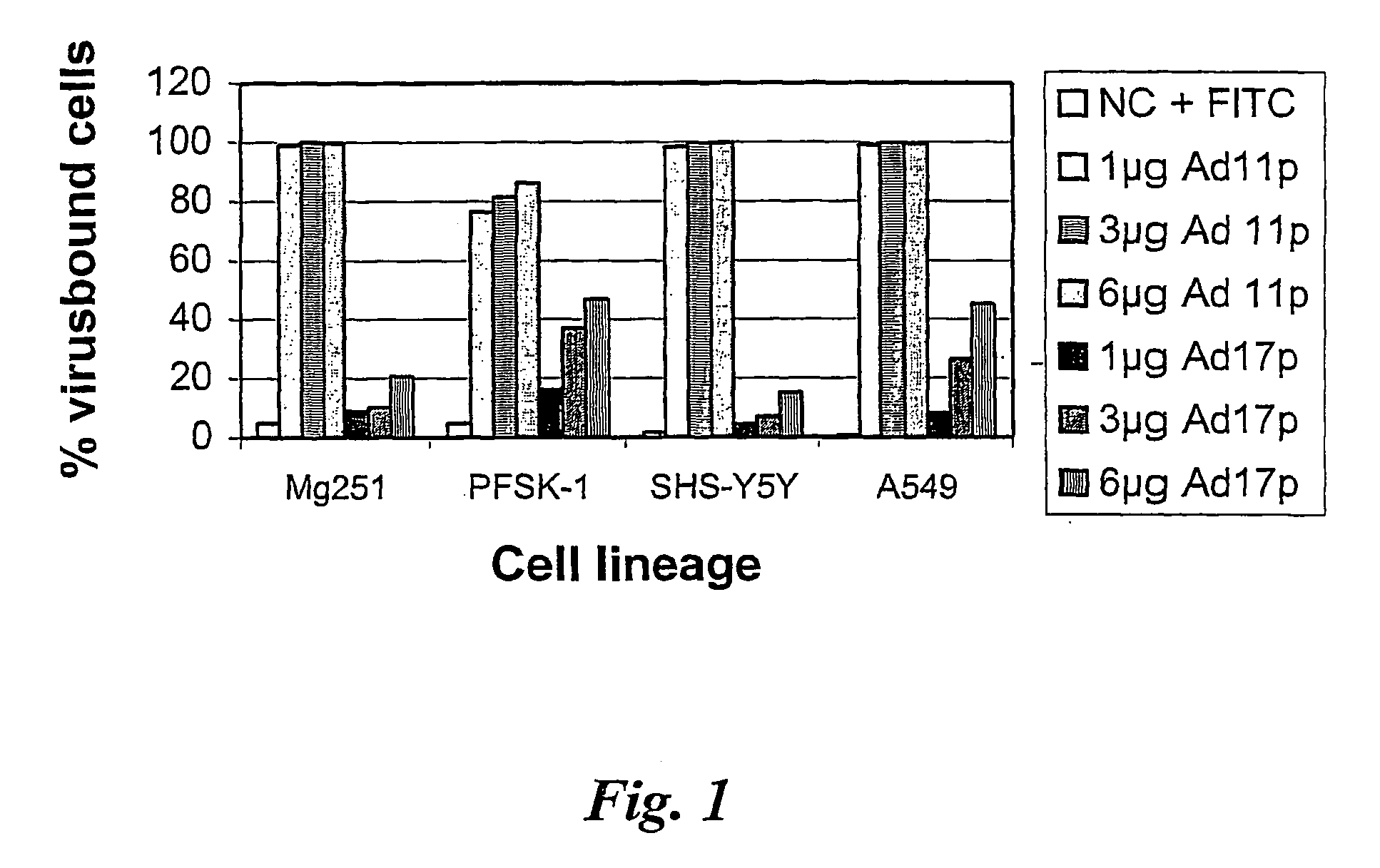
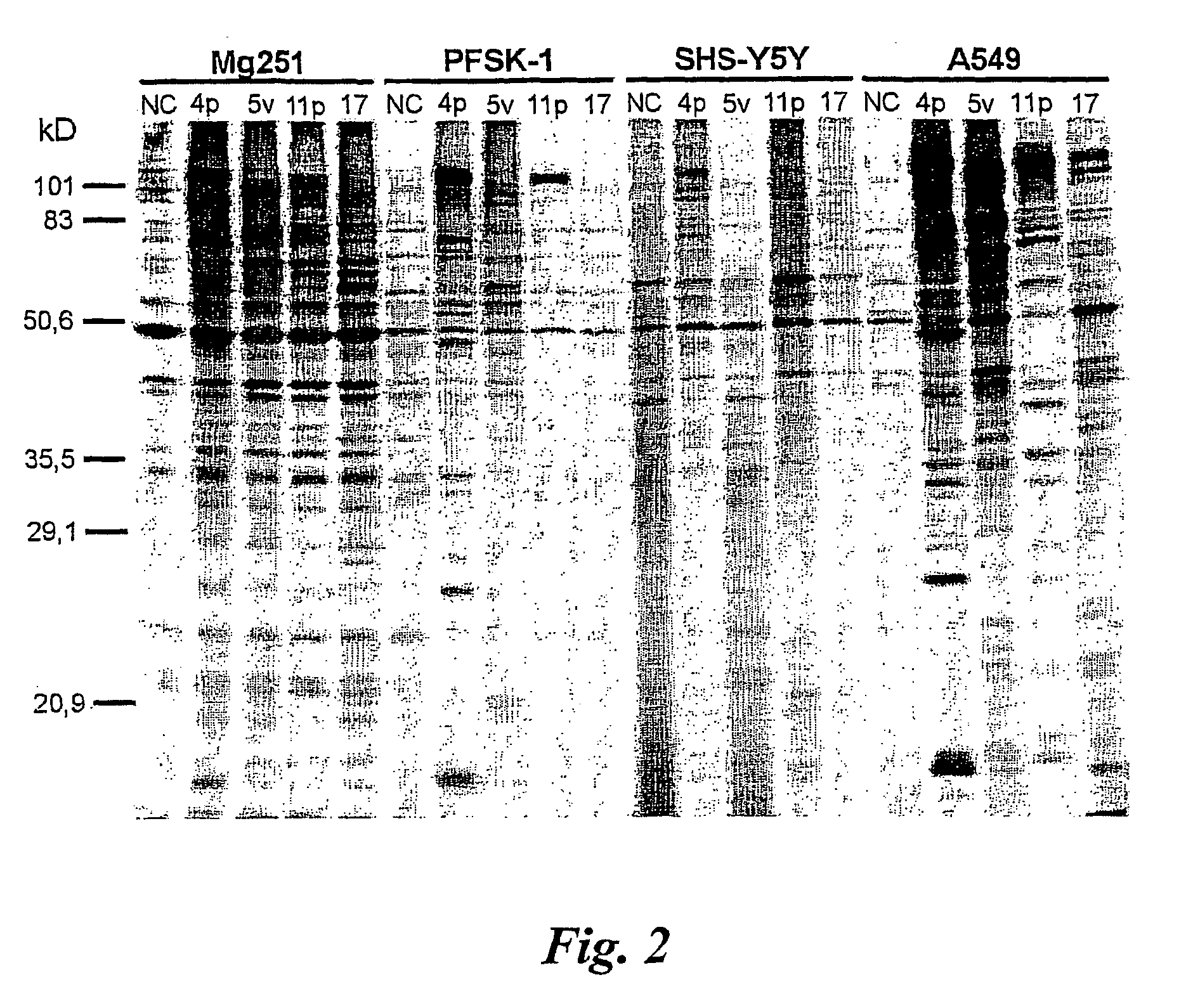
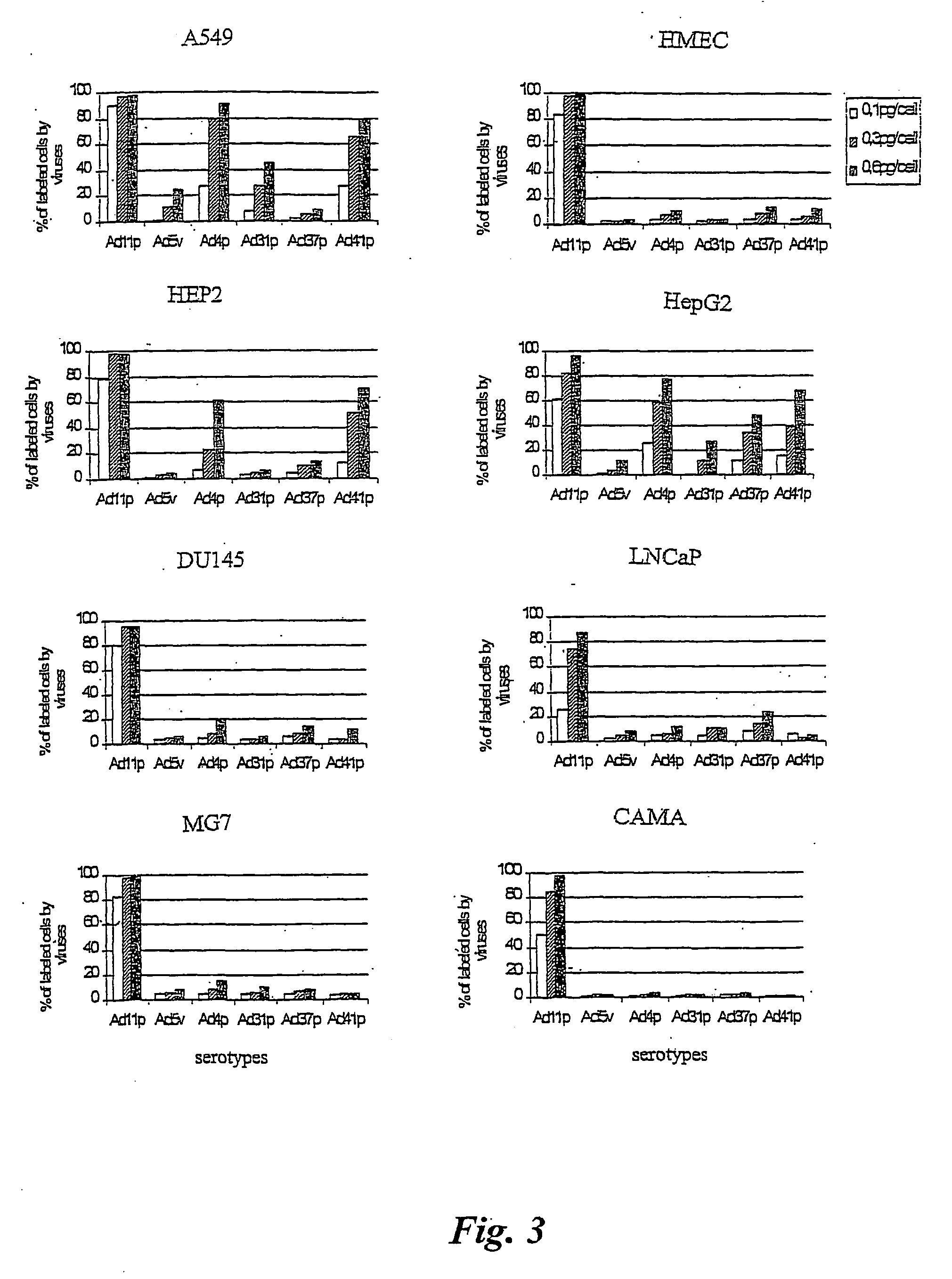
Adenovirus types 11p and 4p show a higher binding affinity and infectivity than type 5 for endothelial and carcinoma cell lines. Adenovirus type 11p shows a stronger binding to cells for neural origin, such as glioblastoma, neuroblastoma and medulloblastoma. The fact that adenovirus type 11 has a comparatively low prevalence in society, together with its high affinity and infectivity, makes it very suitable for use in gene therapy.
Owner:WADELL GORAN +4
Viral vectors for gene therapy
Adenovirus types 11p and 4p show a higher binding affinity and infectivity than type 5 for endothelial and carcinoma cell lines. Adenovirus type 11p shows a stronger binding to cells for neural origin, such as glioblastoma, neuroblastoma and medulloblastoma. The fact that adenovirus type 11 has a comparatively low prevalence in society, together with its high affinity and infectivity, makes it very suitable for use in gene therapy.
Owner:WADELL GORAN +4
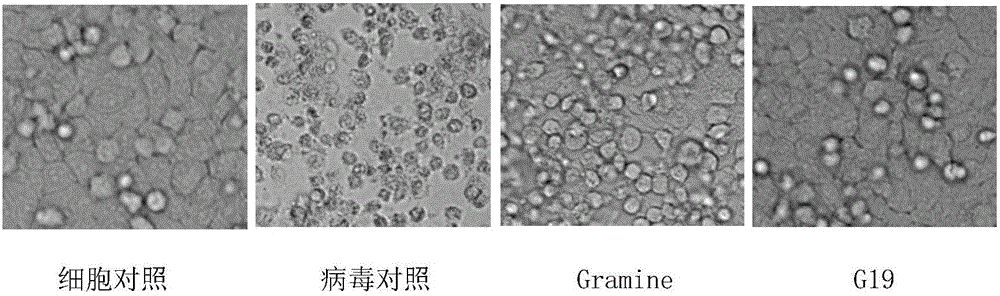
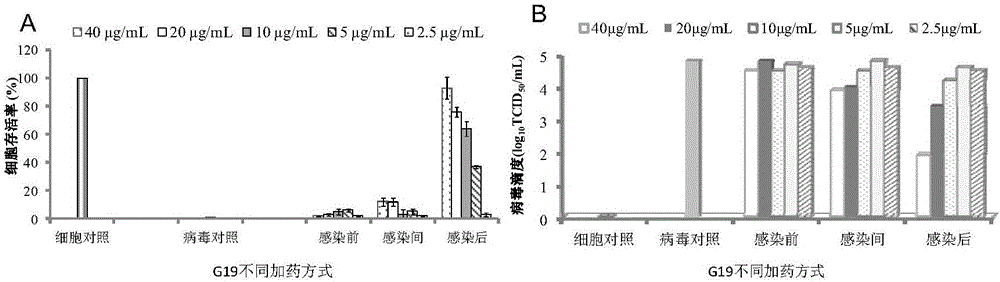
Applications of Gramine and derivatives thereof to preparation of medicaments for resisting adenovirus Type 7
InactiveCN106668002ALow priceEasy to buyOrganic active ingredientsAntiviralsAntiviral drugCytopathic effect
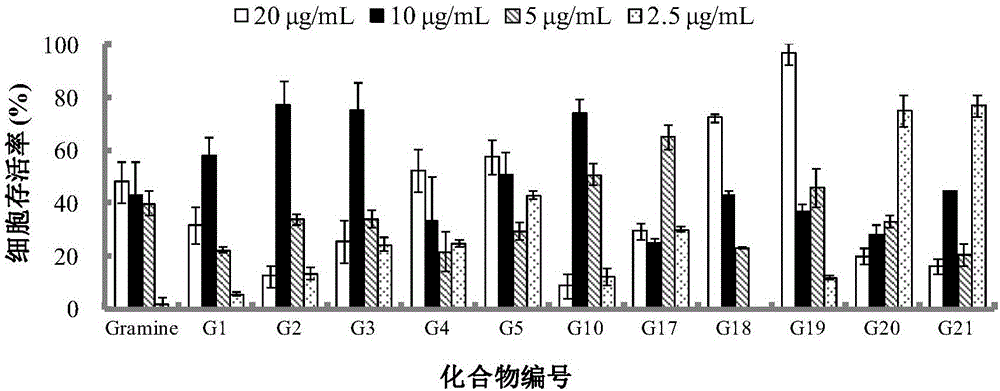
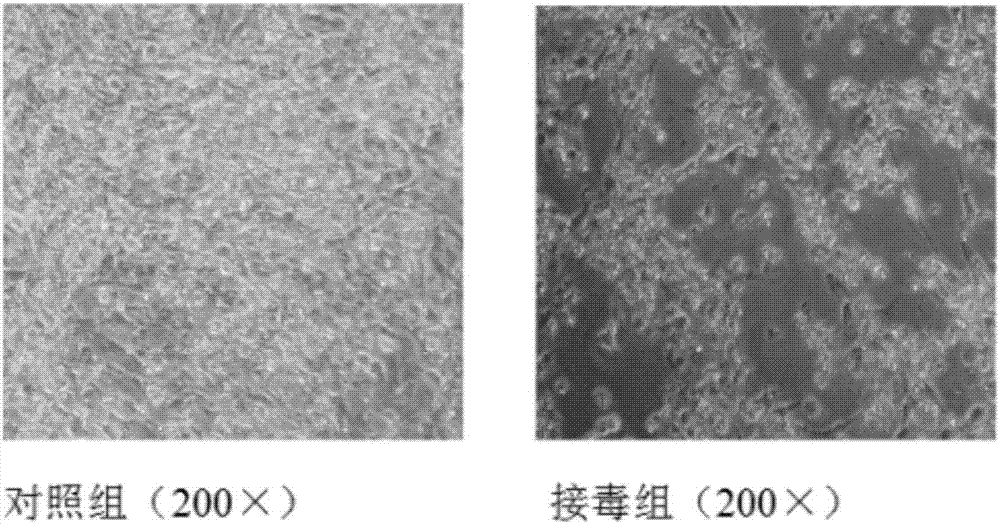
The invention belongs to the field of antiviral drugs. The invention provides applications of Gramine and derivatives thereof to preparation of medicaments for resisting adenovirus Type 7. Gramine derivatives are compounds shown in G1,G2,G3,G4,G5,G10,G17,G18,G19,G20, and G21. Experiments for researching activity of Gramine derivatives for resisting ADV7, pharmacology research of Gramine derivatives for resisting ADV7, and tests of inhibition effects of G19 for ADV7 duplication show that Gramine derivatives can inhibit cytopathic effects (CPE) generated by ADV7 on host cell Hela, enhance cell survival rate, inhibit yield of progeny virus, and mainly inhibit the duplication phase of ADV7 virus in host cells. The Gramine and derivatives thereof have potential for preparing anti-ADV1 virus medicaments, and the compounds have the advantages of simple synthesis process, and economy and fastness; the compounds are easy for large-scale production, and have clinic application prospects.
Owner:HUBEI UNIV OF TECH
Preparation and application of adenovirus parting gene chip
InactiveCN105671212AImprove throughputStrong specificityNucleotide librariesMicrobiological testing/measurementOligonucleotide chipEpidemiologic survey
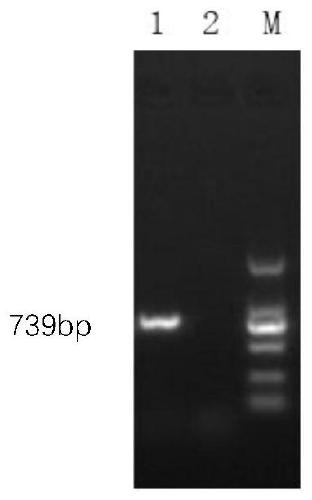
The invention relates to preparation and application of a gene chip capable of detecting adenoviruses in a parting mode.A preparation method comprises the steps of preparing specific primers and probes for different types of adenoviruses, preparing oligonucleotides chips, establishing a plurality of PCR systems, and establishing a hybridization system.The gene chip prepared through the method can screen different types of adenoviruses including a 3-type adenovirus, a 7-type adenovirus, a 14-type adenovirus, an 11-type adenovirus and a 55-type adenovirus.The gene chip has the advantages of quickness, accuracy, high throughput and high specificity.A new detection means is provided for clinical diagnoses of different types of adenovirus infections and epidemiological surveys.
Owner:ACADEMY OF MILITARY MEDICAL SCI
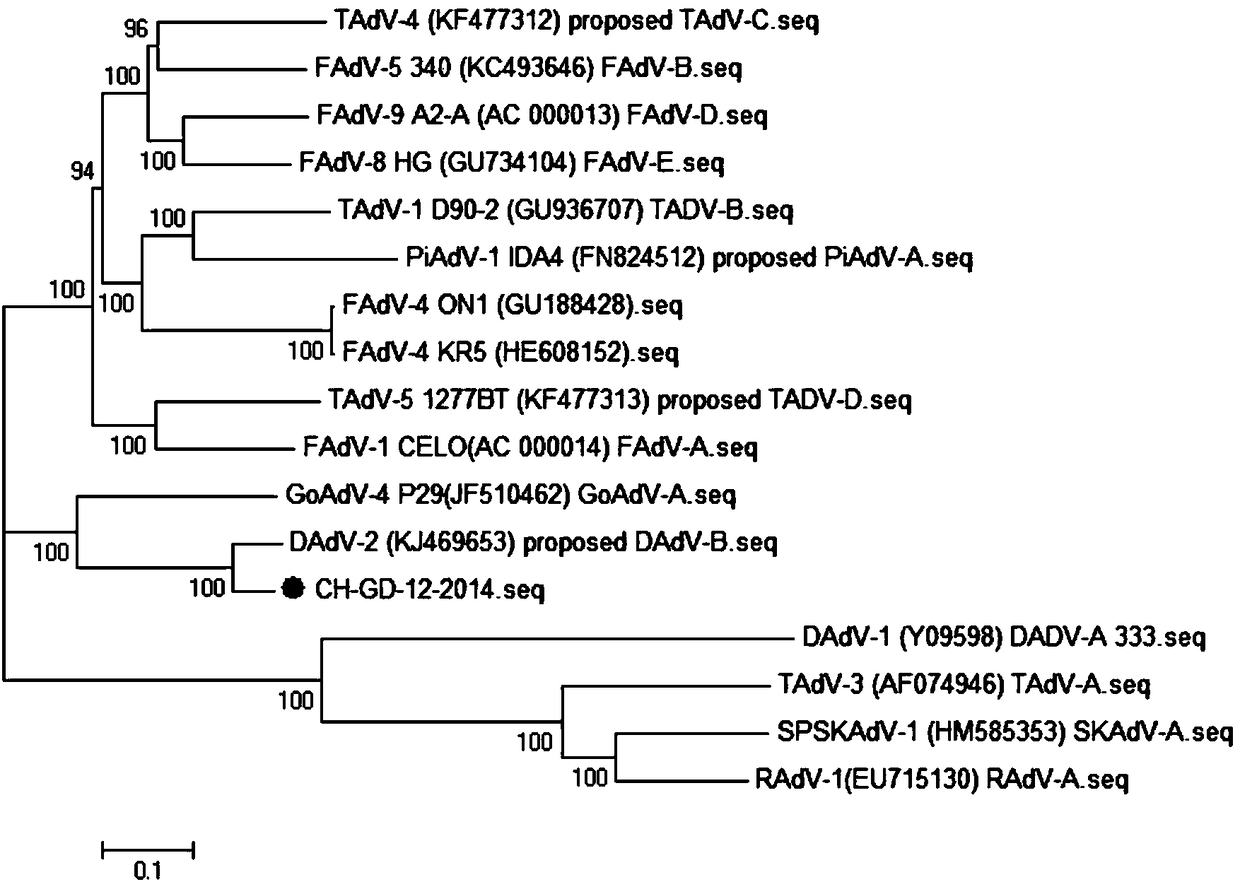
Inactivated vaccine strain CH-GD-12-2014 against duck adenovirus type 3
InactiveCN108330108ANo side effectsProtection against virusesViral antigen ingredientsVirus peptidesDuck adenovirus 1Nucleotide
The invention provides an inactivated vaccine strain CH-GD-12-2014 against the duck adenovirus type 3. The inactivated vaccine strain CH-GD-12-2014 is preserved in China Center for Type Culture Collection, Wuhan University, Wuhan City, China, on November 27, 2017, with an accession number of CCTCC No. V201764. The whole gene sequence of the strain is submitted to Genebank, with an accession numberof KR135164. The invention also provides a polypeptide with an amino acid sequence as shown in SEQ ID No. 2, and a nucleotide sequence encoding the polypeptide, wherein the nucleotide sequence is asshown in SEQ ID No. 1. An inactivated strain provided by the invention is safe to all kinds of ducks and free of side reactions. The vaccine prepared from the inactivated strain is safe and effective,can prevent attacks caused by homologous virulent viruses, and has practical and good application value.
Owner:SOUTH CHINA AGRI UNIV
Complementing cell lines
A packaging cell line that complements recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells that are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 that expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell lines can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. Also, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, and measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Duck adenovirus type 2 inactivated vaccine
ActiveCN107137704AImprove securityGood prospects for commercial developmentViral antigen ingredientsAntiviralsImmune effectsAntigen
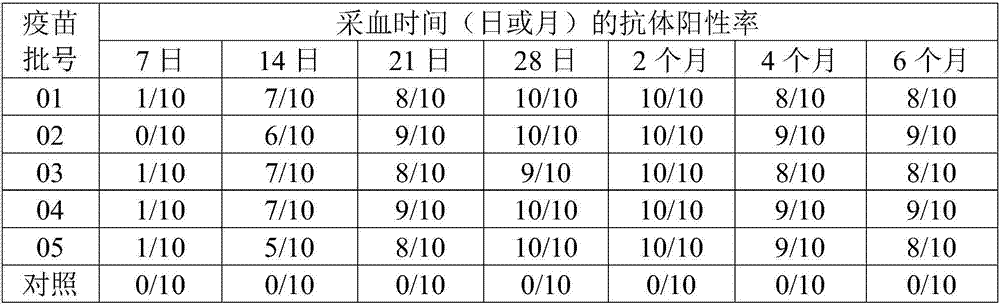
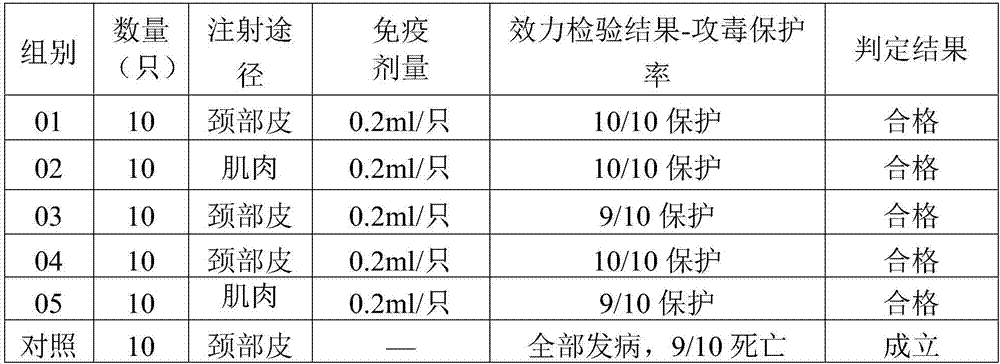
The invention aims at providing a duck adenovirus type 2 inactivated vaccine, wherein an antigen is an inactivated GD strain virus; and the GD strain virus is preserved in China Center for Type Culture Collection in Wuhan University on June 5, 2016 with preservation number of CCTCC No: V201633. The duck adenovirus type 2 inactivated vaccine prepared by the invention is good in safety and free from any local and systemic adverse reactions caused by the vaccine. Based upon analysis on characters, safety test and efficacy test data in a preservation period test, various indexes are stable and effective; and a result of assessing an immune effect of the vaccine by virtue of a serological method and an immune attack method shows that the inactivated vaccine prepared by the invention can achieve effective immune protection on ducks, and the inactivated vaccine has a good commercial development prospect.
Owner:SHANDONG SINDER TECH +1
Duck adenovirus type-3 virus strain and yolk antibody preparation and application thereof
ActiveCN110117576AAvoid infectionImprove featuresEgg immunoglobulinsViral antigen ingredientsYolkVaccine Immunogenicity
The invention discloses a duck adenovirus type-3 virus strain and yolk antibody preparation and application thereof. The duck adenovirus type-3 virus strain is produced by a duck adenovirus type-3 virus GD06 strain immune poultry with the preservation number being CCTCC NO:V201905. The virus strain has the good specificity and immunogenicity. The yolk antibody of the adenovirus type-3 is high in safety, infection of the duck adenovirus type-3 is effectively prevented, the immunity protecting rate is high, and the duck adenovirus type-3 is effectively treated; the virus strain can be used for detecting duck adenovirus type-3 virus and has the wide application prospect.
Owner:GUANGDONG MAIKETE BIOLOGICAL TECH
PCR primer for detecting duck adenovirus type 4 as well as detection method and application of PCR primer
PendingCN112048570AQuick checkEasy to operateMicrobiological testing/measurementMicroorganism based processesApplications of PCRVirology
The invention discloses a PCR primer for detecting duck adenovirus type 4 as well as a detection method and application thereof. The primer capable of rapidly detecting the duck adenovirus type 4 andthe PCR detection method of the primer are established for the first time, operation is easy, cost is low, and consumed time is short. Moreover, the PCR detection method for detecting the duck adenovirus type 4 is high in accuracy, good in specificity and high in sensitivity, the lowest detection limit is 6*10 <1> copies, clinical popularization and application are facilitated, and technical support is provided for carrying out adenovirus type 4 molecular epidemic virology investigation and related detection work in duck flocks.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Conditionally replicating oncolytic adenoviral vector used for expressing two exogenous genes and modified by small peptide, construction method and application thereof
ActiveCN102229961AIncrease the number of expressionsEnhanced inhibitory effectGenetic material ingredientsFermentationFiberSmall peptide
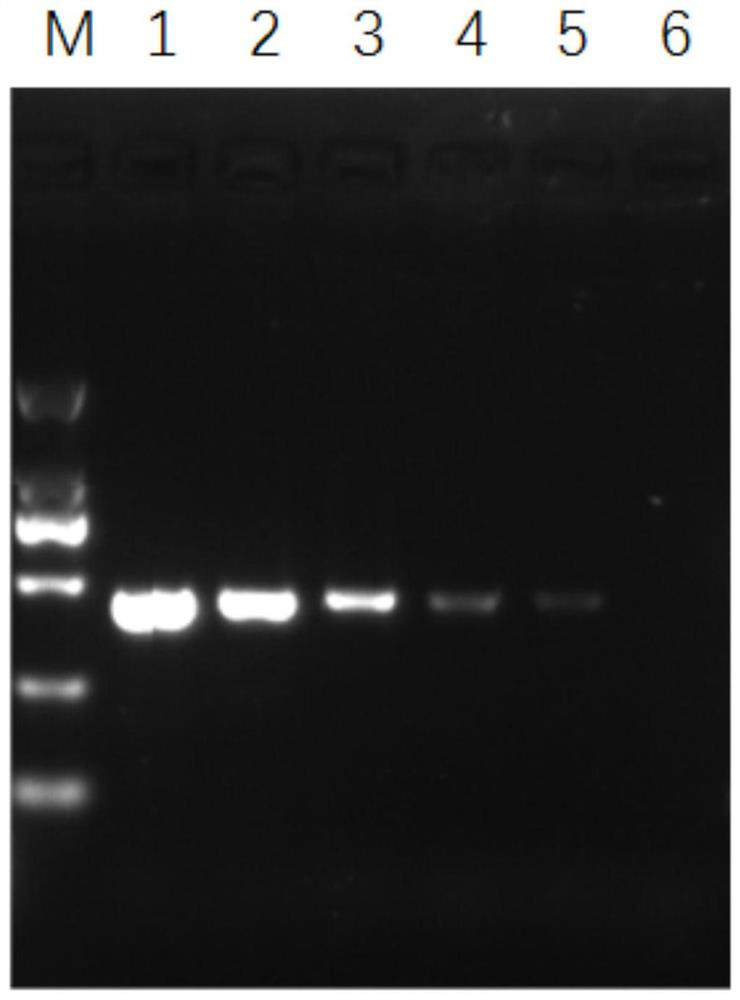
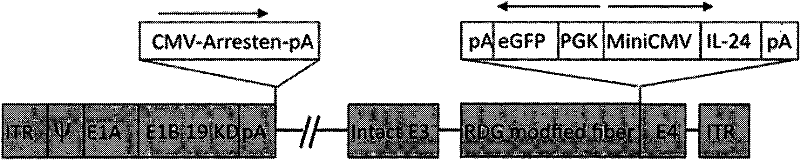
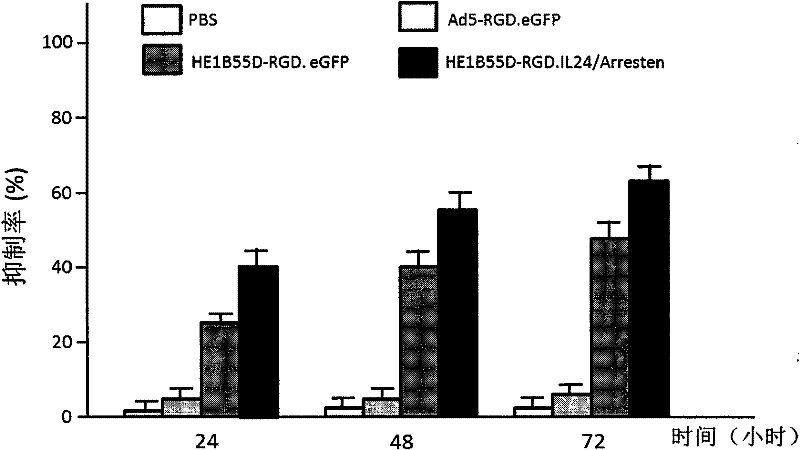
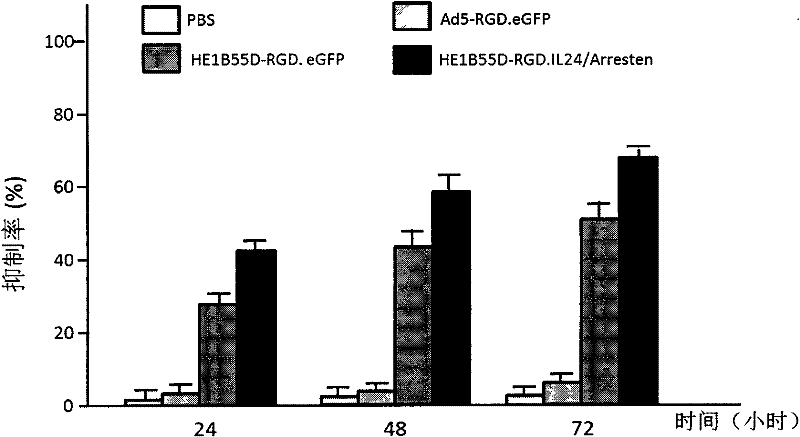
The invention relates to a conditionally replicating oncolytic adenoviral vector used for expressing two exogenous genes and modified by small peptide. Specifically, base 1083bp is deleted in the section of 2245bp-3327bp of human adenovirus type 5 genome, and an expression element expressing a first exogenous gene is inserted into the section of 2245bp-3327bp of human adenovirus type 5 genome. And at 32679bp of adenovirus type 5 genome, i.e. at the position of fibrin HI loop, a code containing a small peptide is inserted. A second exogenous gene of dual expression and an eGFP expression element are inserted into the section of 32787bp-32788bp of human adenovirus type 5 genome. The construction method of the vector includes the steps of: constructing a shuttle missed by pAd5E1B 55kd, constructing a shuttle of the first exogenous gene pHE1B55D-, constructing an adenoviral vector backbone of the first exogenous gene pHE1B55D- / SwaI, constructing a shuttle of the small peptide sequence pshuttle Ad5-E4-fiber-, constructing a shuttle expressing eGFP and the second exogenous gene, and preparing the conditionally replicating oncolytic adenoviral vector used for expressing two exogenous genes and modified by small peptide. The invention also provides the application of the adenoviral vector provided in the invention in preparing medicaments for treating tumours.
Owner:SHAANXI NORMAL UNIV
Primers and probe for rapidly and quantitatively detecting duck adenovirus type 4 and detection method and application thereof
PendingCN111926116AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBiochemistryFluorescent pcr
The invention discloses primers and a probe for rapidly and quantitatively detecting duck adenovirus type 4 and a detection method and application of the primers and the probe. The sequences of the primers are as shown in SEQ ID NO.1-2, and the sequence of the probe is as shown in SEQ ID NO.3. The fluorescent PCR method capable of rapidly and quantitatively detecting the duck adenovirus type 4 inthe clinical sample is established for the first time; the detection method is simple to operate, the whole operation process does not exceed 3 hours, the sensitivity is high, the specificity is good,the repeatability is good, quantitative analysis can be accurately and rapidly carried out with high flux, and the detection method is beneficial to popularization and application in clinical practice.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Conditionally replicating adenovirus to express REIC gene
ActiveUS10071126B2Good anticancer effectGood treatment effectVectorsViral/bacteriophage medical ingredientsAnticarcinogenic EffectCancer cell
An object of the present invention is to provide a conditionally replicating adenovirus having a strong anticancer effect. A conditionally replicating adenovirus to replicate specifically in a cancer cell and express REIC protein or REIC C domain protein, wherein the conditionally replicating adenovirus is obtained by inserting full-length REIC DNA or REIC C domain DNA into a conditionally replicating adenovirus comprising an ITR (inverted terminal repeat) sequence of an adenovirus type 5 genome and insertion of an HRE sequence, an hTERT promoter, a decorin-encoding DNA, and a DNA encoding a peptide comprising an RGD sequence.
Owner:UNIV OKAYAMA +2
Duck adenovirus type 1 virus replication nonessential region fragment screening and its general transporter and recombination obtained therefrom
InactiveCN1936008AFermentationVector-based foreign material introductionScreening methodAdenovirus typing
The invention relates to a selecting method for duck adenovirus 1 type virus replicating non-necessary section segment and the recombination fowl adenovirus gained through transporter gene and the universal transporter gene. It uses duck adenovirus 1 type virus as material to expand the sequence at right side of E4 area by PCR method. Inducing loxP sequence to the two sides of GFP gene expression box and inserting into the expanded segment, the universal transporter gene would be constructed. The transporter gene would gain the recombination adenovirus of stable expression GFP, thus, a replicating non-necessary area would be determined. The universal transporter gene loxP-GFP-loxP sequence side contains a unique enzyme Restriction Enzyme cutting site, and cold insert relative target gene to gain recombination virus without GFP gene. The gene engineer medicine developed from the universal transporter gene would not contain selecting marker gene.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Kit for rapidly and accurately detecting adenovirus type 3 and preparation method thereof
ActiveCN111537711AImprove reliabilityStrong specificityVirus peptidesBiological material analysisAbzymeAntiendomysial antibodies
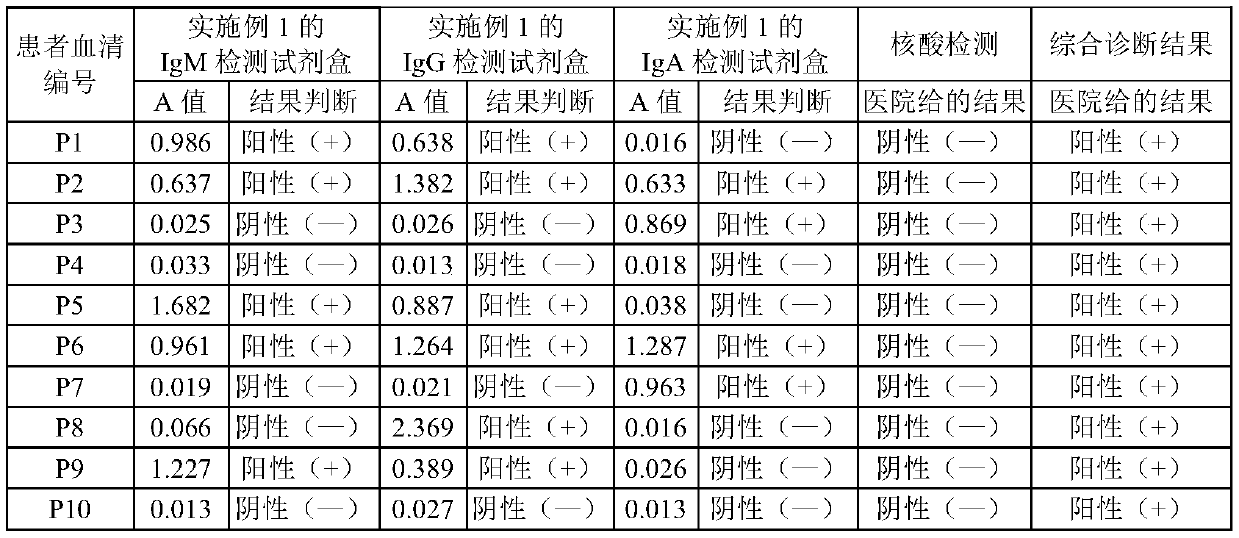
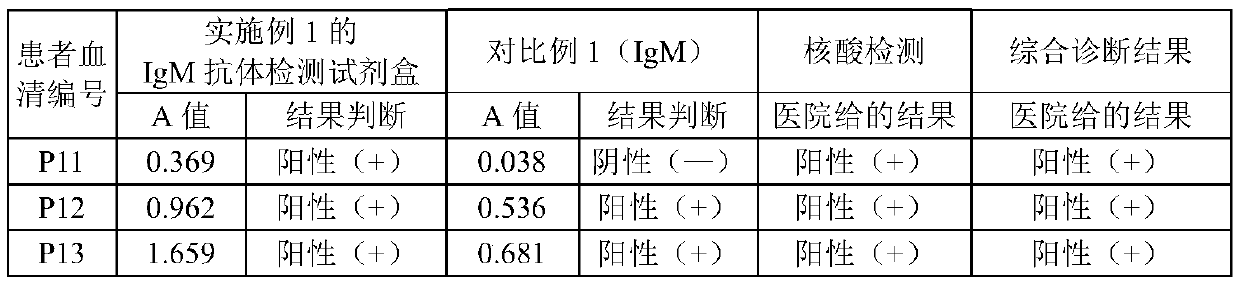
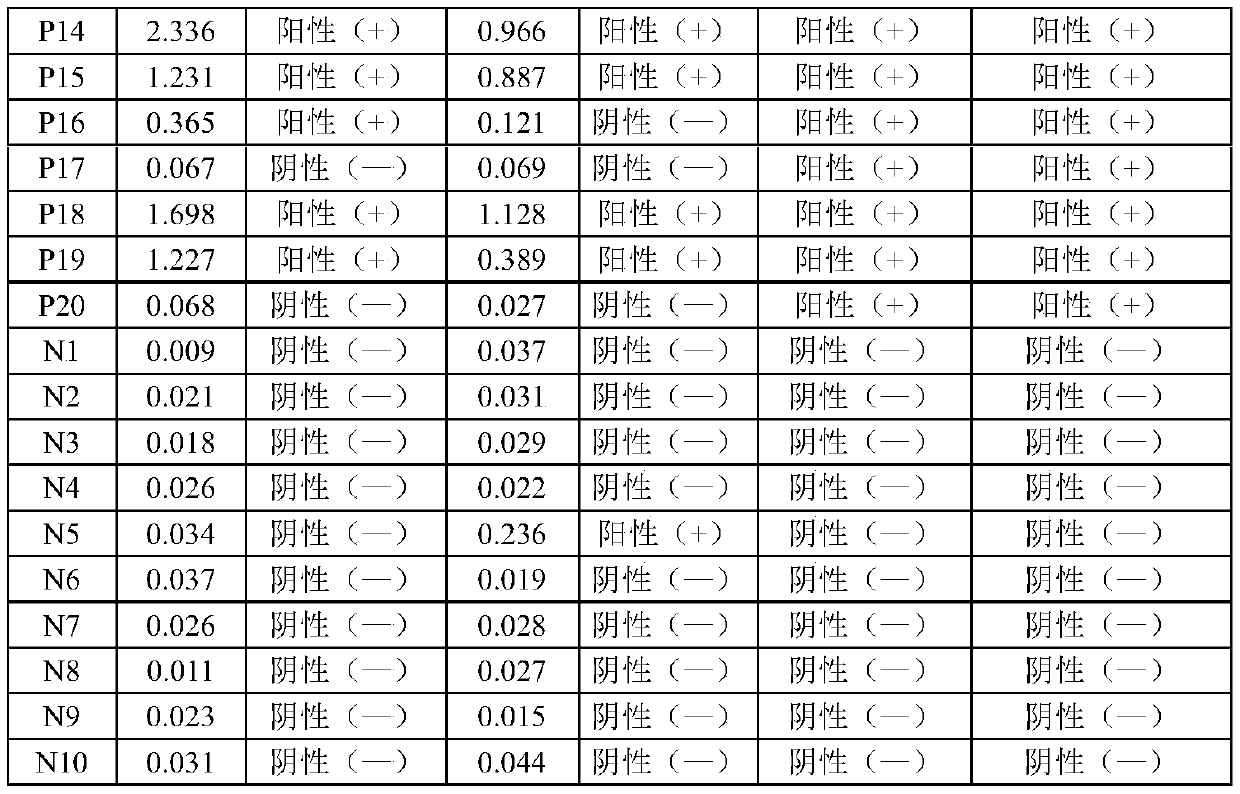
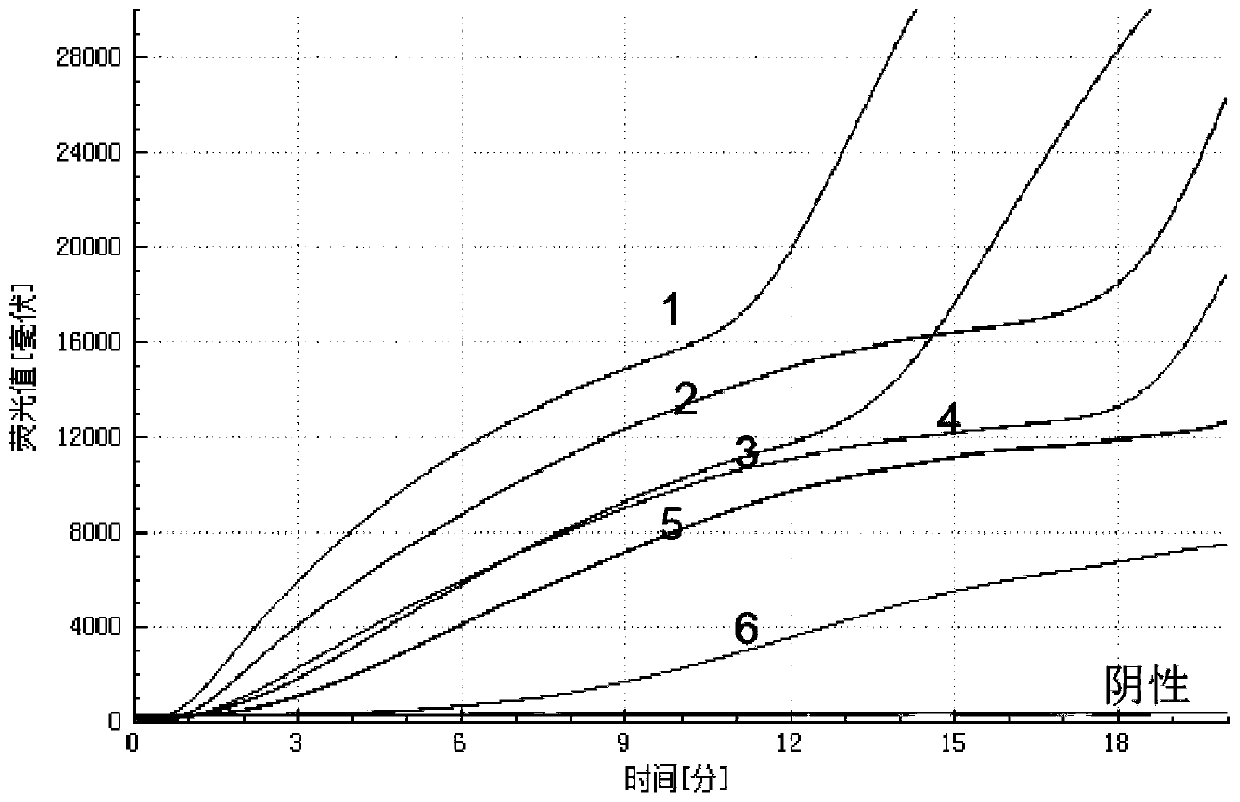
The embodiment of the invention relates to the field of in-vitro detection, in particular to a kit for rapidly and accurately detecting adenovirus type 3 and a preparation method of the kit. The kit provided by the embodiment of the invention comprises an IgM antibody enzyme-labeled working solution, an IgA antibody enzyme-labeled working solution and an IgG antibody enzyme-labeled working solution. The IgG antibody enzyme-labeled working solution comprises a peroxidase-labeled anti-human IgG antibody and an ELISA plate coated with an adenovirus 3 type recombinant antigen; the adenovirus 3 type recombinant antigen comprises one or more of a recombinant antigen A, a recombinant antigen B or a recombinant antigen C. The kit (enzyme linked immunosorbent assay) provided by the invention makesup the blank in the market;, the detection results are comprehensively considered by respectively detecting adenovirus type 3 IgM, IgG and IgA antibodies in human serum or blood plasma, the detectionresults are complementary with each other, the reliability is high, the specificity is good, and the sensitivity is high; the kit can be complementary with nucleic acid detection, and is suitable forclinical diagnosis and epidemiological investigation.
Owner:BEIJING BEIER BIOENG
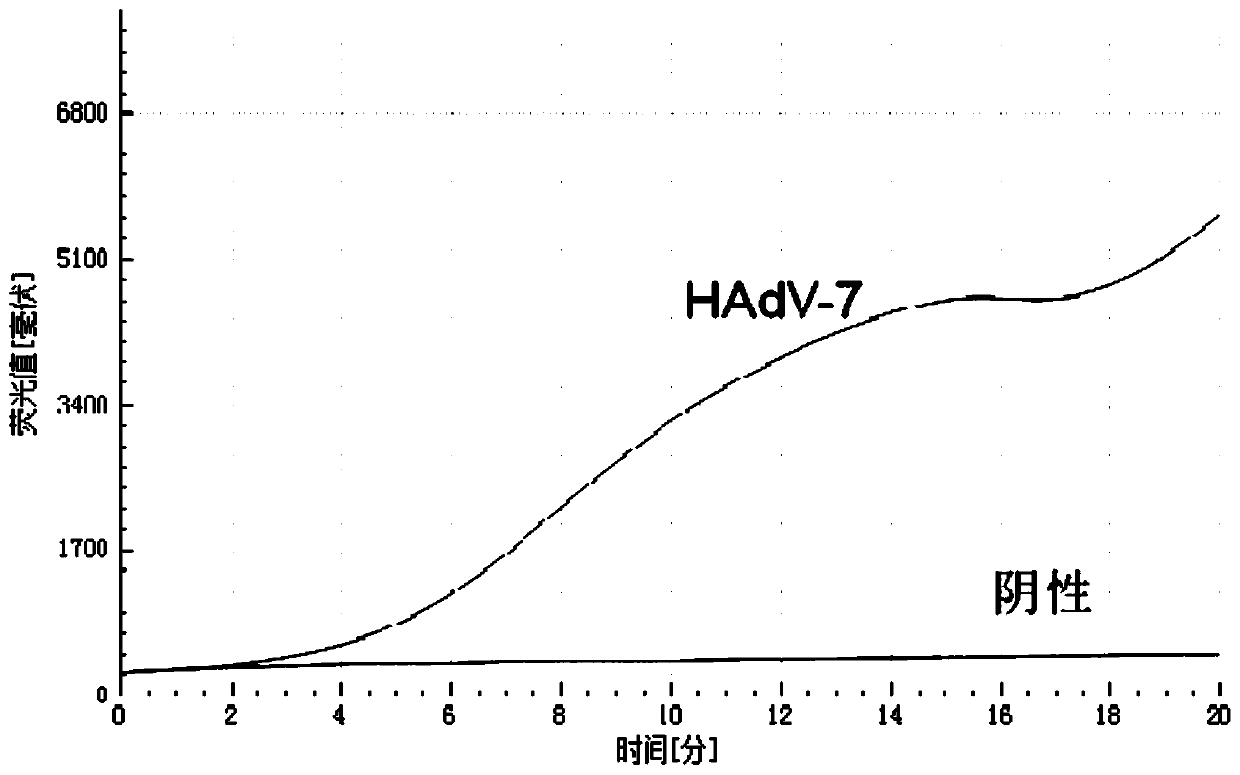
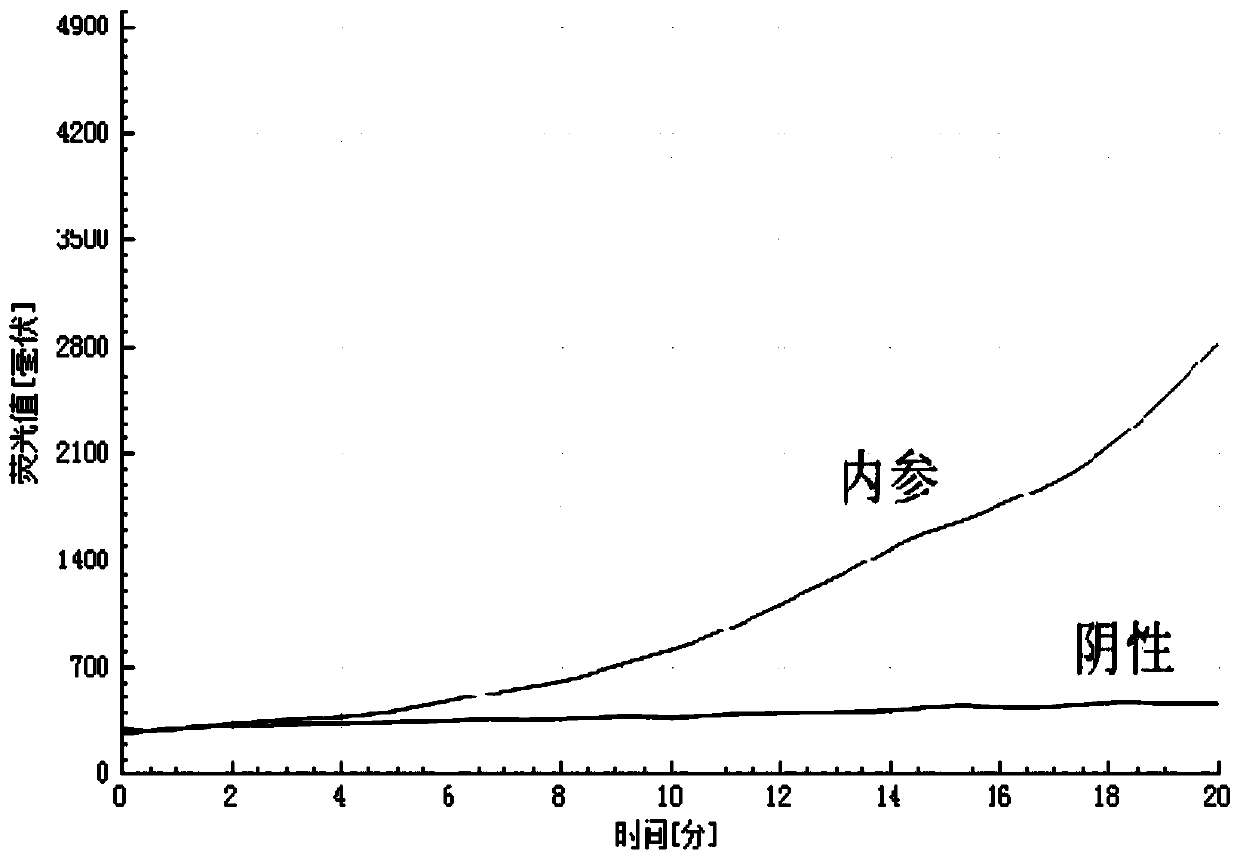
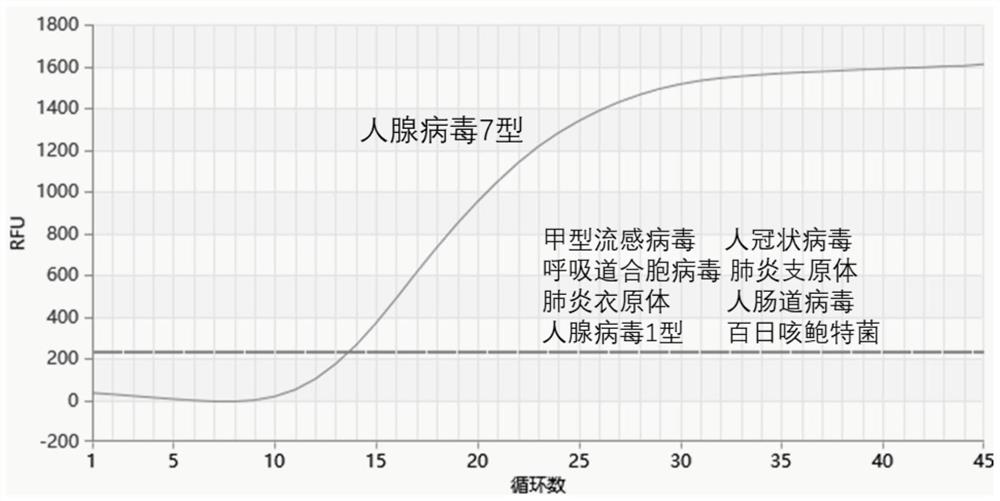
Amplification method of internal reference containing double isothermal nucleic acid for rapidly detecting type-7 adenovirus
ActiveCN110055353AStrong specificityRule out false negativesMicrobiological testing/measurementAgainst vector-borne diseasesNucleotideNucleotide sequencing
The invention provides an amplification method of internal reference containing double isothermal nucleic acid for rapidly detecting type-7 adenovirus and belongs to the technical field of isothermalnucleic acid amplification. According to the amplification method, a specific primer pair and a probe for HAdV-7 detection are designed first through human adenovirus type-7 (HAdV-7) gene sequencing and alignment, an internal reference probe is designed, and the nucleotide sequences of the specific primer pair and the probe are respectively as shown in SEQ ID NO.1-4, an internal reference containing double isothermal nucleic acid amplification system is established, and a kit for detecting adenovirus type 7 is further constructed. The method is carried out under isothermal conditions, the amplification of the type-7 adenovirus and the internal reference DNA can be realized within 5-20min, the sensitivity is high, the specificity is good, false negative and invalid results are eliminated due to the addition of an internal reference, the method is more suitable for the detection of a large number of samples, the clinical application is facilitated, and the method is suitable for the rapid detection of the type-7 adenovirus.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
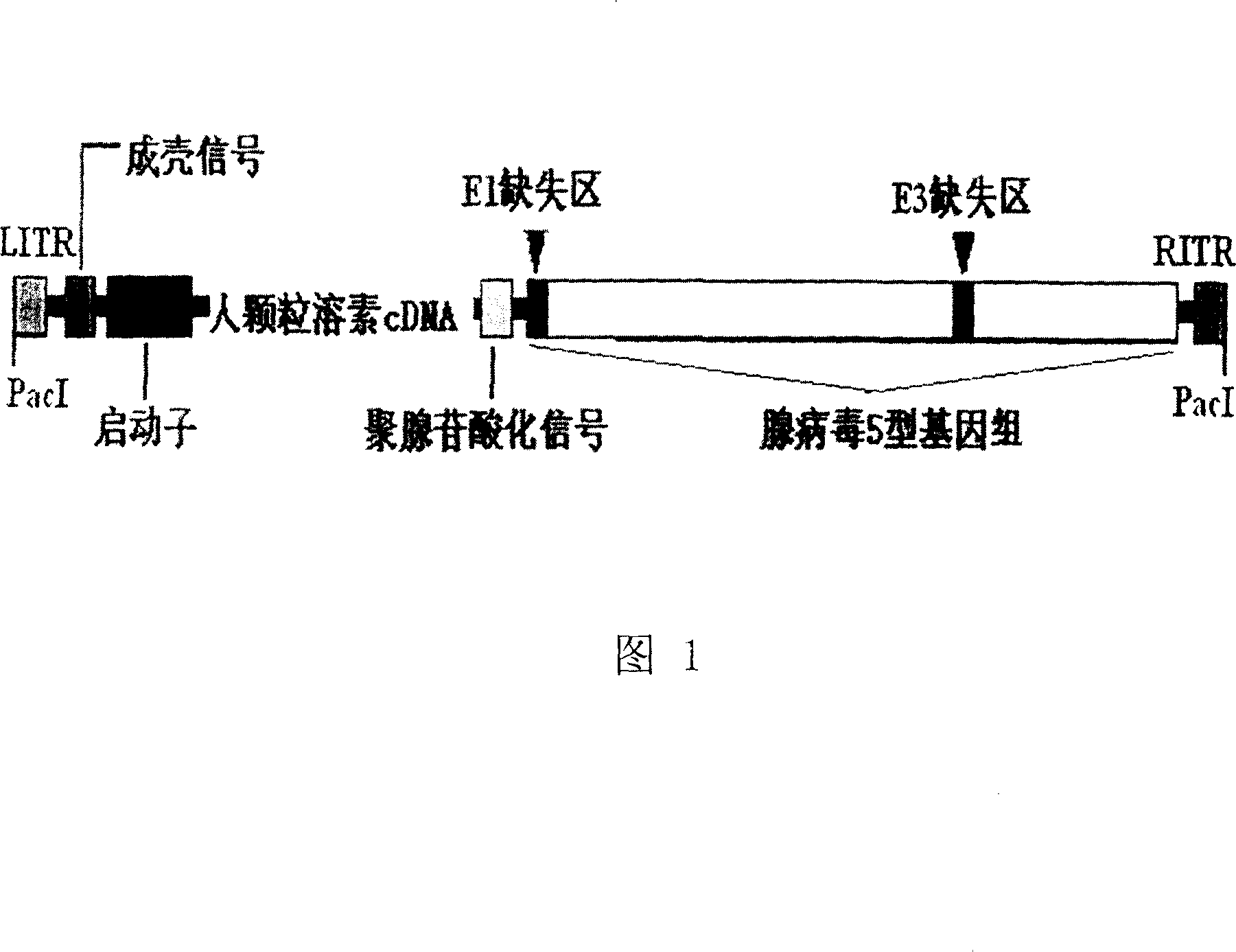
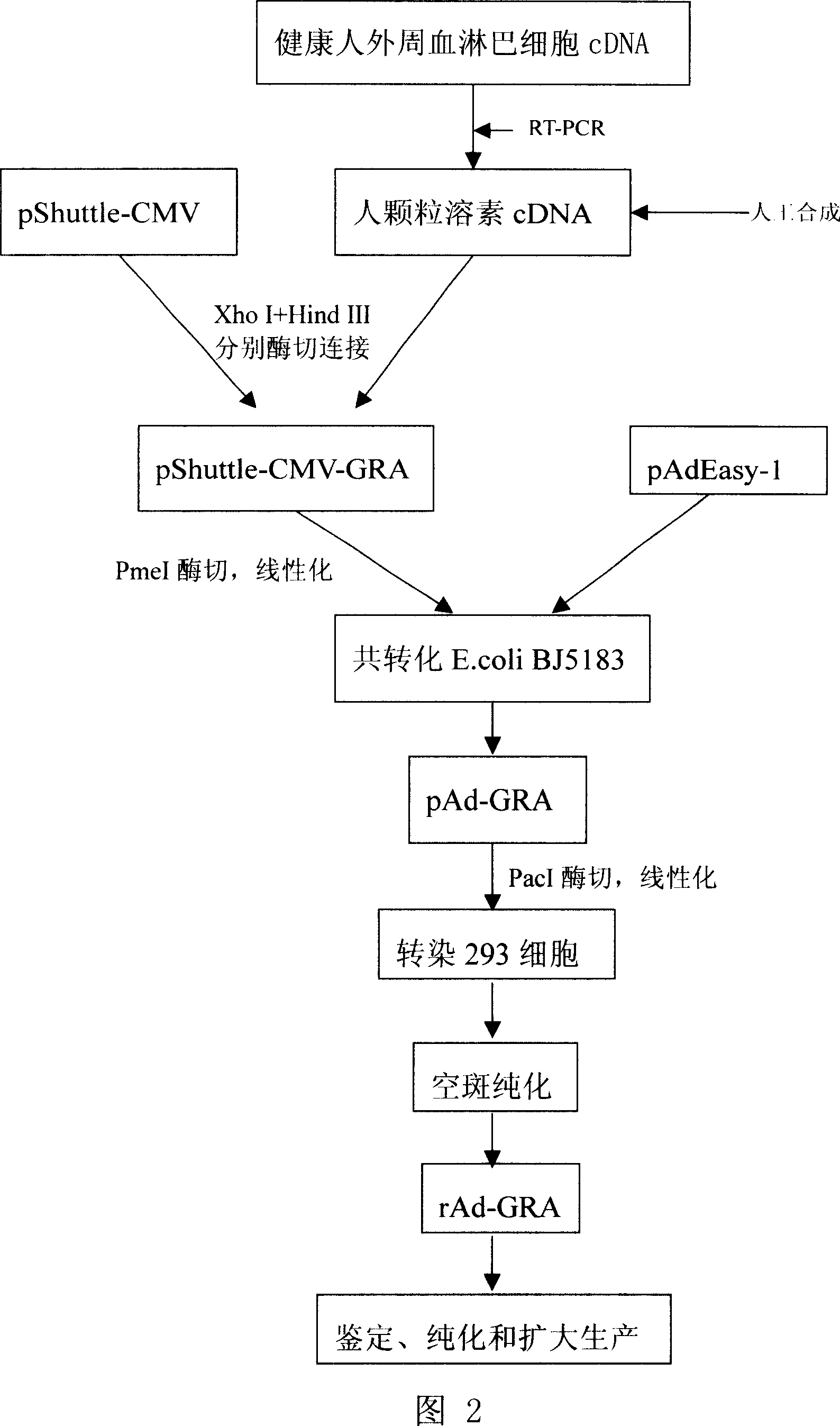
Recombination adenovirus for expressing human particle cytolysin and its preparing method and use
InactiveCN101033464ALow costImprove targetingViruses/bacteriophagesAntibody medical ingredientsPoly-A RNAGenetics
The invention provides a kind of recombinant adenovirus expressing human granulysin, which genome is deleted E1 and E3, and inserted the expression cassette of human granulysin in E1, and the expression cassette includes: promoter sequence, human granulysin coding sequence and polyadenylation signal by turns. The adenovirus type is Ad5. The preparation method includes: a. cloning the gene of human granulysin, b. constructing the plasmid pAd-GRA expressing human adenovirus, c. digesting pAd-GRA with enzyme PacI, conventionally extracting with phenol, chloroform / isoamyl alcohol, washing with ethanol, suspending in TE 50 mu l under sterile conditions, and transferring -293 cells.
Owner:华中科技大学同济医学院
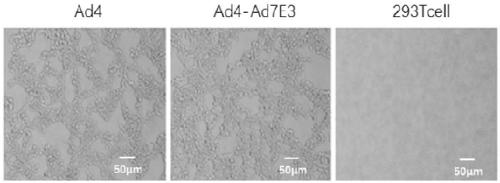
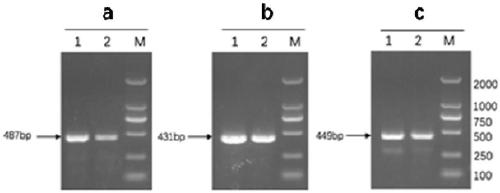
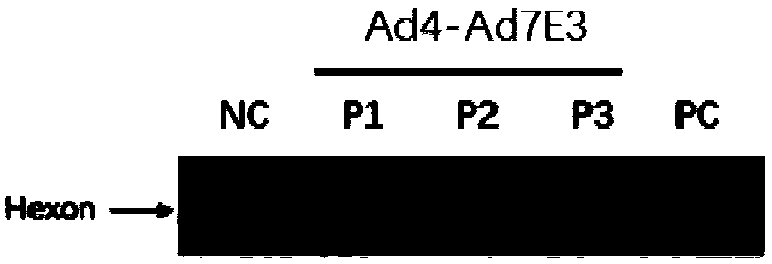
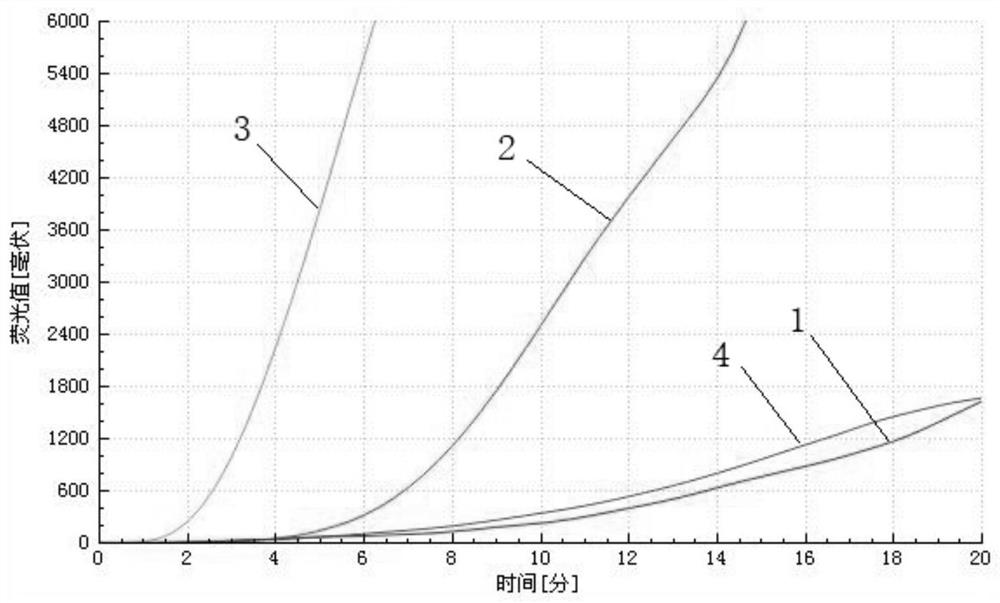
Ad4/Ad7 type bivalent recombinant adenovirus and application thereof
The invention discloses an Ad4 / Ad7 type bivalent recombinant adenovirus and application thereof. The invention provides the recombinant adenovirus, which is characterized in that compared with the genomic DNA of a human type 4 adenovirus, the genomic DNA of the recombinant adenovirus only undergoes the following three mutations: 1, the first to eighth nucleotides mutate into 'CATCATCA' from 'ctatctat'; 2, the 35983th-35990th nucleotides mutate into 'TGATGATG' from 'atagatag'; 3, replacing DNA molecules for encoding a fifth hypervariable region of an adenovirus type 4 hexon with DNA molecules for encoding a fifth hypervariable region of an adenovirus type 7 hexon. The invention has a great application value for the prevention and control of Ad4 adenovirus and Ad7 adenovirus.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Primer probe combination, kit and detection method for detecting duck adenovirus type 3 based on RAA technology
PendingCN112176108AQuick checkAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationHexon geneVirus
The invention discloses a primer probe combination, a kit and a detection method for detecting duck adenovirus type 3 based on an RAA technology. The primer probe combination is designed according toduck adenovirus type 3 virus specific Hexon gene, and the kit and the detection method utilize the primer probe combination to perform RAA reaction on a to-be-detected sample. Whether the to-be-detected sample contains the duck adenovirus type 3 virus or not is judged according to an amplification reaction result. The kit and the detection method are applied to rapid detection of the duck adenovirus type 3 virus, and accurate, rapid, sensitive and convenient detection of the duck adenovirus type 3 virus is realized.
Owner:山东信达基因科技有限公司 +1
Complementing cell lines
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary diploid human cells transformed by adenovirus E1 sequences either operatively linked on one or two DNA molecules, the sequences operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also, a cell line derived from PER.C6 that expresses functional Ad35-E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses. The cell lines can be used to produce human recombinant therapeutic proteins such as human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza, herpes simplex, rotavirus, and measles.
Owner:JANSSEN VACCINES & PREVENTION BV
Fused gene, recombinant expression vector, antigen, preparation method and application
ActiveCN110128545AFree from infectionReduce repetitive workSsRNA viruses negative-senseAntibody mimetics/scaffoldsAgricultural scienceAvian adenovirus
The invention belongs to the technical field of veterinary biological products, and discloses a fused gene. A base sequence is as shown in a sequence table SEQ ID NO: 1. An avian influenza H9 subtypeHA gene, an adenovirus type 4 fiber2 gene and a specific connecting peptide segment gene are fused to form the fused gene. An expression protein antigen corresponding to the fused gene can induce poultry to generate high-level specific antibodies, and the poultry is protected from infection of avian influenza and avian adenoviruses. Besides, the invention further discloses a recombinant expressionvector, an antigen, a preparation method and an application.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +1
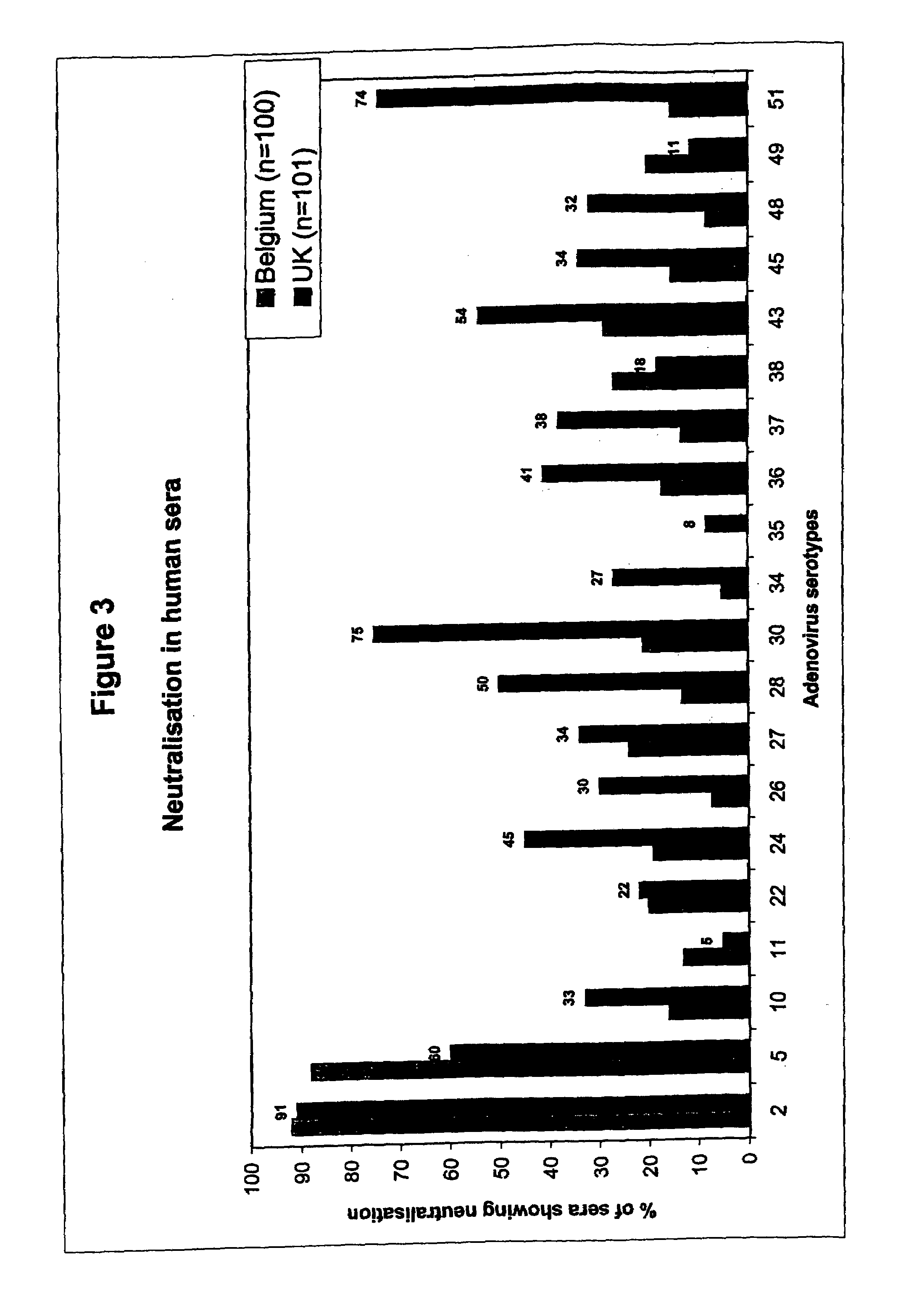
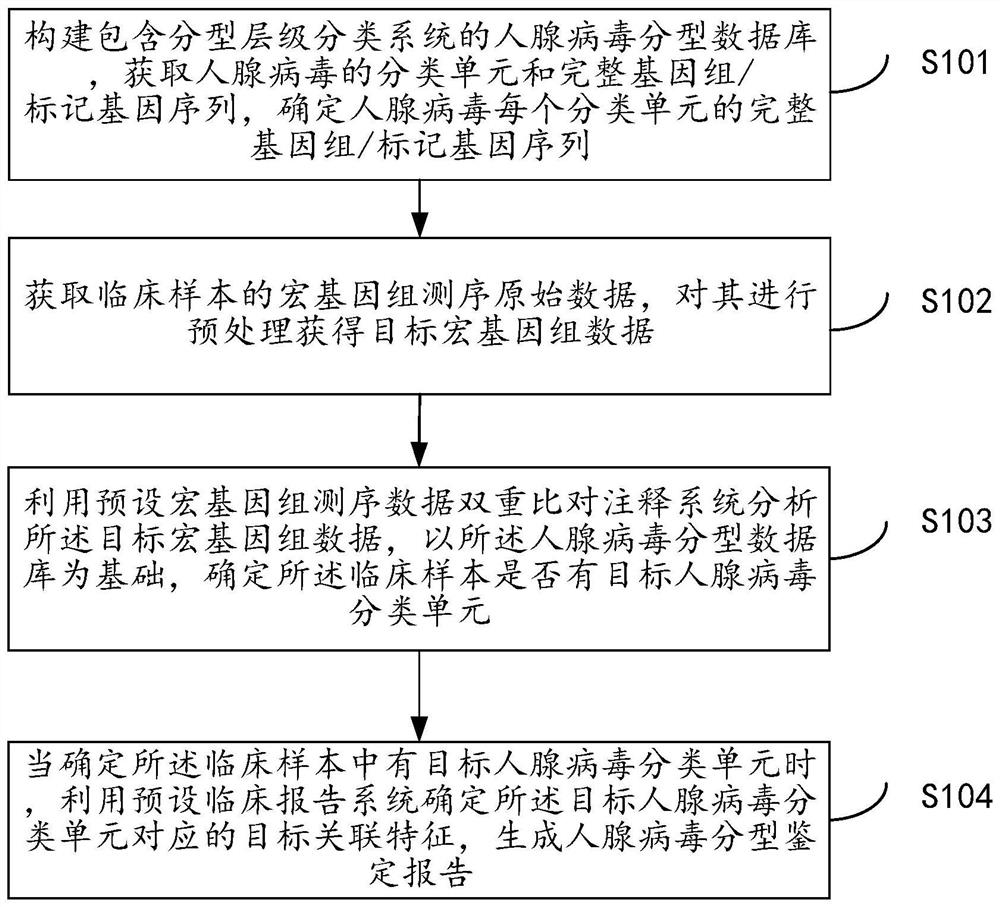
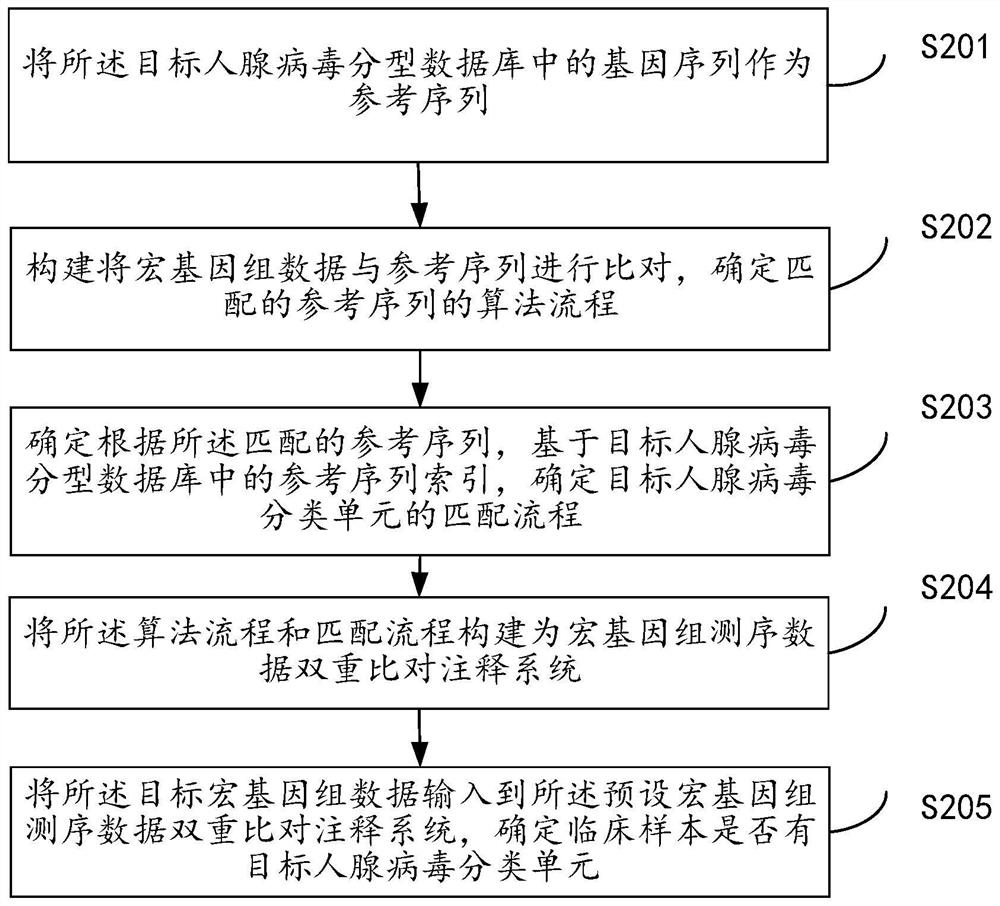
Metagenome-based human adenovirus molecular typing and tracing method and system
The invention discloses a metagenome-based human adenovirus molecular typing and tracing method and system, and the method comprises the steps: constructing a human adenovirus typing database comprising a typing hierarchical classification system, and obtaining a classification unit and a complete genome / marker gene sequence of human adenoviruses, determining a complete genome / marker gene sequence of each classification unit of the human adenovirus, obtaining metagenome sequencing original data of a clinical sample, preprocessing the metagenome sequencing original data to obtain target metagenome data, analyzing the target metagenome data by utilizing a preset metagenome sequencing data dual-comparison annotation system, determining whether the clinical sample has a target human adenovirus classification unit or not, and when it is determined that the clinical sample has the target human adenovirus classification unit, determining a target associated feature corresponding to the target human adenovirus classification unit, and generating a human adenovirus typing identification report. Classification unit (subtype / genotype) typing identification of human adenoviruses can be carried out on clinical infection samples with low virus content.
Owner:HUGOBIOTECH BEIJING CO LTD +2
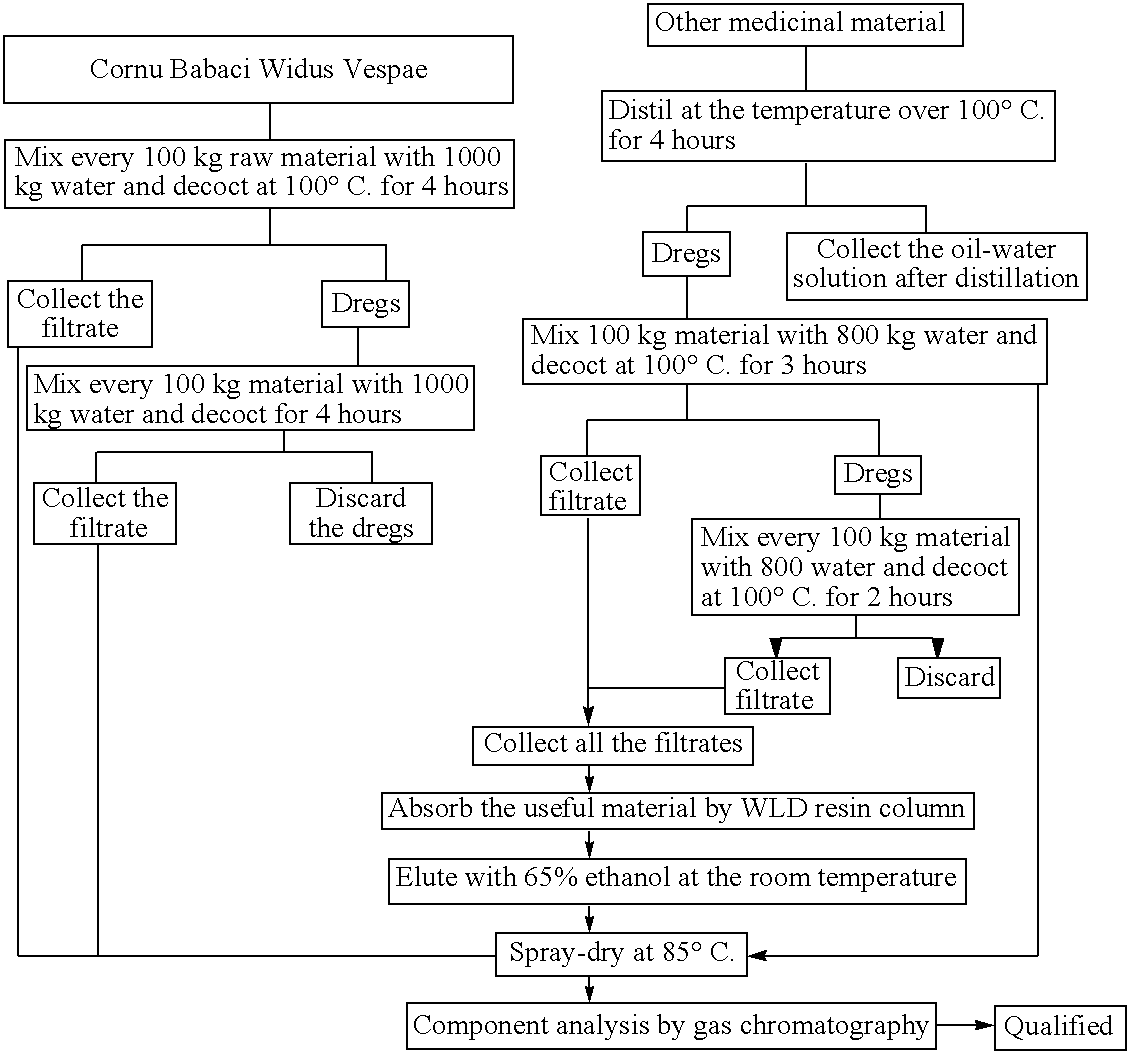
Pharmacological enhancement and manufacturing method of antiviral compound
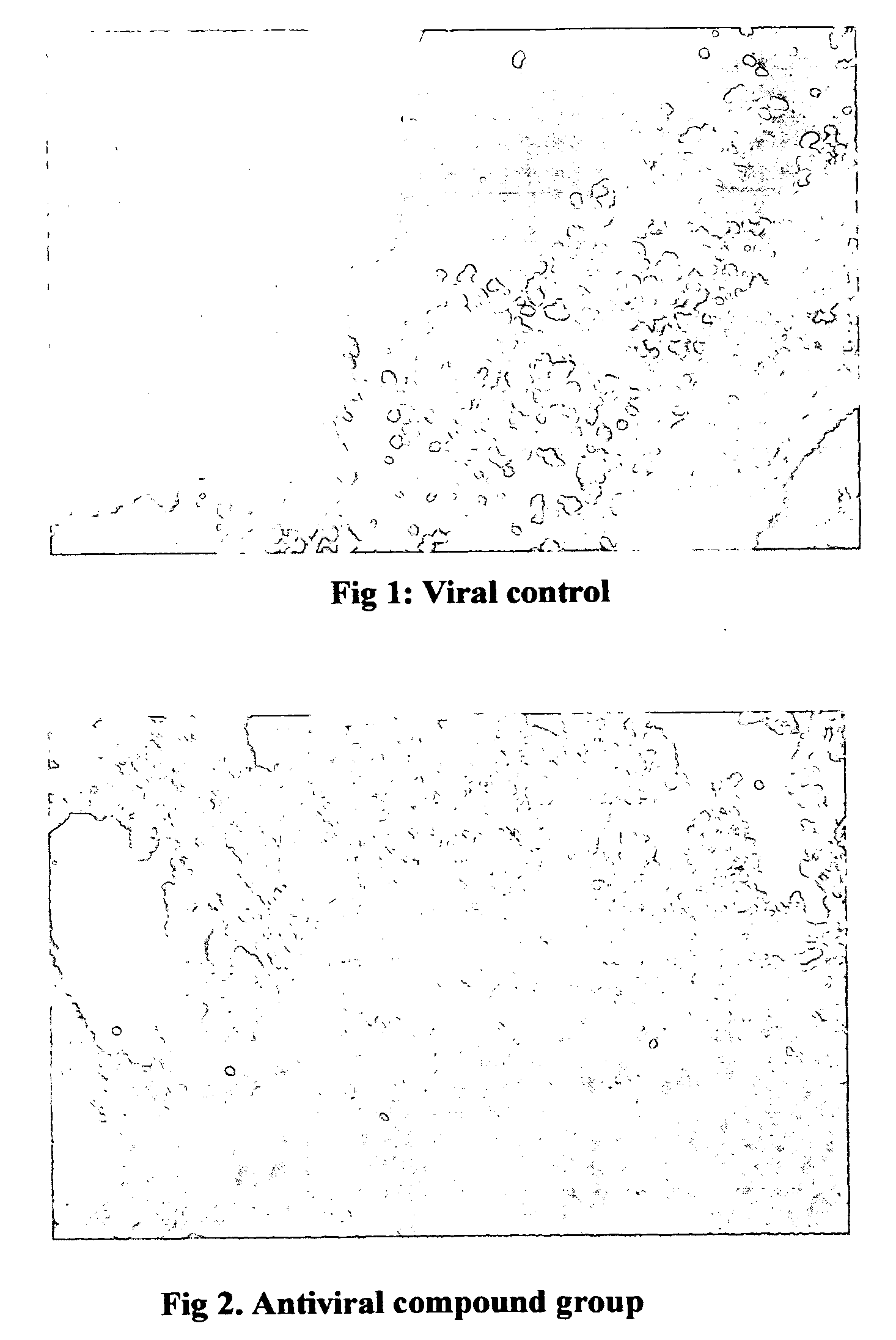
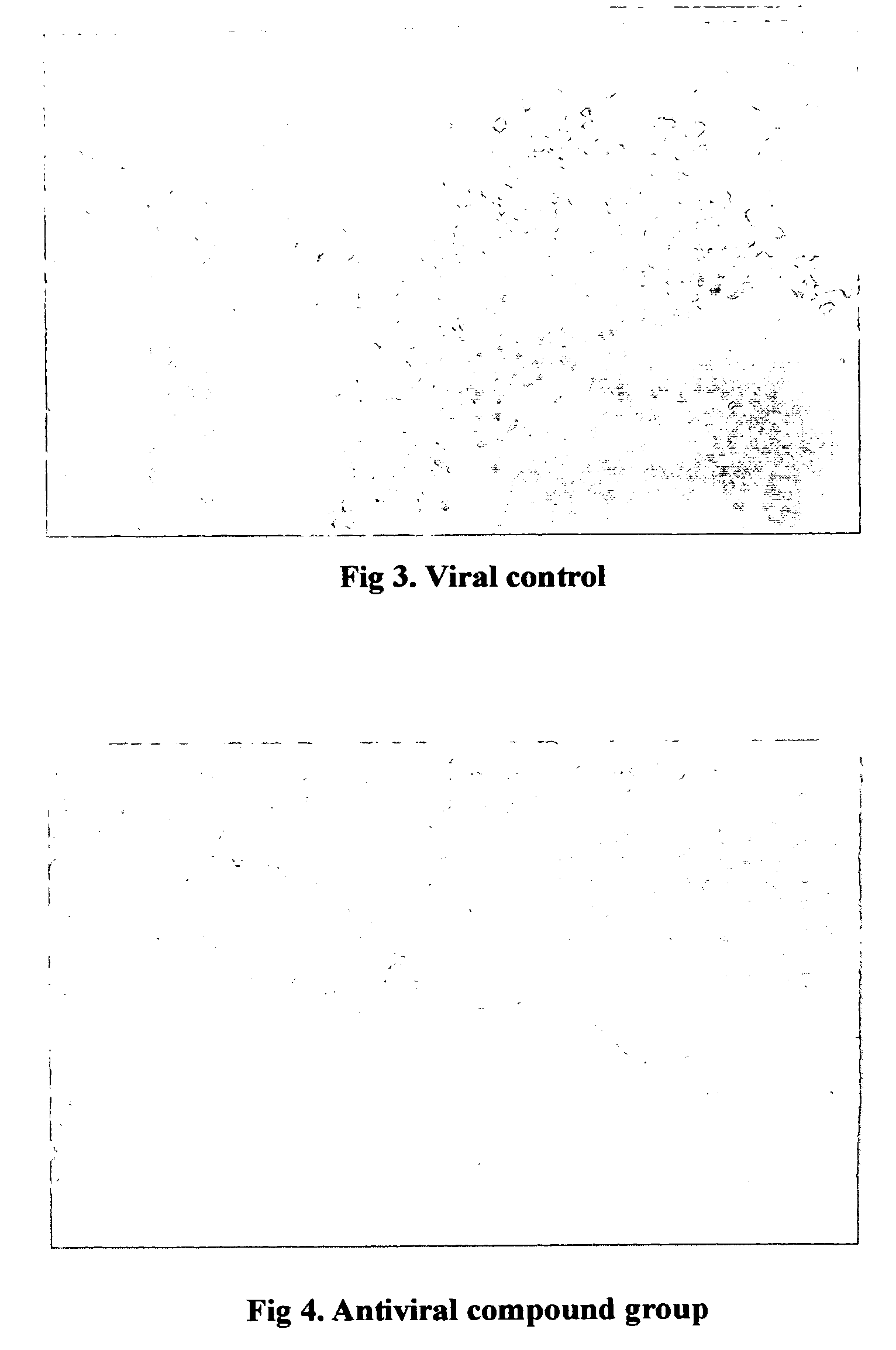
“Pharmacological enhancement and manufacturing method of the antiviral compound” can be categorized into the combined patent of the pharmacological activity as well as method of isolating and purifying the naturing material. Our product adopts 9 medicinal material which are processed strictly. The stable and high quality is ensured by the WLD resin adsorption and gas chromatography. The antiviral prevention and treatment is the most urgent but unsolved problem before the American doctors. The antiviral compound has an unexpected effect on a broad spectrum of viruses including RSV, Adenovirus type 3, Influenza A1 and A3. The antiviral effect on mouse Influenza A1 is very obvious. The definite efficacy in the treatment of acute pharyngitis and tonsillitis have been proved by the clinical trial which showed the total effective rate in the above two disease were 92.3% and 87.5% respectively.
Owner:GONG JIAO +1
Muscovy duck novel adenovirus strain, bivalent inactivated vaccine and preparation method thereof
ActiveCN110628726AImprove securityEffective immune protectionViral antigen ingredientsMicroorganism based processesAntigenImmune effects
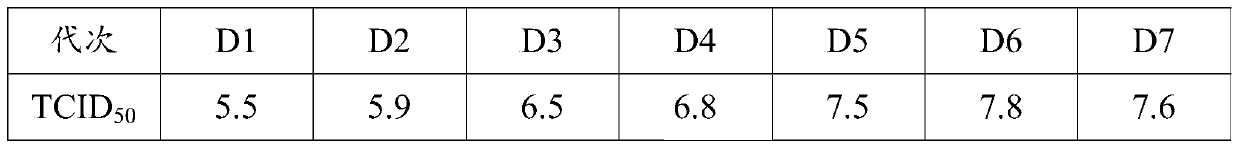
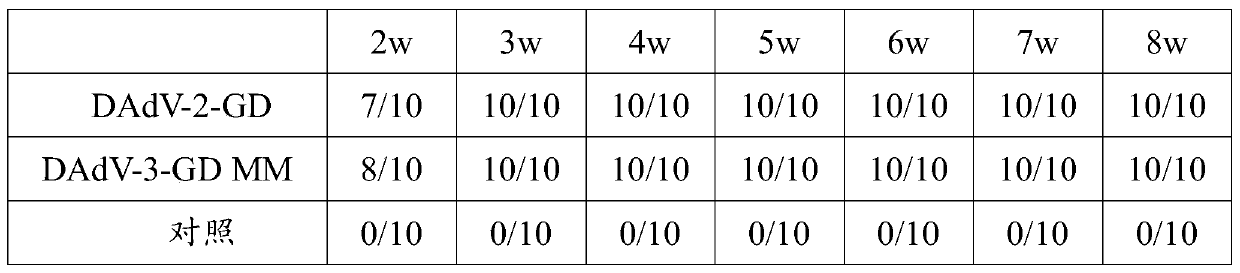
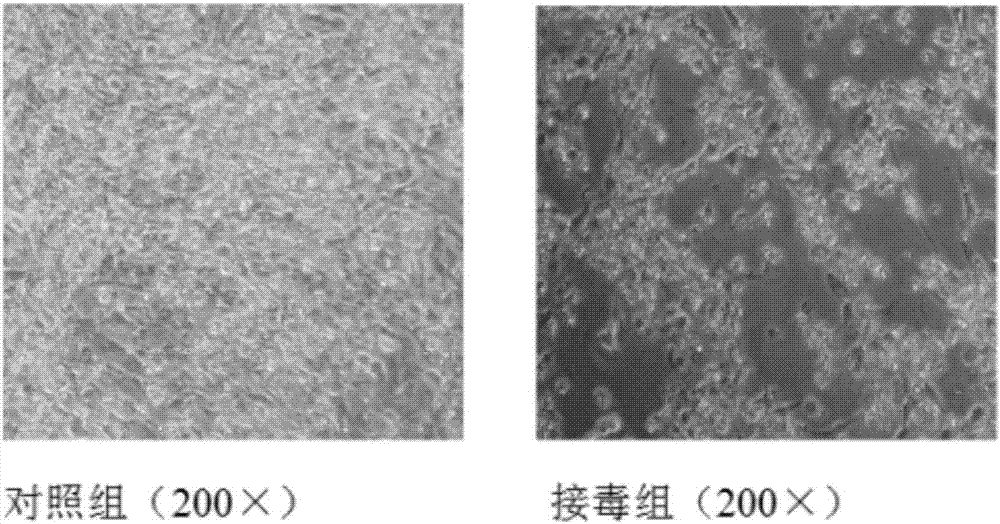
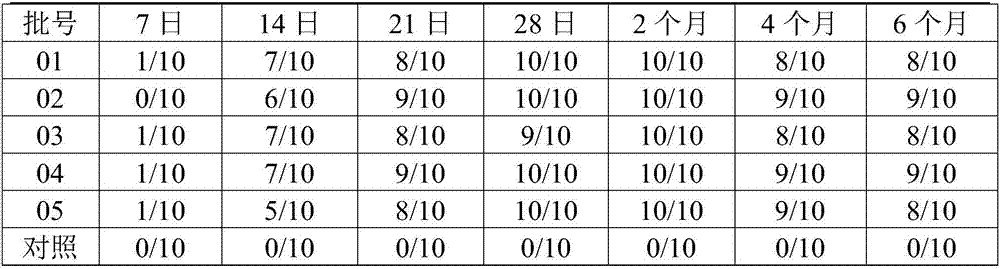
The invention discloses a Muscovy duck novel adenovirus strain, bivalent inactivated vaccine and a preparation method thereof. The bivalent inactivated vaccine is prepared by taking duck adenovirus and DAdV-3-GD MM virus as inactivated antigens. The Muscovy duck adenovirus type II and type III bivalent inactivated vaccine prepared from the screened virus has high safety, and any local and systemicadverse reaction caused by the vaccine does not appear. In the storage life experiment, each index is stable and effective through analysis of character, safety experiment and efficacy experiment data; and through evaluation on the immune effect of the vaccine by a serology method and a immune poison attack method, the Muscovy duck adenovirus type II and type III bivalent inactivated vaccine canprovide effective immune protection on Muscovy ducks and has good commercial development prospect.
Owner:SHANDONG SINDER TECH +1
Duck adenovirus type 2 strain
ActiveCN107142248AImprove securityEffective immune protectionViral antigen ingredientsMicroorganism based processesDiseaseImmune effects
The invention aims at providing a duck adenovirus type 2, wherein the duck adenovirus type 2 is a duck adenovirus type 2 GD strain which is preserved in China Center for Type Culture Collection in Wuhan University on June 5, 2016 with preservation number of CCTCC No: V201633. The duck adenovirus type 2 prepared by the invention can be used for preparing a vaccine for preventing adenovirus type 2 diseases in a group of ducks. The duck adenovirus type 2 inactivated vaccine prepared by the virus screened by the invention is good in safety and free from any local and systemic adverse reactions caused by the vaccine. Based upon analysis on characters, safety test and efficacy test data in a preservation period test, various indexes are stable and effective; and a result of assessing an immune effect of the vaccine by virtue of a serological method and an immune attack method shows that the inactivated vaccine prepared by the invention can achieve effective immune protection on the ducks, and the inactivated vaccine has a good commercial development prospect.
Owner:SHANDONG SINDER TECH +1
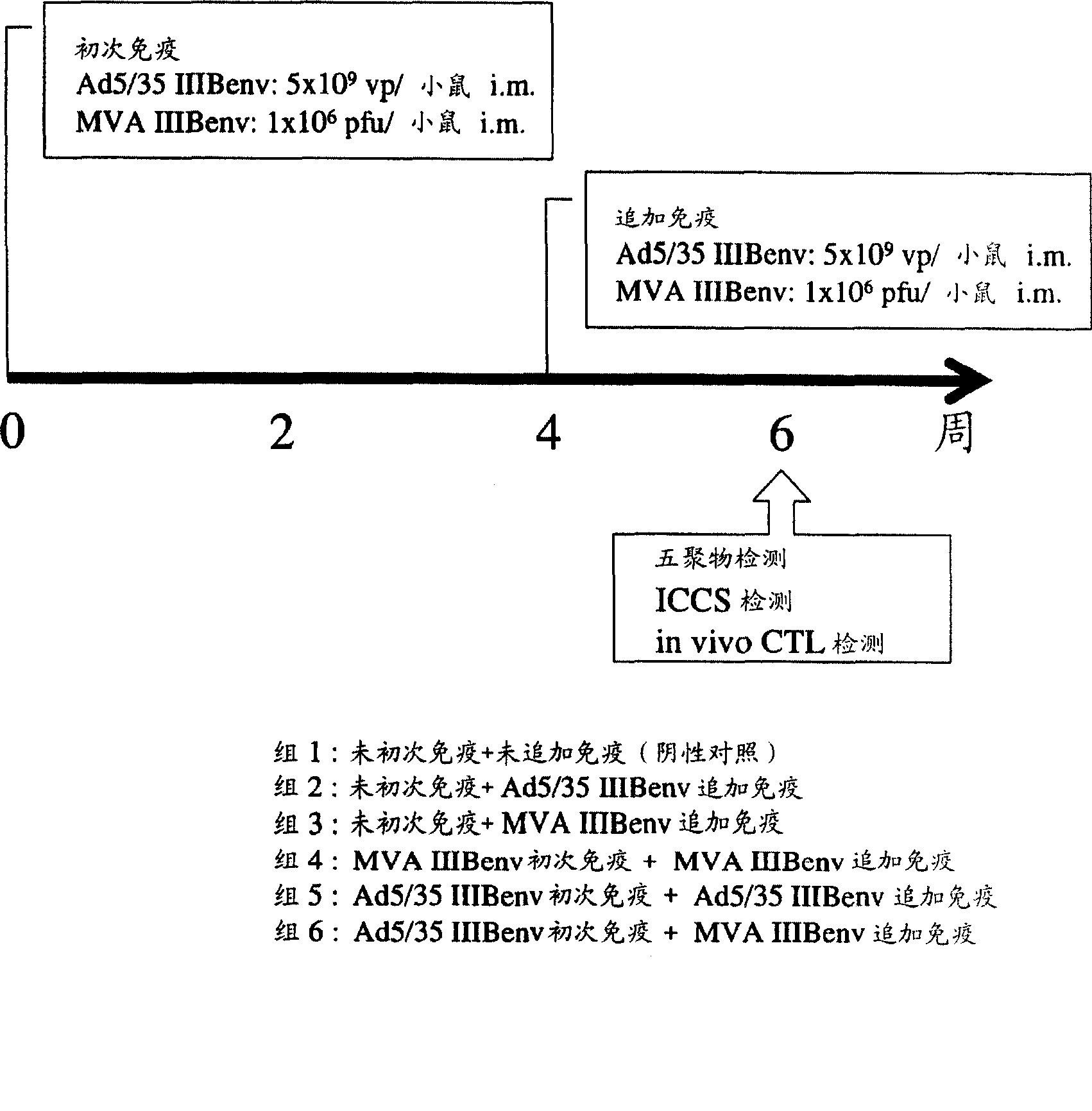
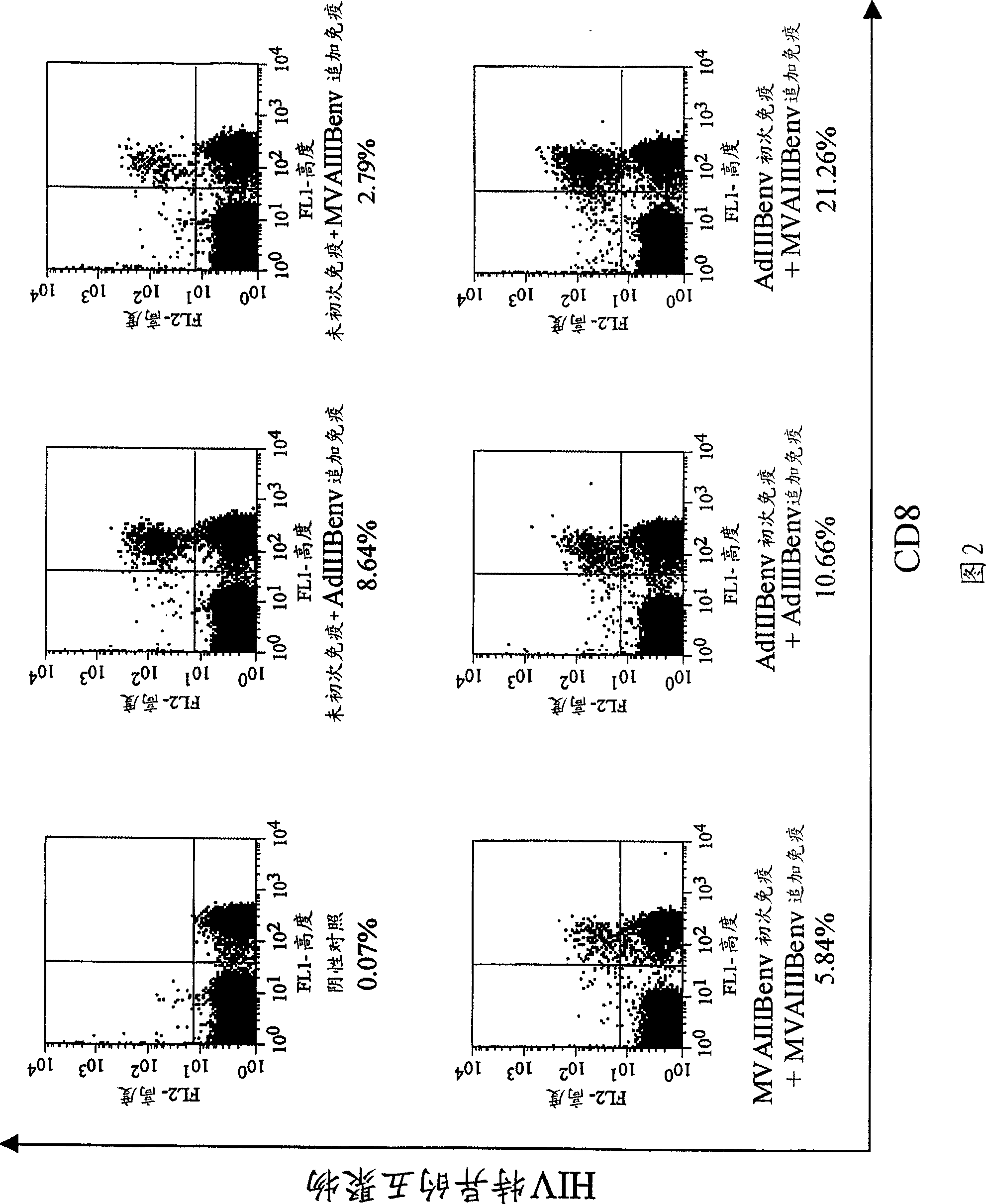
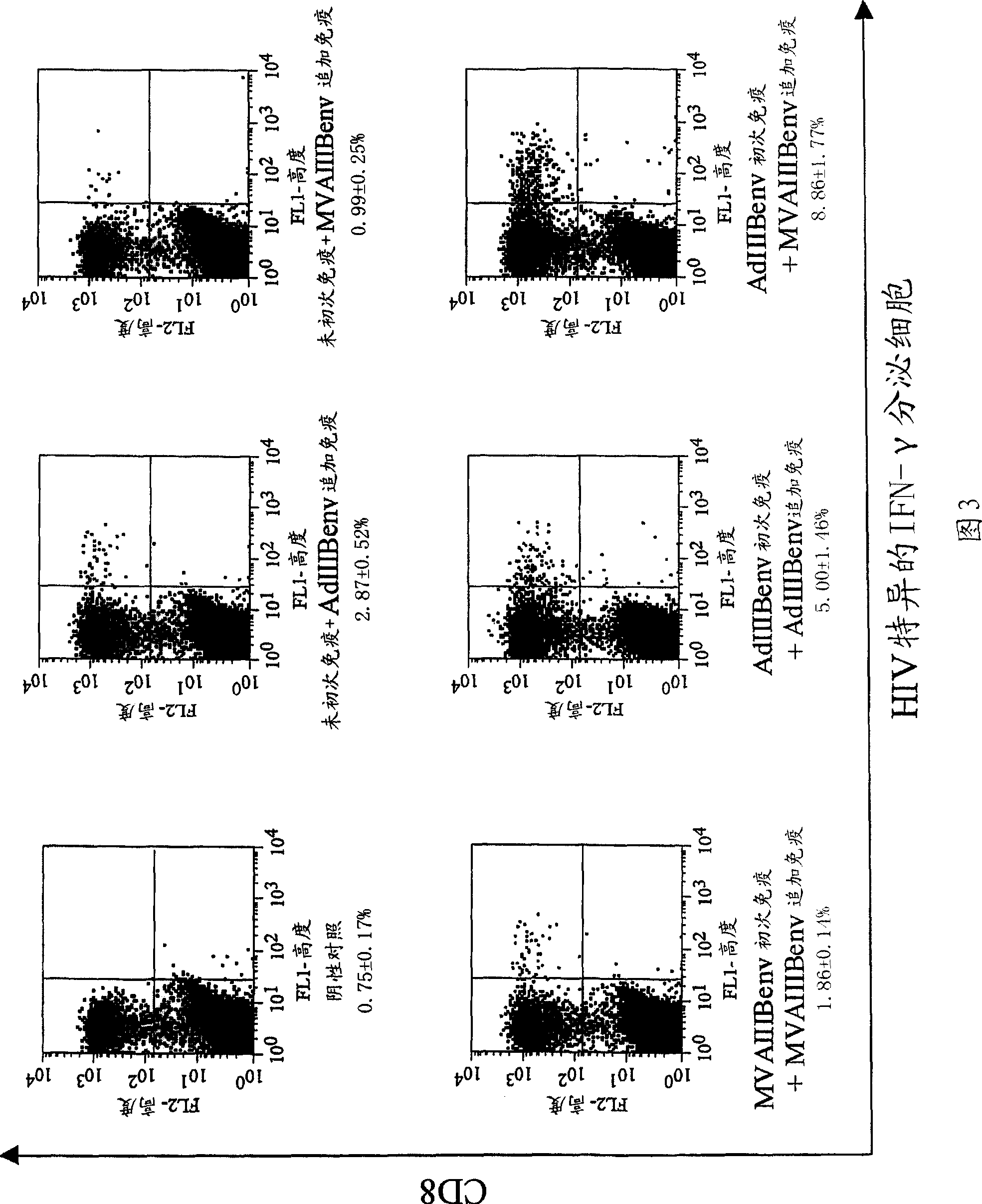
Strong immune induction by using combination of adenovirus type-5/type-35 vector and vaccinia virus mva vector
InactiveCN101394868AEnhance immune responseOrganic active ingredientsViral antigen ingredientsAnti-HIV AgentVaccinia
Disclosed is an effective and inexpensive anti-HIV agent. The anti-HIV agent comprises a combination of a recombinant viral vector having the structural gene of HIV inserted into a chimeric virus and a recombinant viral vector having the structural gene of HIV inserted into a modified vaccinia virus Ankara, wherein the chimeric virus is prepared by substituting a fiber in an adenovirus type-5 by a fiber derived from an adenovirus type-35.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Duck adenovirus type I Penton protein as well as preparation method and application thereof
InactiveCN111704656ANo transmembrane domainGuaranteed neutralityVirus peptidesMicroorganism based processesCell freeFree protein
The invention belongs to the field of protein engineering, and particularly relates to duck adenovirus type I Penton protein as well as a preparation method and application thereof. The inventor, by analysis, finds that the Penton protein has the advantages of hydrophilicity, no transmembrane structural domain, no signal peptide prediction and the like, and full-length expression is selected. A Penton target fragment is obtained through artificial synthesis after sequence optimization, and the expression mode of the protein is cell-free protein expression. Compared with truncated expression ofantigen dominant epitopes of traditional Penton protein, the artificially synthesized and expressed Penton recombinant protein is high in accuracy, the neutrality of the protein is guaranteed, the trouble of high mutation rate is avoided, and the application to the development of an antibody detection technology is better facilitated. In addition, an expression system has unique advantages of saving time and improving the total yield of soluble full-length protein. Furthermore, the expression system is low in cost, large in expression quantity and high in Western Blot detection reactogenicity.
Owner:ANYANG INST OF TECH
Duck adenovirus type 3 strain, duck adenovirus egg yolk antibody as well as preparation method and application of duck adenovirus type 3 strain and duck adenovirus egg yolk antibody
The invention discloses a duck adenovirus type 3 strain, a duck adenovirus egg yolk antibody as well as a preparation method and application of the duck adenovirus type 3 strain and the duck adenovirus egg yolk antibody. The duck adenovirus type 3 strain is a duck adenovirus type 3 YJ strain and is preserved in the China General Microbiological Culture Collection Center on November 18, 2020, and the preservation number of the duck adenovirus type 3 strain is CGMCC No.21093; and the nucleotide sequence of the HEXON gene of the duck adenovirus 3 type YJ strain is as shown in SEQ ID NO. 1. The adenovirus 3 type YJ strain is obtained through separation, and the egg yolk antibody which can be used for treating the duck adenovirus 3 type virus and is efficient, safe and free of toxic and side effects is prepared.
Owner:重庆永健生物技术有限责任公司
Probe and kit for detecting human adenovirus 7 and use method of kit
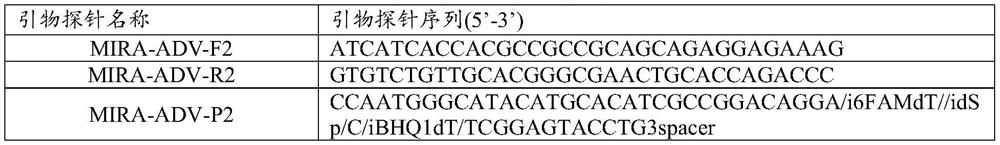
PendingCN113355324AShort reaction timeLow reaction temperatureMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid detectionGene
The invention provides a probe and kit for detecting human adenovirus 7 and a use method of the kit. The kit is a human adenovirus 7 type nucleic acid detection kit based on an MIRA fluorescence method. The primer of the MIRA is designed according to the specific sequence of the hexon gene of the adenovirus type 7. The nucleic acid detection kit is rapid, specific and sensitive and does not need complex instruments and equipment.
Owner:国药(武汉)医学实验室有限公司
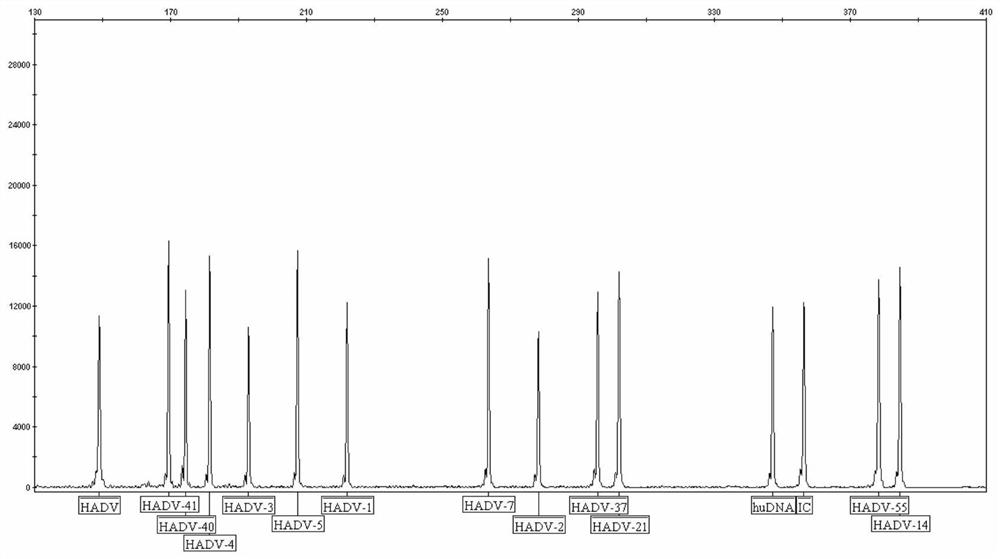
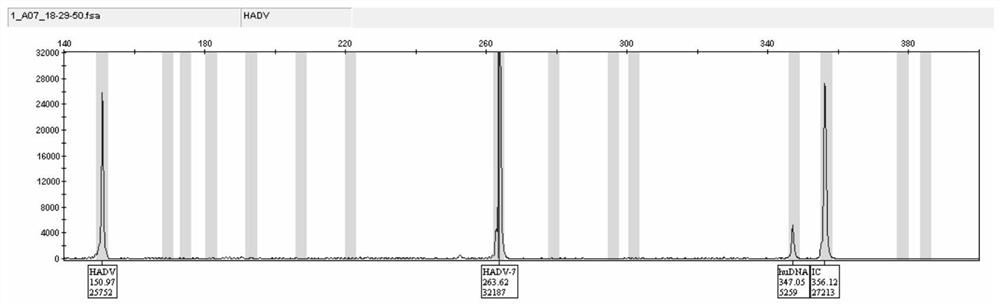
Capillary-electrophoresis-based adenovirus typing detection kit and use method therefor
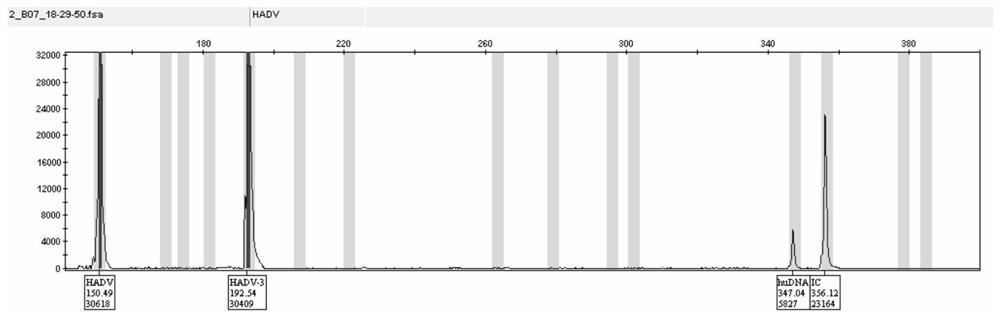
InactiveCN112813194AHigh sensitivityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationHuman DNA sequencingMultiplex
The invention discloses a capillary-electrophoresis-based adenovirus typing detection kit and a use method therefor. The kit comprises a PCR primer group; and the PCR primer group comprises 12 kinds of adenovirus serotype forward and reverse primers, total adenovirus forward and reverse primers, human genome internal-reference forward and reverse primers and PCR internal-reference forward and reverse primers. The use method for the kit comprises the following steps: (1) pretreating a sputum or throat swab sample, and then, extracting nucleic acid from the sample; (2) carrying out multiplex PCR amplification on the extracted nucleic acid; (3) separating a PCR product by employing a capillary electrophoresis method; and (4) analyzing and reading a result. According to the kit and the use method therefor, 12 kinds of adenovirus serotypes are subjected to typing detection rapidly through combining multiplex PCR with a capillary electrophoresis analysis method. The kit has the advantages of high sensitivity, high specificity, high flux and low cost and can be used for rapidly and synchronously detecting a variety of adenovirus serotypes, and defects of the traditional methods are overcome.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com