Muscovy duck novel adenovirus strain, bivalent inactivated vaccine and preparation method thereof
A bivalent inactivated vaccine and adenovirus technology, applied in the field of veterinary biological products, can solve the problems of economic losses in the Muscovy duck breeding industry, and achieve good commercial development prospects and good safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Virus isolation and identification
[0027] 1.1 Isolation and identification of duck adenovirus type 2
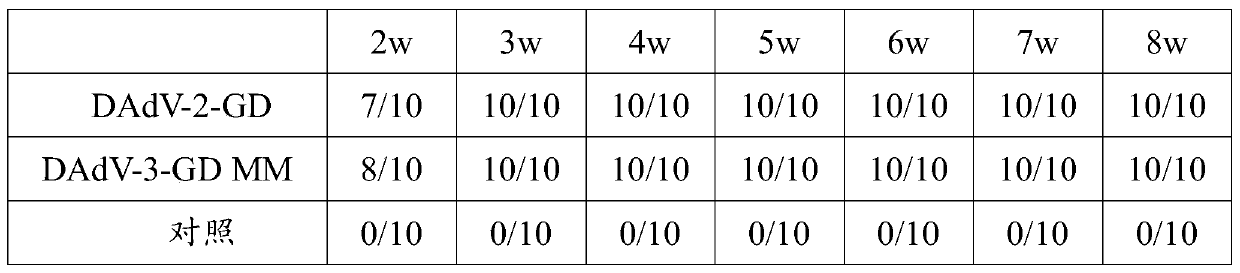
[0028] The disease materials were collected from 12-day-old Muscovy ducks in a Muscovy duck farm in Guangdong, and the liver, spleen, kidney and other tissues of the diseased Muscovy ducks were collected and grinded, and the non-toxic drug containing double antibodies was added at a ratio of 1:3 (w / v). Bacteria were homogenized in PBS, freeze-thawed 3 times, and then centrifuged at 8000r / min for 10 minutes. The supernatant was sterilized by a 0.22um filter and stored for later use. Inoculate muscovy duck embryo fibroblasts with 1% virus filtrate, culture in serum-free M199 culture medium, and culture at 37°C for 60-78 hours, obvious cytopathic changes may appear, collect virus fluid, and take supernatant to extract DNA, using the Hexon protein gene specificity, was identified by sequencing, which proved that the virus was duck adenovirus type 2, named DAdV-2-GD stra...
Embodiment 2
[0032] Preparation of DAdV-2-GD Strain Virus Seeds
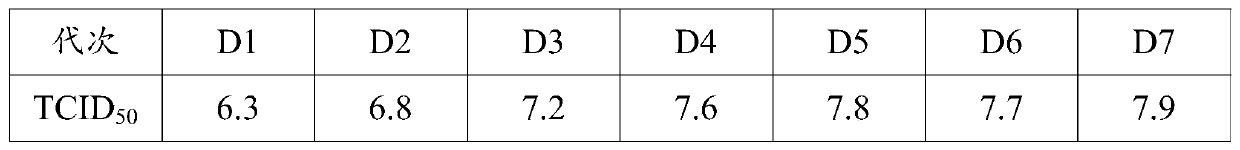
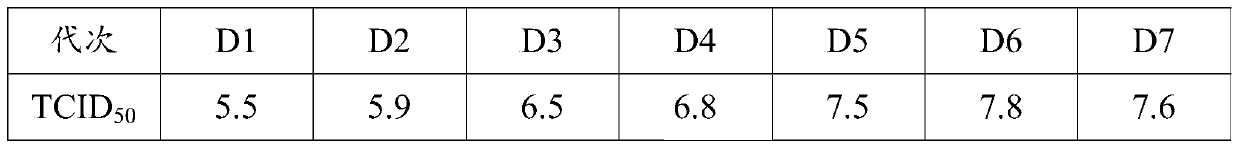
[0033] Select muscovy duck embryo fibroblasts that have grown into a single layer, discard the original culture medium, add serum-free M199 maintenance solution with a final concentration of 1% virus species (the third generation of the original virus species of GD strain), and culture at 37°C for 60 After ~78 hours, obvious cytopathy can appear, and harvest when the cytopathy reaches more than 80%. According to this method, 10 passages are continuously passed, and the cytotoxicity of the 10th passage is used to inoculate the LMH cells grown to a single layer at a ratio of 1-3% of the culture medium, and the original culture medium is discarded. DMEM / F12 virus maintenance solution without bovine serum, 37°C, 5% CO 2 Under the condition of static culture for 72-96h, the virus liquid was harvested, and the freeze-thaw was repeated 1-2 times. The harvested virus liquid was continuously inoculated with LMH cells for 3 to 5 gen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com