Recombination adenovirus for expressing human particle cytolysin and its preparing method and use
A technology of recombinant adenovirus and granulysin, applied in the field of gene therapy, can solve the problems of difficult to reach the target site of treatment, high treatment cost and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Cloning of human granulysin gene
[0031] Referring to the GenBank granulysin cDNA sequence, the structural sequence of the 419th to 640th nucleotide sequence was taken to artificially synthesize the gene fragment, and the synthesized gene fragment was cloned into the vector pUC57 and confirmed by sequencing. Entrust Shanghai Biological Engineering Company to complete. The gene sequence of the granulysin is as follows (sequence listing sequence 3):
[0032] 5'GGCCGTGACTACAGGACCTGTCTGACGATAGTCCAAAAACTGAAGAAGATGGTG
[0033] GATAAGCCCACCCAGAGAAGTGTTTCCAATGCTGCGACCCGGGTGTGTAGGACGGG
[0034] GAGGTCACGATGGCGCGACGTCTGCAGAAATTTCATGAGGAGGTATCAGTCTAGAG
[0035] TTACCCAGGGCCTCGTGGCCGGAGAAACTGCCCAGCAGATCTGTGAGGACCTCAG
[0036] G3'.
Embodiment 2
[0037] Example 2. Construction of an adenoviral vector expressing human granulysin
[0038] The primers for amplifying the human granulysin gene fragment were designed. The upstream primer contained the start code XhoI and ATG, and the downstream primer contained the TAA termination code and HindIII. The target gene was amplified by PCR method and cloned into the pZero-T vector (completed by Beijing Baosai Biological Company). After the enzyme digestion identification was confirmed, the sequence was determined. See sequence 1 for the gene sequence of the cloned and expressed granulysin. The corresponding amino acid sequence of the expression product is shown in sequence 2 of the sequence listing.
[0039] After XhoI and HindIII digestion and recovery of specific fragments, it was subcloned into the corresponding restriction site of the adenovirus transfer vector pShuttle-CMV (provided by Qbiogene, 6.6 kb), confirmed by restriction digestion and named pShullte-CMV-GRA. pShul...
Embodiment 3
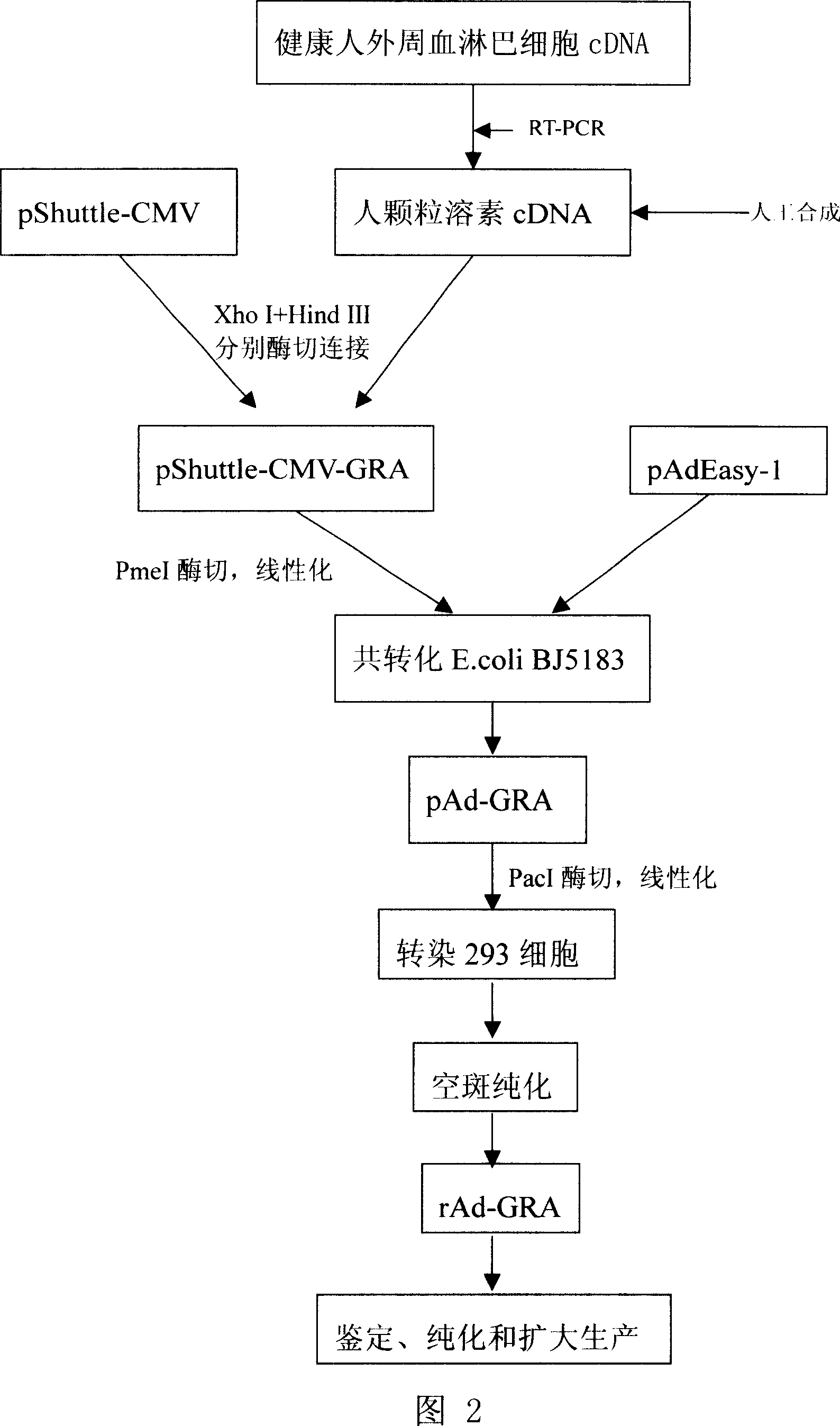
[0040] Example 3. Preparation, amplification and titer detection of recombinant adenovirus expressing human granulysin (see Figure 2: production process flow chart)
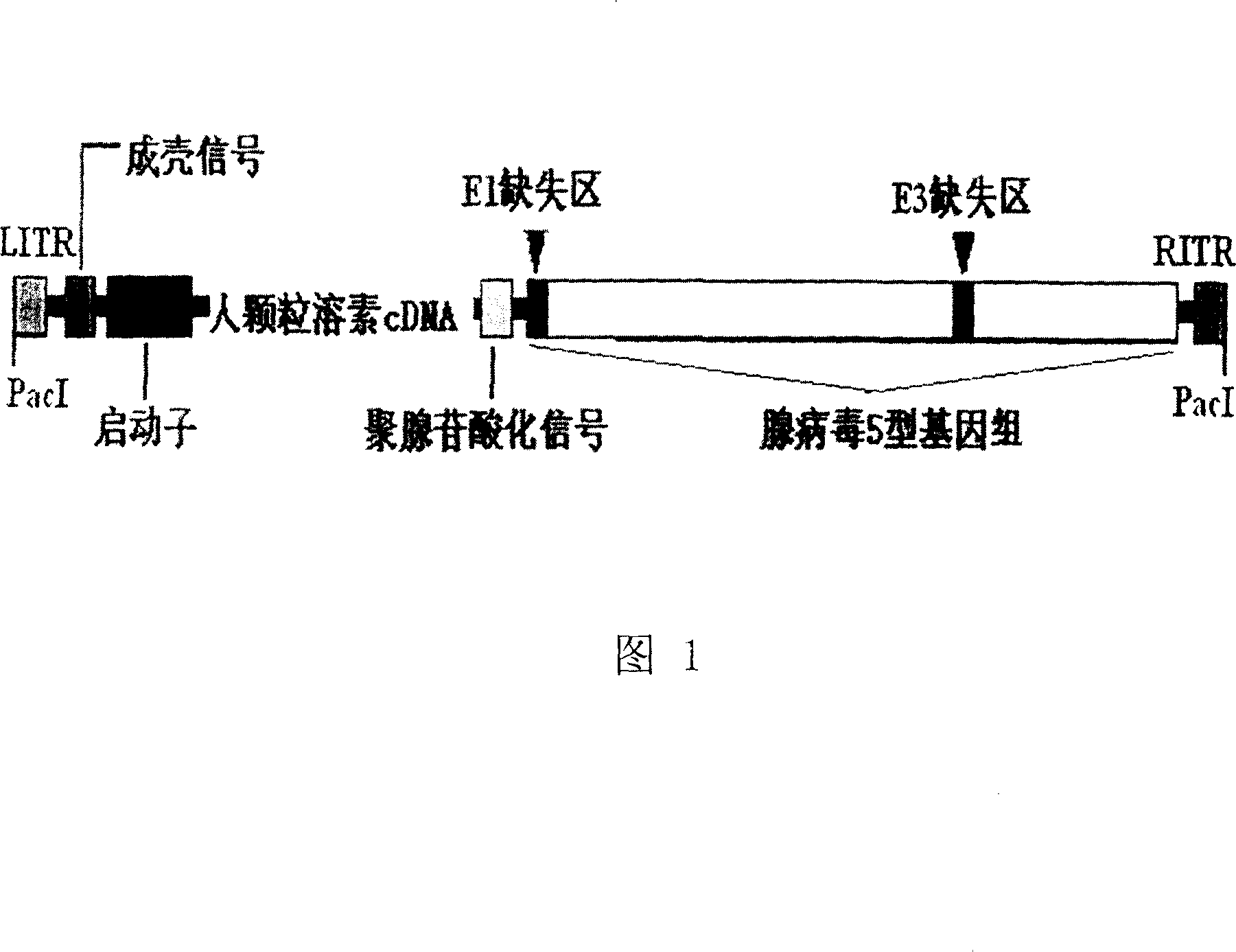
[0041] 20 μg of recombinant adenovirus plasmid was digested with PacI, extracted with conventional phenol, chloroform / isoamyl alcohol, washed with ethanol, resuspended in 50ul TE under sterile conditions, and transfected into -293 cells according to the calcium phosphate method in the manual to obtain human-expressing particles Lysin recombinant adenovirus rAd5-GRA (see Figure 1: Schematic diagram of the structure of recombinant human granulysin adenovirus).
[0042] The recombinant adenovirus rAd5-GRA is used as an active ingredient to prepare nasal drops, sprays, injections and oral preparations according to conventional medical methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com