Inactivated vaccine strain CH-GD-12-2014 against duck adenovirus type 3
A technology of inactivated vaccines and adenoviruses, applied in the direction of vaccines, viruses, viral peptides, etc., can solve the problems of lack of sequence and endanger the development of poultry industry, and achieve the effect of wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
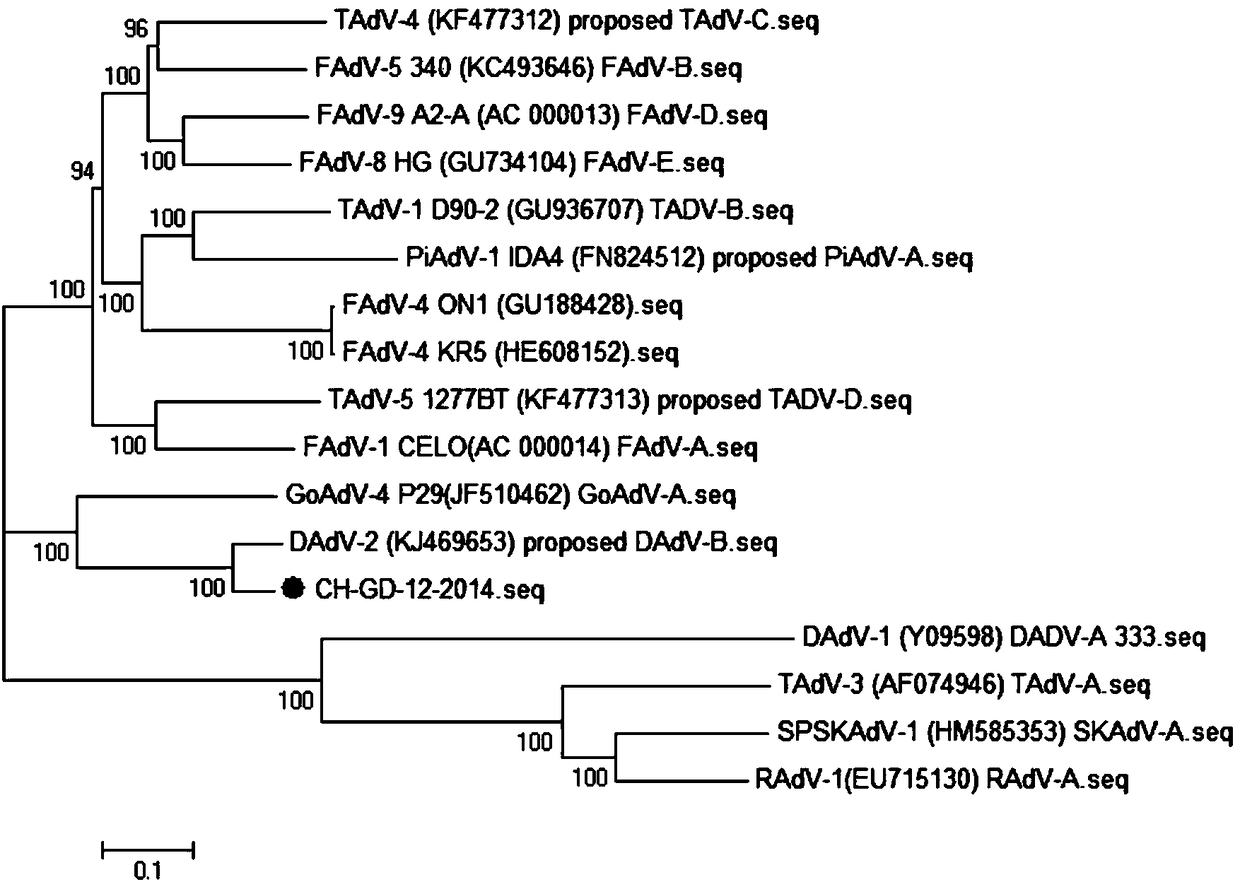
[0019] Example 1: Isolation and Identification of Duck Adenovirus Type 3 CH-GD-12-2014 Strain
[0020] CH-GD-12-2014 isolated and identified sick duck livers collected from duck farms in 2014. The specific process is as follows:
[0021] a. Virus isolation and culture
[0022] a.1 Culture of duck embryo fibroblasts
[0023] Aseptically collect 11-day-old non-immune fertilized duck embryos, remove the head, wings, claws and internal organs of the duck embryos, wash 3 times with PBS solution, and cut them to less than 3mm with sterilized scissors 3 After the tissue block, add PBS solution and shake gently, then add 0.25% trypsin, and digest in a water bath at 37°C until the liquid becomes uniform, then add serum to terminate the digestion. This process generally takes 20-30min; After filtering with a 100-mesh cell sieve, collect the filtrate in a 5mL centrifuge tube, centrifuge at 8000 rpm for 5min, remove the supernatant, add DMEM, and dilute to 5×10 5 Individuals / mL (contai...
Embodiment 2
[0039] Example two: research on duck adenovirus type 3 CH-GD-12-2014 inactivated vaccine
[0040] a. Physicochemical detection of virus
[0041] The results of physical and chemical tests show that CH-GD-12-2014 is resistant to acid and alkali, and is not sensitive to temperature. Ethyl ether, acetone, and chloroform have little effect on CH-GD-12-2014. Ultraviolet radiation and formaldehyde can make CH- GD-12-2014 inactivation;
[0042] b. Determination of virus content
[0043] Determination of TCID of CH-GD-12-2014 strain virus 50 , the virus was diluted 10 times with sterilized normal saline, and the diluted virus was inoculated into a single layer of DEF cells on 96 wells, and each dilution was inoculated in a vertical row with a total of 8 wells, each well was 0.1m1, and a control group was set ; After 2 hours of incubation, suck out the virus solution and add 100 μL of DMEM maintenance solution containing 2% serum to each well, at 37°C, CO 2 After culturing in a con...
Embodiment 3
[0050] Embodiment three CH-GD-12-2014 inactivated vaccine preparation
[0051] a. Preparation of CH-GD-12-2014 virus liquid
[0052] After thawing the DEF cell virus solution collected in the sixth generation, centrifuge at 10,000 rpm for 10 min, filter it with a microporous membrane with a pore size of 0.22 μm, and inoculate it into 200 75 cm cell culture flasks with 80%-90% monolayer DEF cells, 1ml per bottle, after inoculation, place at 37°C, CO 2 After culturing in the incubator for 3 hours, add DMEM maintenance solution (containing 2% serum) and incubate for 4-5 days. After the virus solution has been frozen three times and thawed three times, centrifuge at 10,000 rpm for 10 minutes to take the supernatant and filter it with a microporous membrane with a pore size of 0.22um. Collect cell venom, take samples for inspection, and store at -20°C for later use;
[0053] b. CH-GD-12-2014 venom inspection
[0054] The harvested virus liquid was subjected to sterility test and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com