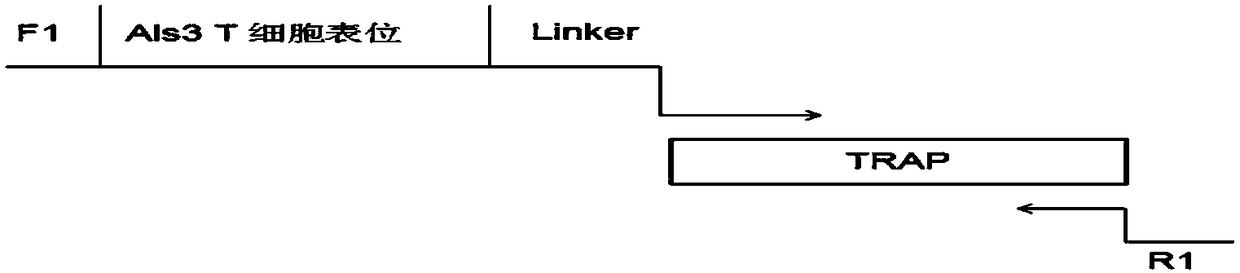
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32results about How to "Strong immune protection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Recombinant porcine pseudorabies virus for expressing GP protein of porcine reproductive and respiratory syndrome virus, and application
ActiveCN110628730AInviolableStrong targetingSsRNA viruses positive-senseViral antigen ingredientsAntigenVirulent characteristics
The invention provides a recombinant porcine pseudorabies virus for expressing GP protein of a porcine reproductive and respiratory syndrome virus, and an application. A PRV virus strain genome is quickly edited through a Crispr / Cas9 gene editing technique and a Cre / lox recombination system, virulence genes gE, gI and TK of the PRV virus strain genome are subjected to fixedpoint deletion, and an antigenic gene of a NADC30-like strain is subjected to fixedpoint insertion at a gG position. According to the recombinant porcine pseudorabies virus for expressing GP protein of a porcine reproductiveand respiratory syndrome virus disclosed by the invention, non-transmembrane regional coding sequences of GP3 protein, GP4 protein, GP5 protein and GP6 protein of an epidemic PRRSV strain PRRSV NADC30-like are selected as antigenic genes for the first time, the kinds of antigens are more comprehensive, and the antigenic genes have higher applicability on current PRRSV epidemic situations, and arebetter in immunoprotection effects. A live vaccine provided by the invention can protect target animals from being invaded by PRV and PRRSV in a more pointed manner, and a powerful tool is provided for preventing and controlling epidemic situations of porcine pseudorabies and porcine reproductive and respiratory syndromes in China.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Infectious bursal disease virus strain A11 and application thereof
ActiveCN105802920AStrong immune protectionResistance to virulent attacksViral antigen ingredientsMicroorganism based processesMicroorganismBiological property
The invention discloses an infectious bursal disease virus strain A11 and application thereof, and belongs to the field of the microbial technology. Infectious bursal disease viruses are separated from infectious bursal disease vaccine immunity failure chicken flocks appearing in recent years, biological characteristic analysis is carried out on obtained virus isolate strains, and the infectious bursal disease virus strain A11 with good biological characteristics is screened out from the obtained virus isolate strains and is a currently-popular infectious bursal disease virus with super-strong virulence. The virulence of the virus is attenuated with a chicken embryo and cell continuous passage method to culture the low-virulence strain A11, the low-virulence strain A11 has super-strong reproductivity in cells, the virus yield in cells reaches up to 10<11> TCID50 / 0.1 ml, immune protection resisting infectious bursal disease viruses can be rapidly generated by immunizing chicks with the strain A11, immune protection can be achieved after 1 week, and the strain A11 has huge application value in developing new specific infectious bursal disease vaccines.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Attenuated live vaccine for preventing infection of toxoplasma gondii and application of attenuated live vaccine
InactiveCN107281477AAvoid infectionPrevent acute infectionProtozoa antigen ingredientsSolution deliveryAcute infectionAttenuated Live Vaccine
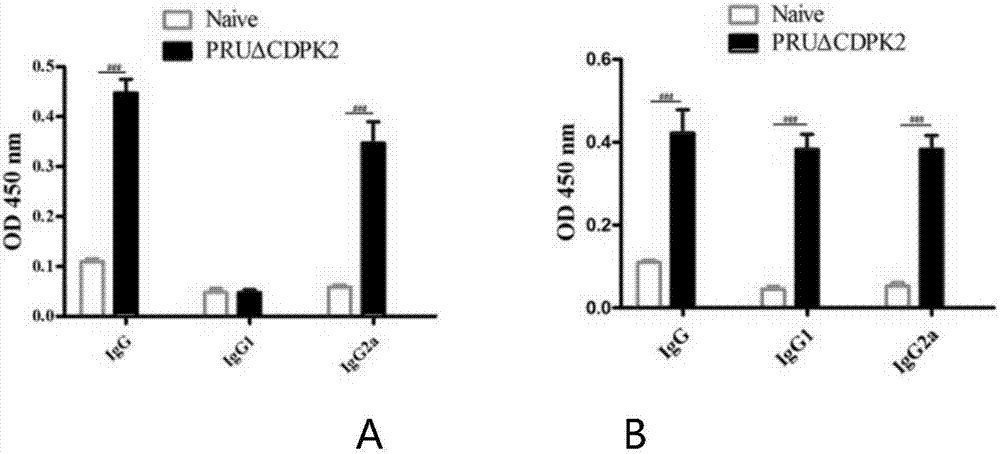
The invention discloses an attenuated live vaccine for preventing the infection of toxoplasma gondii and application of the attenuated live vaccine. The attenuated live vaccine is a toxoplasma gondii attenuated strain PRU delta CDPK2 tachyzoite suspension prepared by adopting PBS as a solvent. An immune dosage form of the attenuated live vaccine is determined, an immune inoculation program and an inoculation amount of the vaccine are ascertained, the attenuated live vaccine has higher immunity protection effect for the acute infection, chronic infection and congenital infection of the toxoplasma gondii by virtue of an animal experiment, not only can prevent the normal infection of the toxoplasma gondii, but also can effectively prevent the vertical propagation of the toxoplasma gondii by virtue of a mother-infant route, is attenuated live vaccine with great application value, can be used for preventing the chronic infection, acute infection and congenital infection of the toxoplasma gondii, and can effectively prevent the toxoplasma gondii.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
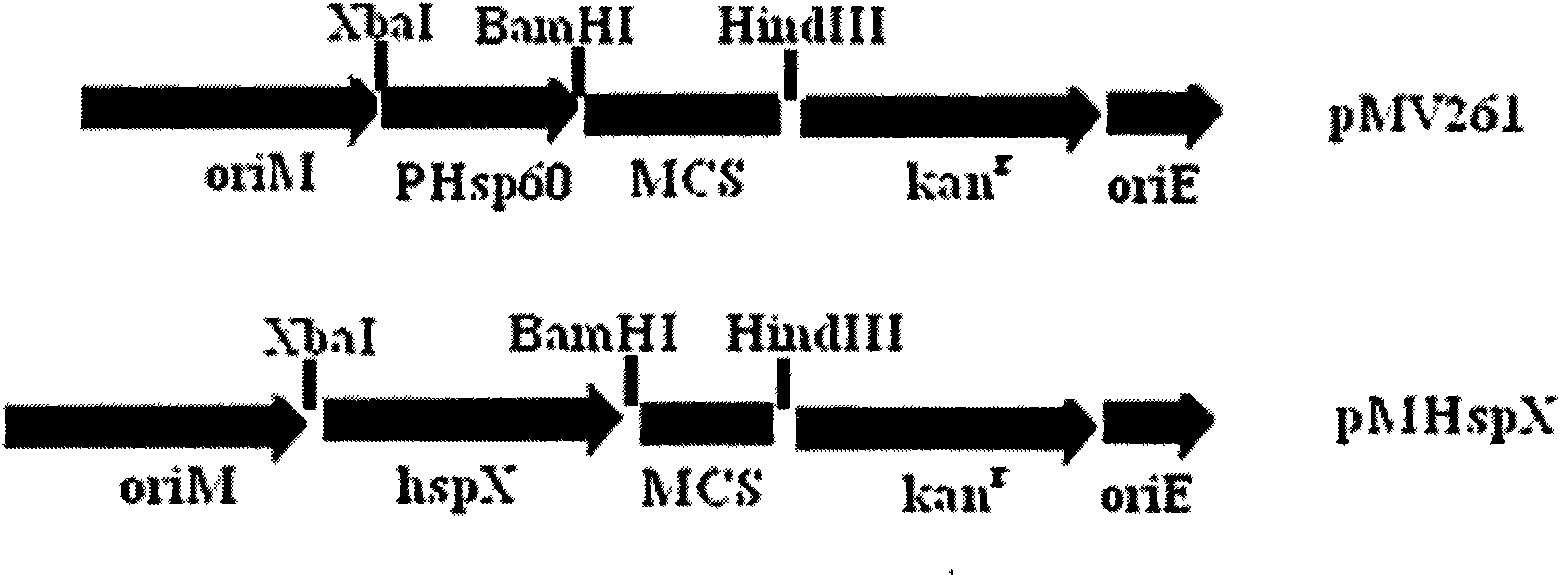
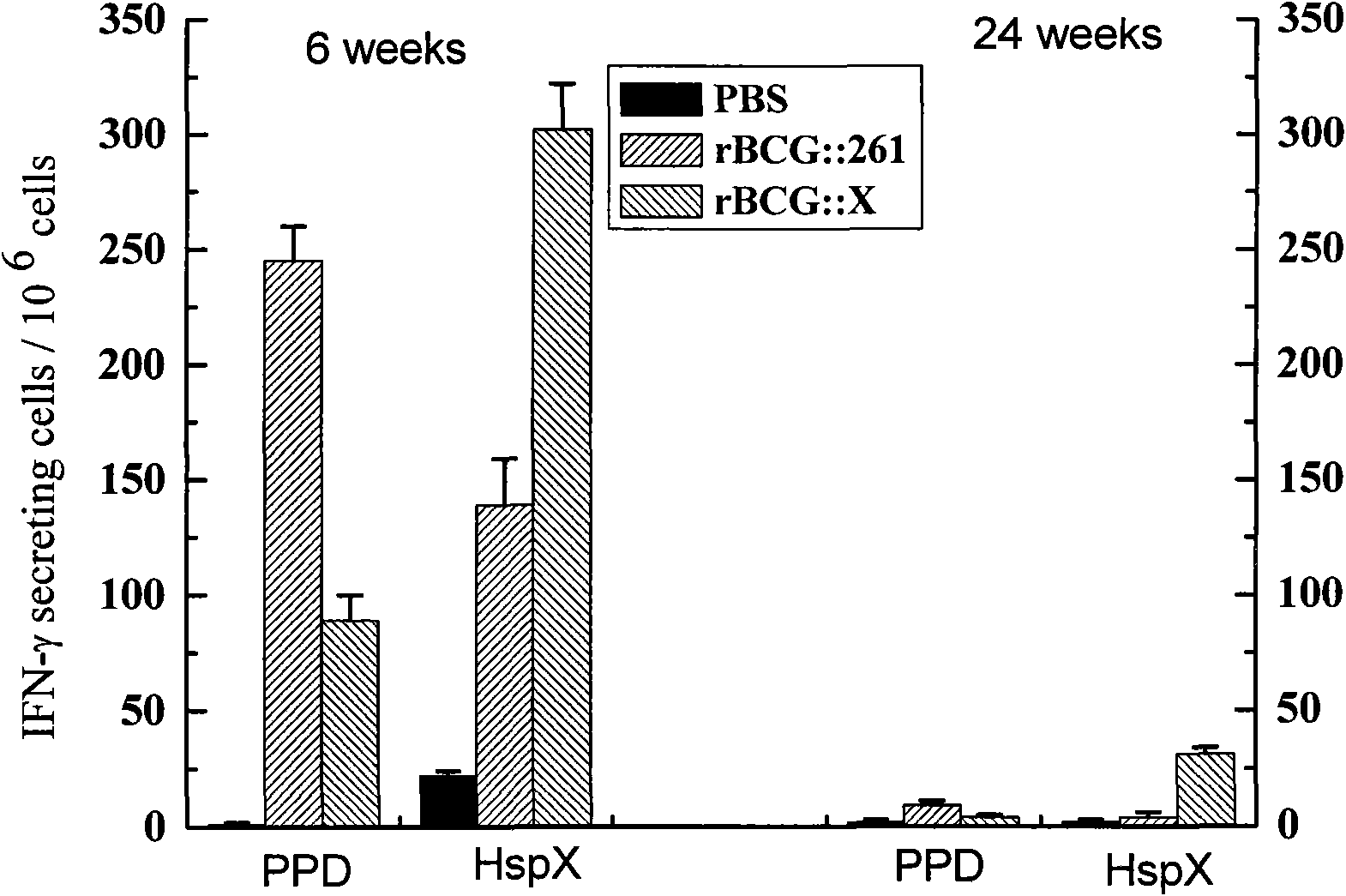
Recombinant BCG vaccine rBCG::X
InactiveCN101921801AStrong protective effectStrong immune protectionAntibacterial agentsBacterial antigen ingredientsCell immune responseShuttle plasmid
The invention provides an anti-tubercle bacillus recombinant BCG vaccine rBCG::X. The recombinant escherichia coli-mycobacteria shuttle plasmid containing a coded HspX protein gene is transformed into the BCG vaccine to form the recombinant BCG vaccine rBCG::X; the recombinant BCG vaccine rBCG::X realizes the over expression of the HspX protein, can obviously induce the organism to generate the cellular immune response aiming at the HspX protein after animal immunity, and generate stable and endurable anti-infective protection, thus overcoming the shortcoming that the BCG vaccine has short protection period and unstable protection effect on the adult tuberculosis and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
Preparation of immuno-stimulation composition for hemolycin in monad
InactiveCN1583171AImprove immunityStrong synergismAntibacterial agentsBacterial antigen ingredientsImmune effectsOrganic acid
An immunostimulating complex with stable and durable immune effect is prepared from hygrophilic monodas through liquid culturing, centrifugal separating of supernatant, treating it by saturated ammonium sulfate solution, suspending its deposit by PBS dialyzing to obtain toxic, concentrating, adding organic acid, adding Mega-10, treating at 37 deg.C for 4 hr, adding Quil A, cholesterol and lecithin, treating by organic acid, and dialyzing.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +3
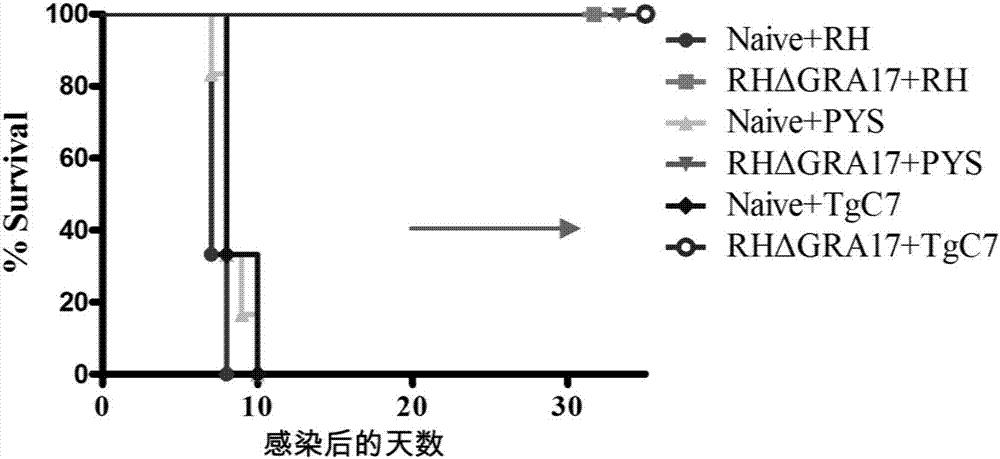
Attenuated live vaccine used for preventing toxoplasma infection and application of attenuated live vaccine
InactiveCN107308444AAvoid infectionPrevent acute infectionProtozoa antigen ingredientsAntiparasitic agentsAcute infectionSolvent
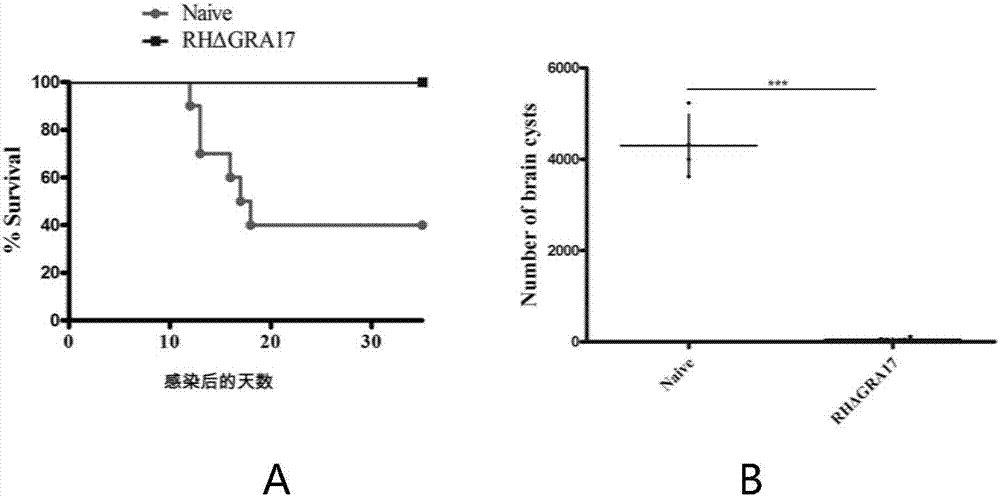
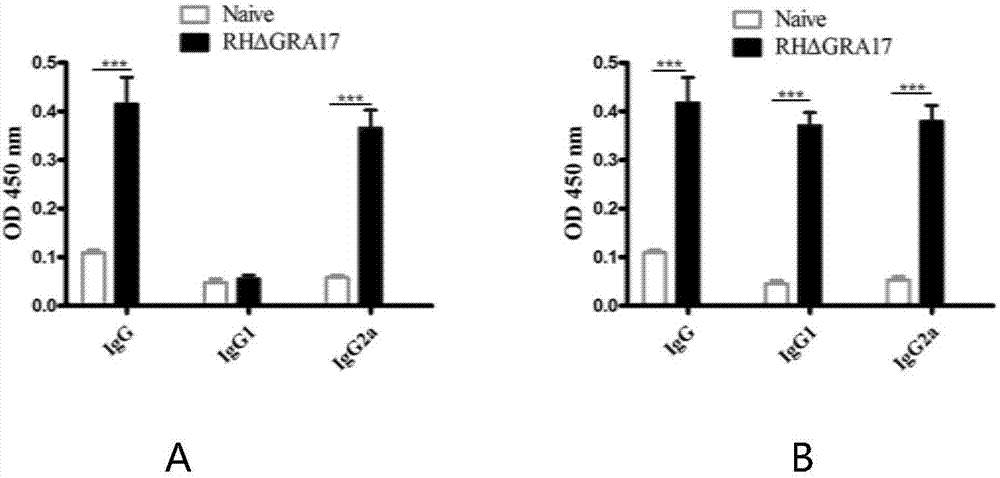
The invention discloses an attenuated live vaccine used for preventing toxoplasma infection and application of the attenuated live vaccine. The attenuated live vaccine takes PBS as a solvent to prepare a tachyzoite suspension of a toxoplasmosis attenuative strain RHdetltaGRA17. An immune preparation for the attenuated live vaccine is established, the immunization procedure and the inoculum size of the vaccine are explored, and the animal experiment shows that the attenuated live vaccine has a stronger immunoprotection function for the acute infection, the chronic infection and the congenital infection of toxoplasmosis, the normal infection of the toxoplasmosis is prevented, meanwhile, the mother-to-fetus vertical propagation of the toxoplasmosis is effectively stopped, and the vaccine has a huge application value, and can be used for preventing the acute infection, the chronic infection and the congenital infection of toxoplasmosis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Expression vector of novel coronavirus vaccine, construction method and application of expression vector and vaccine
PendingCN113186223AStrong immune protectionSsRNA viruses positive-senseViral antigen ingredientsEnzyme digestionReceptor
The invention is applicable to the technical field of biology, and provides an expression vector of a new coronavirus vaccine, a construction method and application of the expression vector and the vaccine, and the construction method of the expression vector comprises the following steps: connecting nucleotide sequences for expressing S protein and NP protein of a new coronavirus by using 2A peptide to synthesize a fusion gene; two ends of the fusion gene respectively comprise two restriction enzyme cutting sites, and the fusion gene is loaded to a plasmid to obtain a recombinant plasmid; carrying out double enzyme digestion on the recombinant plasmid, and carrying out gel cutting to recover a target gene segment; carrying out double enzyme digestion on original plasmids, and carrying out gel cutting to recover carrier fragments; and connecting the target gene fragment with the vector fragment to obtain the expression vector. According to the embodiment of the invention, the coronavirus S protein receptor binding region and the NP protein are expressed at the same time, so that cells infected by the expression vector not only can induce antibody reaction, but also can induce T cell reaction, humoral immunity and cellular immunity are effectively induced, and stronger immune protection is provided for subjects.
Owner:浙江格源致臻生物医药科技有限公司
Haemonchus contortus nano-material subunit vaccine and application thereof
ActiveCN111558034AEnhance specific immune responseProlong or enhance specific immune responseProtozoa antigen ingredientsPeptidesDendritic cellAdult worm
The invention discloses a Haemonchus contortus nano-material subunit vaccine and applications thereof. The Haemonchus contortus nano-material subunit vaccine is a subunit vaccine prepared by wrappinghaemonchus contortus recombinant protein HCA59 with nano-material PLGA, the recombinant HCA59 protein has an amino acid sequence as shown in SEQ ID NO. 1. The subunit vaccine can be used for preventing haemonchus contortus infection of sheep. The particle size of the nano subunit vaccine is 75 to 402nm; the Haemonchus contortus nano-material subunit vaccine has good biocompatibility and unique physicochemical properties, has the advantages of targeting property, slow release property, safety, high efficiency and the like, and can be decomposed and metabolized in an animal body; the vaccine hasan irritant antigen effect of stimulating dendritic cells, can promote the capability of converting mononuclear cells into the dendritic cells, has relatively strong immunoprotection capability, andcan remarkably reduce the ovum discharge rate and adult reduction rate of goats infected with haemonchus contortus clinically.
Owner:NANJING AGRICULTURAL UNIVERSITY
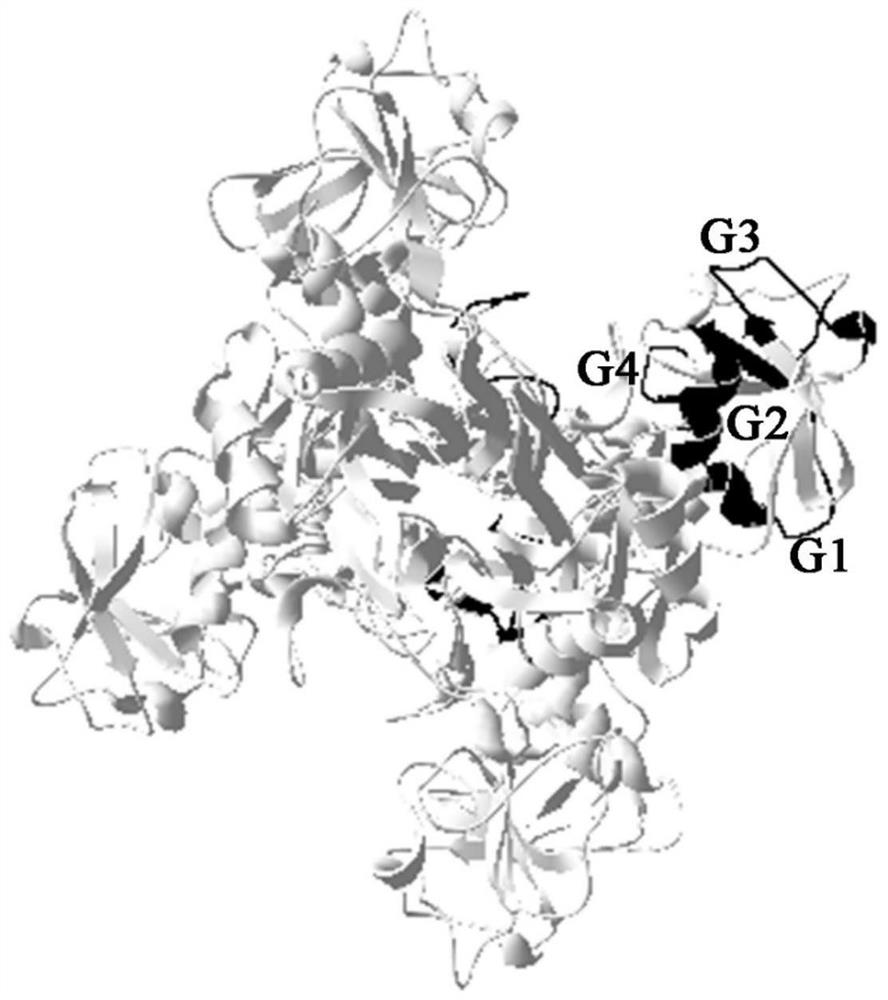
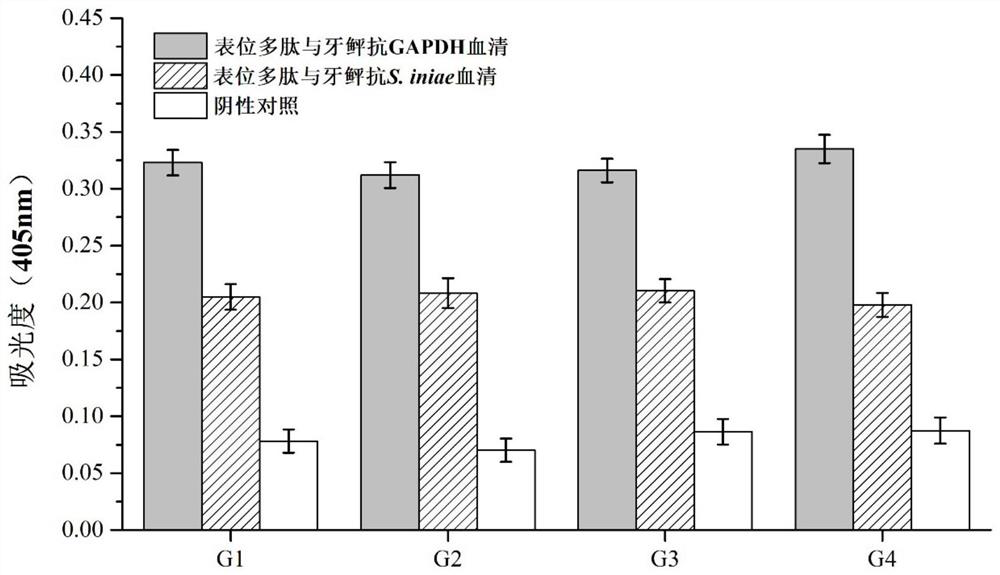
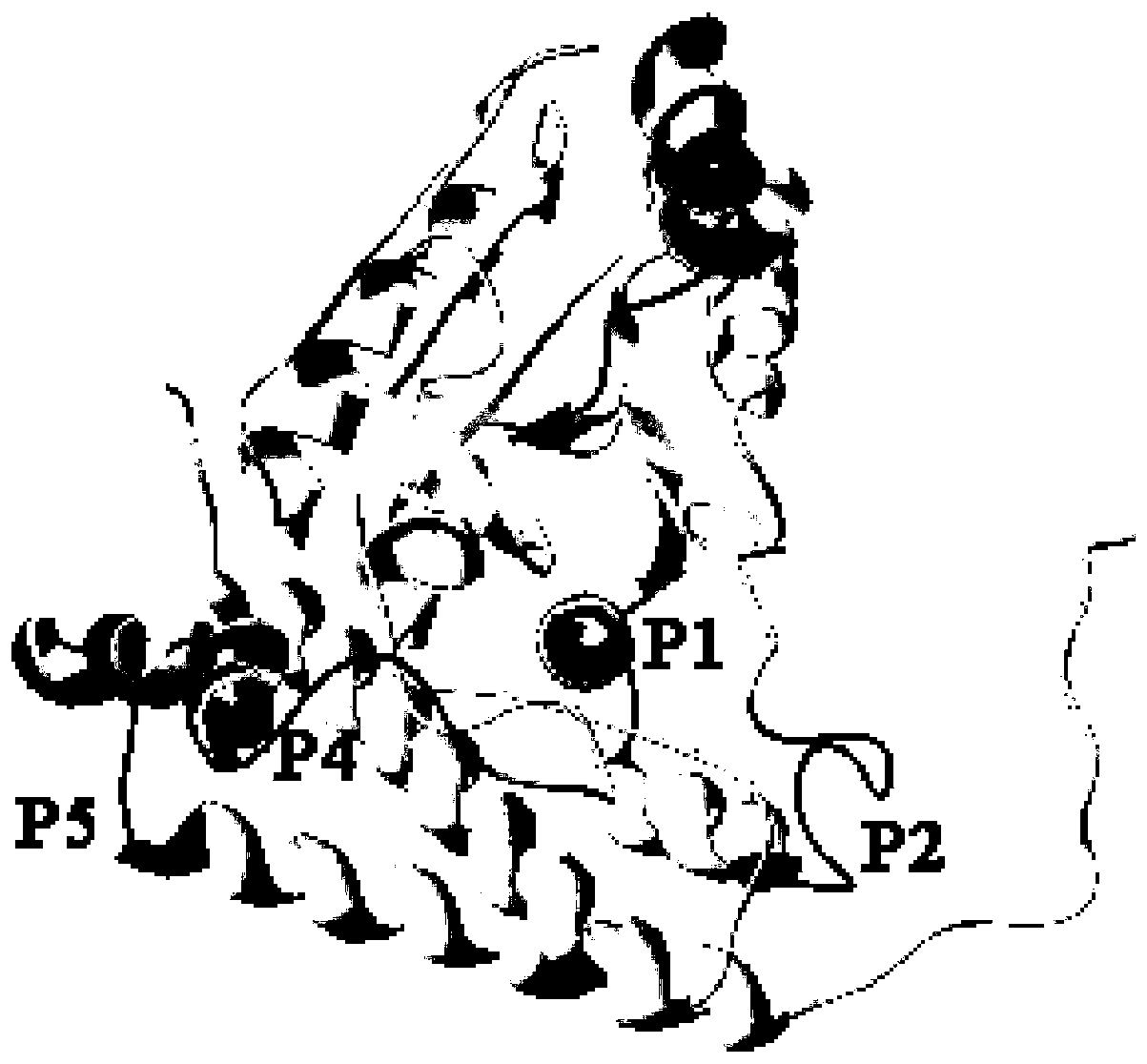
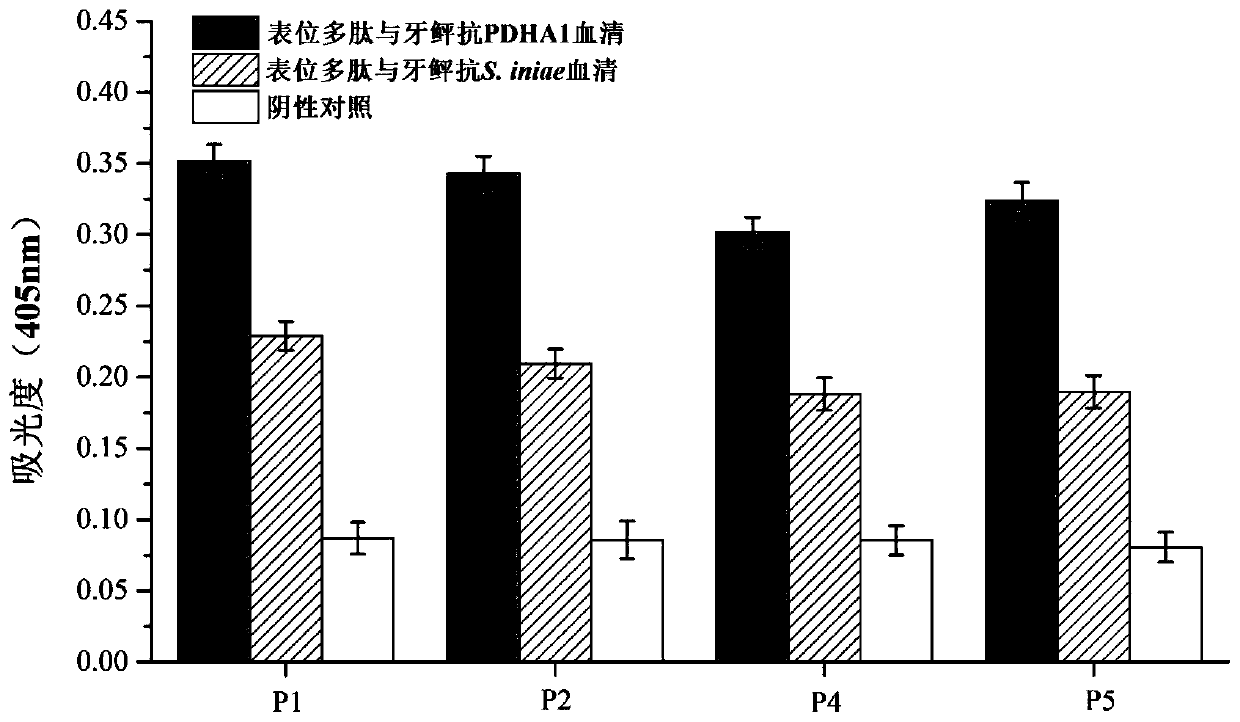
Paralichthys olivaceus streptococcus iniae GAPDH series-connection multi-epitope polypeptide and application thereof
ActiveCN110372797AStrong immune protectionGood effectAntibacterial agentsBacterial antigen ingredientsProtein subunitMicrobiology
The invention provides a paralichthys olivaceus streptococcus iniae GAPDH series-connection multi-epitope polypeptide and application thereof. The provided series-connection multi-epitope polypeptideis formed by binding four antigen epitope peptides through peptide joints, wherein the amino acid sequences of the four antigen epitope peptides are shown in SEQID NO:1-4 respectively. One specific amino acid sequence of the series-connection multi-epitope polypeptide is SEQID NO:8. The invention further provides a streptococcus iniae GAPDH multi-epitope vaccine prepared by using the series-connection multi-epitope polypeptide as an antigen and a vaccine adjuvant. The multi-epitope vaccine can provide strong immune protection for paralichthys olivaceus resistance to S.iniae infection, and theeffect is significantly higher than that of a GAPDH recombinant protein subunit vaccine and a S.iniae inactivated vaccine.
Owner:OCEAN UNIV OF CHINA
Riemerella anatipestifer DNA vaccine based on RA OmpA gene as well as preparation method and identification method of riemerella anatipestifer DNA vaccine
ActiveCN112843225AStrong immune protectionGood immune protectionAntibacterial agentsBacterial antigen ingredientsOmpa geneRiemerella anatipestifer
The invention discloses a riemerella anatipestifer DNA vaccine based on an RA OmpA gene, wherein the riemerella anatipestifer DNA vaccine comprises an eukaryotic expression plasmid pVAX1-OmpA of the RA OmpA gene; during inoculation, subcutaneous immunization is carried out on the neck of a duckling by using a recombinant eukaryotic expression plasmid PVAX1-OmpA of 100 [mu]g / duckling. The invention also discloses a preparation method and an identification method of the riemerella anatipestifer DNA vaccine based on the RA OmpA gene. The eukaryotic expression plasmid pVAX1-OmpA containing the RA OmpA gene are researched and developed, and the recombinant plasmid PVAX1-OmpA immunizes the duckling so as to induce the generation of a specific immune antibody, so that the strong immune protection effect can be generated, and the recombinant plasmid can be adopted as a candidate strain of the novel DNA vaccine so as to lay the foundation for the further development and research of the novel RA gene engineering vaccine. Compared with the prior art, the riemerella anatipestifer DNA vaccine provided by the invention has a better immune protection effect.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
A kind of recombinant BCG and its application
ActiveCN108949783BImprove defectsConsistent structureAntibacterial agentsBacterial antigen ingredientsEscherichia coliBacterial strain
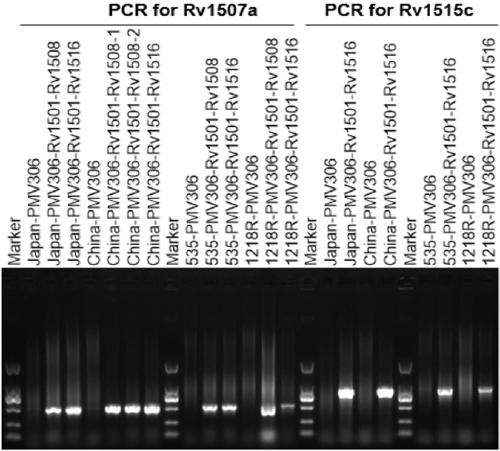
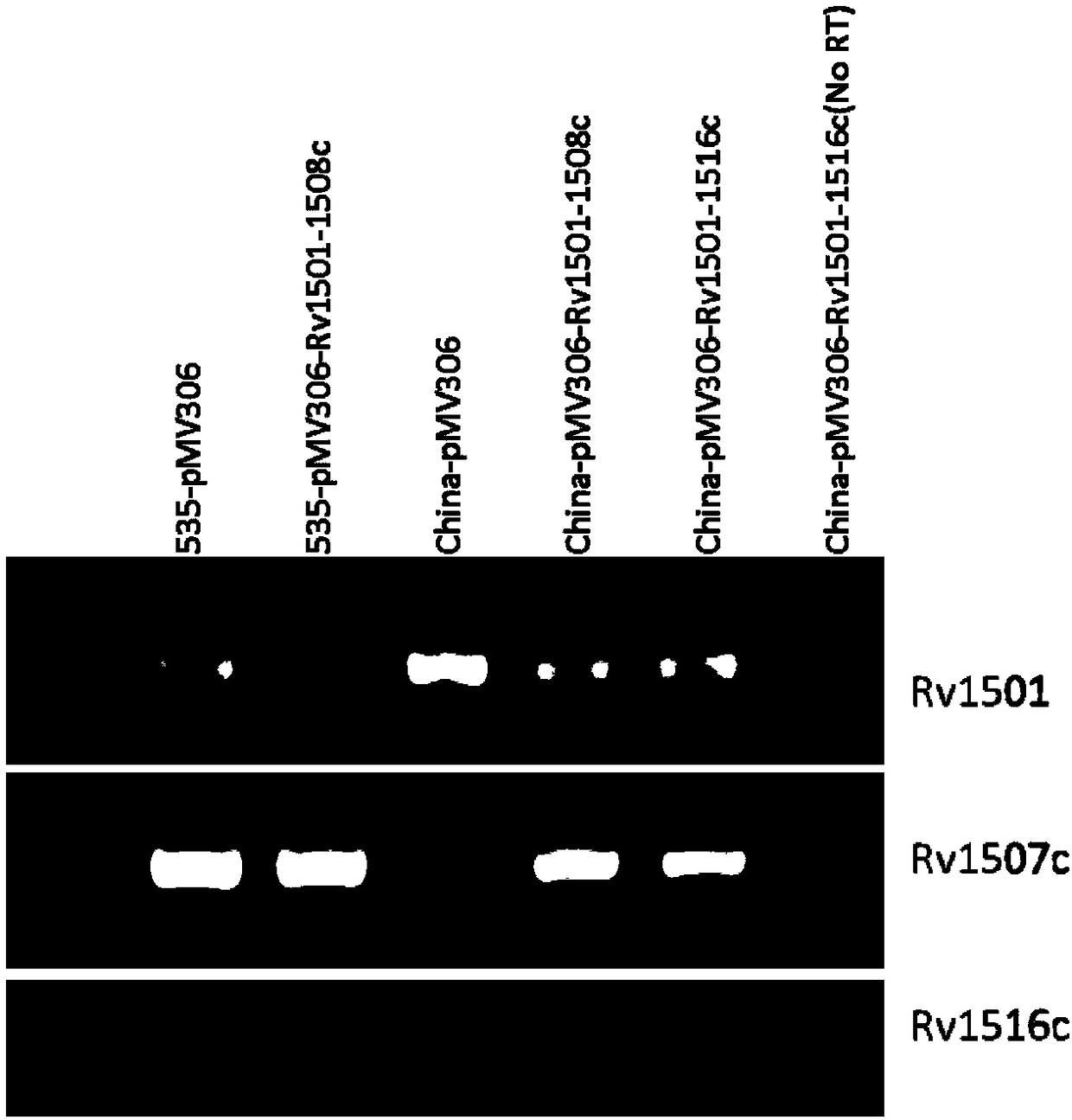
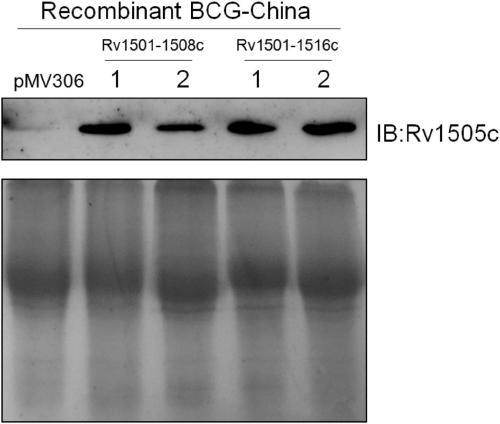
The invention belongs to the technical field of gene engineering and tuberculosis vaccine, and provides a recombinant bacillus calmette guerin (BCG) vaccine containing mycobacterium tuberculosis genome RD4 zone related coding genes. According a preparation method, the recombinant bacillus calmette guerin vaccine is produced through transformation of recombinant Escherichia coli-mycobacterium shuttle plasmids containing genes used for coding of mycobacterium tuberculosis genome RD4 zone proteins into bacillus calmette guerin vaccine. The recombinant bacillus calmette guerin vaccine is capable of realizing RD4 zone gene and protein expression. After immunization of animals with the recombinant bacillus calmette guerin vaccine, the safety of recombinant BCG bacterial strain containing complete RD4 zone is not reduced, the safety of recombinant BCG bacterial strain containing a part of RD4 zone (Rv1501-Rv1508c) is increased obviously. The recombinant BCG bacteria strain containing the complete or a part of RD4 zone genes possess better anti-infection protection effects. The recombinant bacillus calmette guerin vaccine can be used in prevention or treatment of tuberculosis.
Owner:FUDAN UNIV
Bovine rotavirus fusion protein and calf diarrhea polyvalent vaccine
ActiveCN111518222AStrong immune protectionImproving immunogenicityAntibacterial agentsSsRNA viruses positive-senseBovine rotavirusEscherichia coli
The invention provides a bovine rotavirus fusion protein and a calf diarrhea polyvalent vaccine, and relates to the technical field of molecular biology. The bovine rotavirus fusion protein contains aVP6 fragment, and the VP6 fragment contains an amino acid sequence as shown in SEQ ID NO.4, wherein at least one loop region in the following (a)-(c) is replaced with an epitope derived from bovine coronavirus and / or an epitope derived from escherichia coli: (a) 168th-177th amino acid residues with an amino acid sequence as shown in SEQ ID NO.1; (b) 194th-205th amino acid residues with an amino acid sequence as shown in SEQ ID NO.2; and (c) 296th-316th amino acid residues with an amino acid sequence as shown in SEQ ID NO. 3. The bovine rotavirus fusion protein contains a plurality of epitopes, and a host can generate a plurality of antibodies after the host is immunized.
Owner:天康制药股份有限公司
Recombinant bacillus calmette guerin vaccine, and applications thereof
ActiveCN108949783ADoes not compromise or enhances securityImprove securityAntibacterial agentsBacterial antigen ingredientsBacterial strainTGE VACCINE
The invention belongs to the technical field of gene engineering and tuberculosis vaccine, and provides a recombinant bacillus calmette guerin (BCG) vaccine containing mycobacterium tuberculosis genome RD4 zone related coding genes. According a preparation method, the recombinant bacillus calmette guerin vaccine is produced through transformation of recombinant Escherichia coli-mycobacterium shuttle plasmids containing genes used for coding of mycobacterium tuberculosis genome RD4 zone proteins into bacillus calmette guerin vaccine. The recombinant bacillus calmette guerin vaccine is capable of realizing RD4 zone gene and protein expression. After immunization of animals with the recombinant bacillus calmette guerin vaccine, the safety of recombinant BCG bacterial strain containing complete RD4 zone is not reduced, the safety of recombinant BCG bacterial strain containing a part of RD4 zone (Rv1501-Rv1508c) is increased obviously. The recombinant BCG bacteria strain containing the complete or a part of RD4 zone genes possess better anti-infection protection effects. The recombinant bacillus calmette guerin vaccine can be used in prevention or treatment of tuberculosis.
Owner:FUDAN UNIV
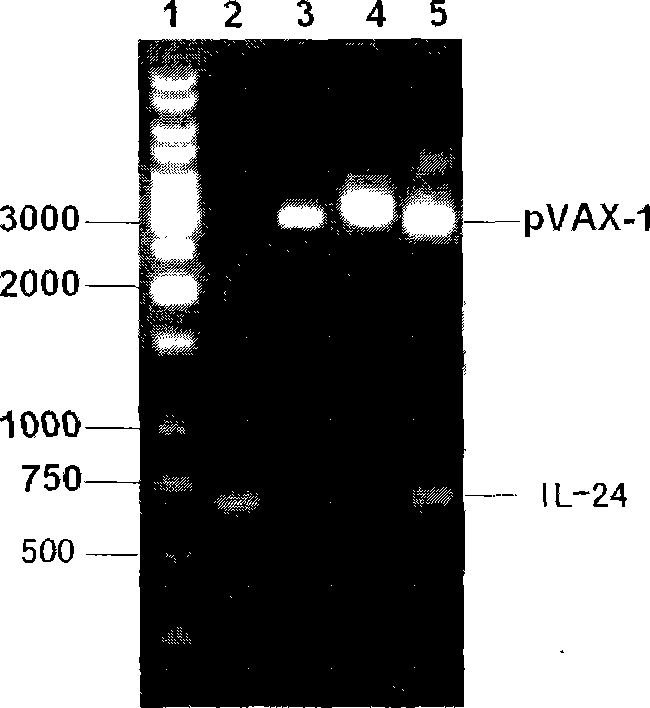
Intensified tubercle-resisting action of human interleukin 24
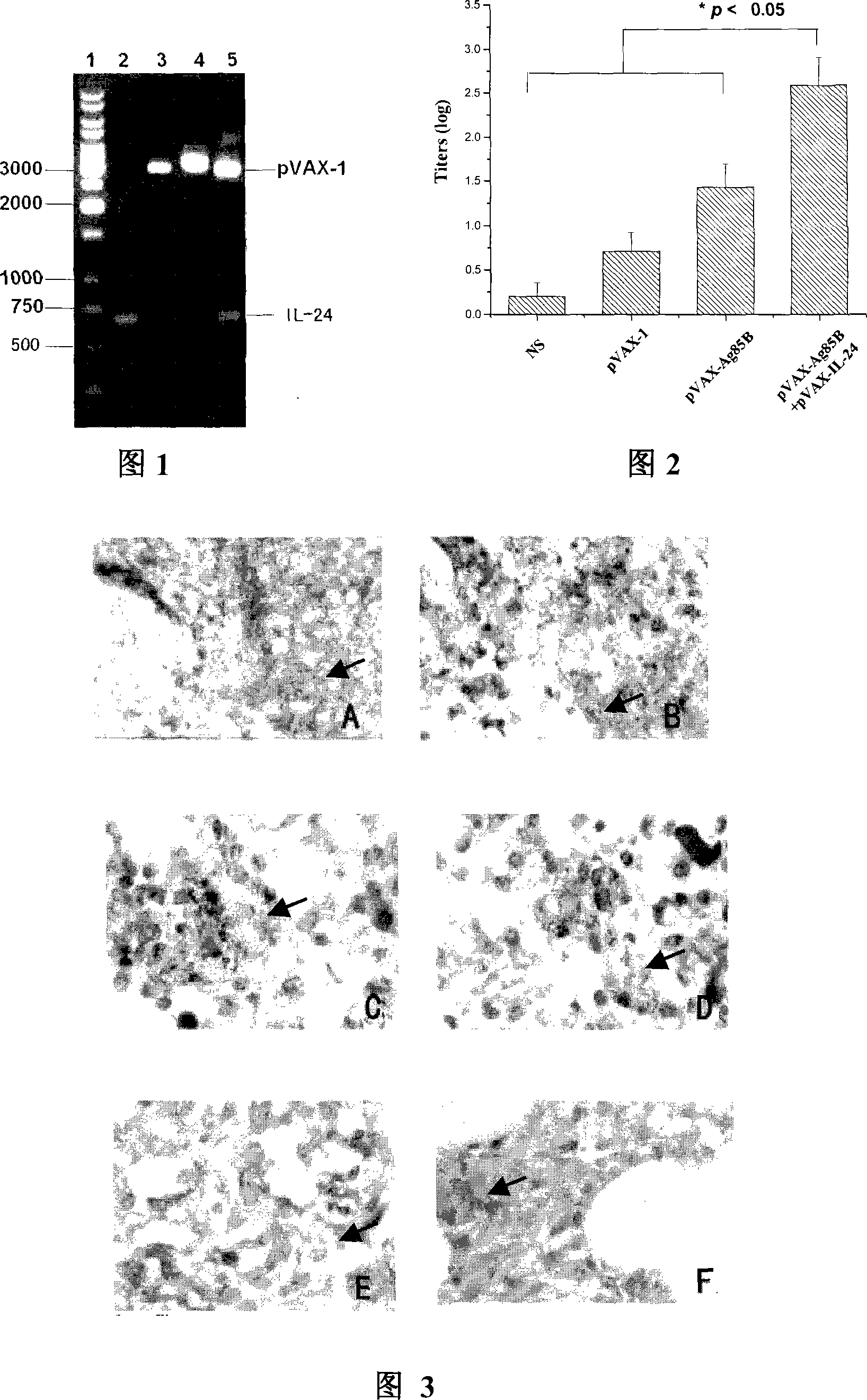
InactiveCN101070542AImprove protectionImprove immunityAntibacterial agentsPeptide/protein ingredientsProtein moleculesInterleukin 24
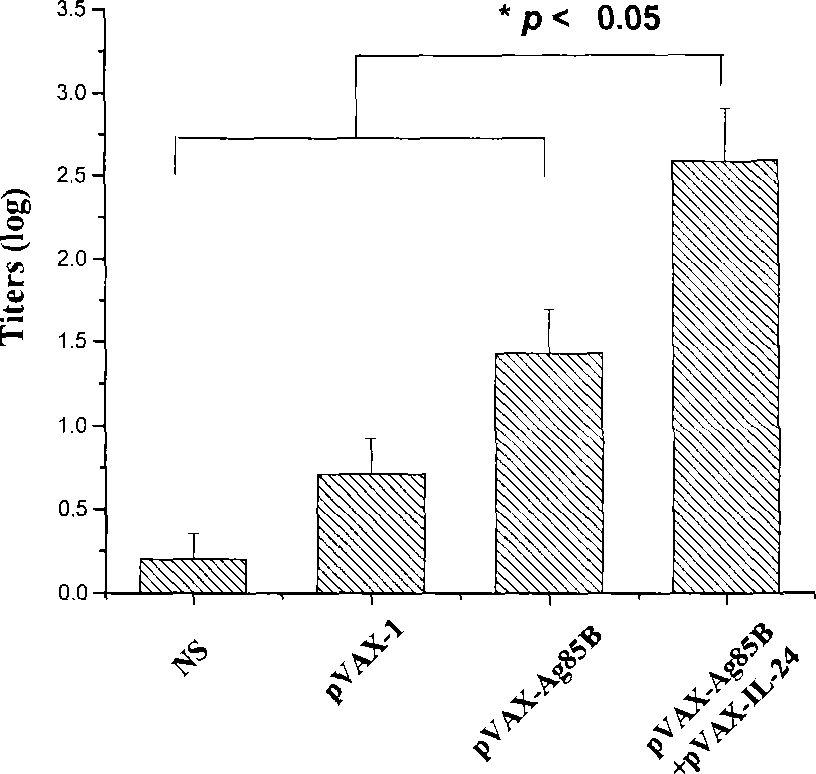
The present invention discloses the human interleukin-24 is shown in SEQ ID NO.1 nucleotide sequence, the sequence containing human interleukin-24 protein molecules of 601 base pairs. Human interleukin-24 recombinant plasmid pVAX1-IL-24, the building will include the use PCR IL-24cDNA from pcDNA3.1-IL-24 amplification and purification, in the purified PCR products, to really build nuclear expression vector pVAX-1, also includes recombinant plasmid pVAX1-IL-24 Construction and preparation steps. Construction of the present invention of pVAX1-IL-24 of the eukaryotic expression vector for in vivo expression in the IL-24 protein, a Th1-type cell-mediated immunity, its joint tuberculosis DNA vaccine Ag85B with immunization, than separate DNA vaccine Ag85B immune used with stronger anti-tuberculosis infection role, and enhance Ag85B resistance tuberculosis infection immune protective effect of IL-24 with that as an immune adjuvant, with the increase of anti-tuberculosis immune protection role.
Owner:WUHAN UNIV
Avian infectious bronchitis virus (IBV)-like particle and preparation method and application thereof
ActiveCN112625096AAvoid reorganizationModification preventionSsRNA viruses positive-senseViral antigen ingredientsBaculovirus expressionInfectious bronchitis virus
The invention discloses an avian infectious bronchitis virus (IBV)-like particle. The virus-like particle is prepared by the following steps of: transferring IBV-S, IBV-M and IBV-E genes into a baculovirus expression system respectively to obtain three recombinant rod particles; transfecting the three recombinant rod particles into insect cells respectively to obtain recombinant baculovirus rHBM-S, rHBM-M and rHBM-E; and then carrying out primary construction through a co-infection form. The virus-like particle is applied to preparation of vaccines and has a great development potential.
Owner:GUANGXI UNIV
Lawsonia intracellularis flgE recombinant protein and lawsonia intracellularis antibody detection kit
ActiveCN112940089ALow false positive detectionConvenient work arrangementsBacteria peptidesBiological testingLawsonia intracellularisAmino acid
The amino acid sequence of the lawsonia intracellularis flgE recombinant protein is as shown in SEQ ID NO. 1. The kit prepared from the lawsonia intracellularis flgE recombinant protein is high in specificity and good in sensitivity and repeatability, can be used for detecting lawsonia intracellularis antibodies and discovering infection of swinery in the early stage, is high in detection speed, can be used for evaluating the LI infection state of the swinery, and is convenient for guiding protection, prevention and control of infection of the lawsonia intracellularis.
Owner:湖南康保特生物科技有限公司 +1
Haemonchus contortus recombinant ARF1 protein nanometer subunit vaccine and application thereof
ActiveCN111529698AProlong or enhance specific immune responseTargetedHydrolasesMicroorganism based processesGlycolic acidEngineering
The invention discloses a haemonchus contortus recombinant ARF1 (ADP-ribosylation factor) protein nanometer subunit vaccine and application thereof. The haemonchus contortus nanometer material subunitvaccine is formed in a way that the recombinant ARF1 protein is coated with a PLGA (poly (lactic-co-glycolic acid)) nanometer material. The experiment utilizes the rARF1-PLGA nanometer subunit vaccine constructed by the ARF1 protein to act in the body of a mouse to induce the immune response effect of the mouse, and an experiment in the body of a goat proves that the rARF1-PLGA nanometer subunitvaccine has an immunoprotection function for an organism. The recombinant ARF1 protein is coated by the PLGA nanometer material to form the nanometer subunit vaccine so as to provide a reference for the preventive utilization of the haemonchus contortus infection of the goat.
Owner:NANJING AGRICULTURAL UNIVERSITY
A flounder streptococcus dolphin gapdh tandem multi-epitope polypeptide and its application
ActiveCN110372797BStrong immune protectionGood effectAntibacterial agentsBacterial antigen ingredientsTGE VACCINEImmune protection
Owner:OCEAN UNIV OF CHINA
Polyglucose and its extracting method and application
InactiveCN101104648BStrong immune protectionSimple extraction methodOrganic active ingredientsImmunological disordersDiseaseVitis vinifera
Owner:XINJIANG MEDICAL UNIV
Preparation of immuno-stimulation composition for hemolycin in monad
InactiveCN1292794CStrong immune protectionImprove immunityAntibacterial agentsBacterial antigen ingredientsImmune effectsOrganic acid
An immunostimulating complex with stable and durable immune effect is prepared from hygrophilic monodas through liquid culturing, centrifugal separating of supernatant, treating it by saturated ammonium sulfate solution, suspending its deposit by PBS dialyzing to obtain toxic, concentrating, adding organic acid, adding Mega-10, treating at 37 deg.C for 4 hr, adding Quil A, cholesterol and lecithin, treating by organic acid, and dialyzing.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +3
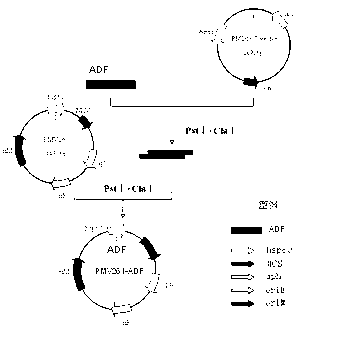
Chicken Eimeria tenella actin depolymerizing factor (ADF) recombinant bacillus calmette-guerin and preparation method thereof
InactiveCN102552893BImprove stabilityEasy to solveAntiparasitic agentsAntibody medical ingredientsActin depolymerizationEnzyme digestion
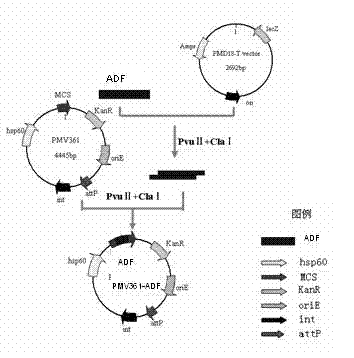
The invention provides chicken Eimeria tenella actin depolymerizing factor (ADF) recombinant bacillus calmette-guerin and a preparation method thereof. The preparation method comprises obtaining Eimeria tenella oocyst, extracting ribose nucleic acid (RNA), reversely transcribing the RNA into complementary deoxyribose nucleic acid (cDNA), designing primers according to an open reading frame of a cloned Eimeria tenella ADF gene sequence to perform polymerase chain reaction (PCR), enabling amplification products obtained by amplifying ADF genes to be connected with a PMD-18 vector to perform clone, performing enzyme digestion on a plasmid with correct sequencing identification, recycling a target fragment, enabling the plasmid to be respectively connected with an escherichia coli-mycobacterium shuttle expression vector PMV 261 and an integrated expression vector PMV 361 which use the same enzyme to perform enzyme reaction, and then obtaining the positive chicken Eimeria tenella recombinant bacillus calmette-guerin through resistance screening and PCR identification. The live vector vaccine is good in stability, easy to transport, store and produce, free of purification, capable of being directly used for immune protection tests, removing complex processes of post-processing of proteins and greatly reducing cost, and suitable for vast rural areas.
Owner:JILIN UNIV
Fusion protein of bovine rotavirus and multiple vaccine against calf diarrhea
ActiveCN111518222BStrong immune protectionImproving immunogenicityAntibacterial agentsSsRNA viruses positive-senseBovine rotavirusEscherichia coli
The invention provides a bovine rotavirus fusion protein and calf diarrhea multiple vaccine, and relates to the technical field of molecular biology. The bovine rotavirus fusion protein contains a VP6 fragment, and the VP6 fragment contains the amino acid sequence shown in SEQ ID NO.4, and at least one loop region in the following (a) to (c) is replaced with an antigenic expression derived from bovine coronavirus and / or antigenic epitopes derived from Escherichia coli: (a) amino acid residues 168‑177, amino acid sequence such as SEQ ID NO.1; (b) amino acid residues 194‑205, amino acid sequence such as SEQ ID NO.1 ID NO.2; (c) 296‑316th amino acid residue, the amino acid sequence is as shown in SEQ ID NO.3. The bovine rotavirus fusion protein contains multiple antigenic epitopes, and after immunizing the host, the host can produce multiple antibodies.
Owner:TIAN KANG ZHI YAO GU FEN YOU XIAN GONG SI
Infectious bursal disease virus a11 strain and its application
ActiveCN105802920BStrong immune protectionResistance to virulent attacksViral antigen ingredientsMicroorganism based processesInfectious bursal disease virus IBDVEmbryo
The invention discloses an infectious bursal disease virus strain A11 and application thereof, and belongs to the field of the microbial technology. Infectious bursal disease viruses are separated from infectious bursal disease vaccine immunity failure chicken flocks appearing in recent years, biological characteristic analysis is carried out on obtained virus isolate strains, and the infectious bursal disease virus strain A11 with good biological characteristics is screened out from the obtained virus isolate strains and is a currently-popular infectious bursal disease virus with super-strong virulence. The virulence of the virus is attenuated with a chicken embryo and cell continuous passage method to culture the low-virulence strain A11, the low-virulence strain A11 has super-strong reproductivity in cells, the virus yield in cells reaches up to 10<11> TCID50 / 0.1 ml, immune protection resisting infectious bursal disease viruses can be rapidly generated by immunizing chicks with the strain A11, immune protection can be achieved after 1 week, and the strain A11 has huge application value in developing new specific infectious bursal disease vaccines.
Owner:JIANGSU ACAD OF AGRI SCI
Intensified tubercle-resisting action of human interleukin 24
InactiveCN101070542BIncrease aggressivenessStrong immune protectionAntibacterial agentsPeptide/protein ingredientsProtein moleculesInterleukin 24
The present invention discloses the human interleukin-24 is shown in SEQ ID NO.1 nucleotide sequence, the sequence containing human interleukin-24 protein molecules of 601 base pairs. Human interleukin-24 recombinant plasmid pVAX1-IL-24, the building will include the use PCR IL-24cDNA from pcDNA3.1-IL-24 amplification and purification, in the purified PCR products, to really build nuclear expression vector pVAX-1, also includes recombinant plasmid pVAX1-IL-24 Construction and preparation steps. Construction of the present invention of pVAX1-IL-24 of the eukaryotic expression vector for in vivo expression in the IL-24 protein, a Th1-type cell-mediated immunity, its joint tuberculosis DNA vaccine Ag85B with immunization, than separate DNA vaccine Ag85B immune used with stronger anti-tuberculosis infection role, and enhance Ag85B resistance tuberculosis infection immune protective effect of IL-24 with that as an immune adjuvant, with the increase of anti-tuberculosis immune protection role.
Owner:WUHAN UNIV
Truncated rotavirus vp8 protein and use thereof
ActiveCN105085639BHigh yieldHigh purityViral antigen ingredientsVirus peptidesRotavirus gastroenteritisVirus Protein
Disclosed are a truncated rotavirus VP8 protein, fusion protein containing the truncated protein, conjugate containing the truncated protein, coding sequences of the truncated protein and the fusion protein and preparation methods therefor, and pharmaceutical compositions and vaccines containing the truncated protein, the fusion protein or the conjugate. The truncated protein, the fusion protein, the conjugate, the pharmaceutical compositions and the vaccines can be used for preventing, relieving or treating rotavirus infection and diseases caused by rotavirus infection, for example, rotavirus gastroenteritis and diarrhea. Also disclosed are uses of the truncated protein, the fusion protein, and the conjugate for preparing pharmaceutical compositions or vaccines.
Owner:XIAMEN UNIV +1
Attenuated Strain of Streptococcus agalactiae from Tilapia and Its Application
ActiveCN104152368BStrong immune protectionHighly effective immune protectionAntibacterial agentsBacterial antigen ingredientsBacteroidesPhosphate
The invention relates to the disease prevention and control technology of aquaculture animals, in particular to the attenuated strain of Streptococcus agalactiae derived from tilapia and its application. The attenuated strain of Streptococcus agalactiae derived from tilapia is YM001, which is preserved in CCTCC, and the preservation number is: CCTCC M 2014045. After the attenuated strain was activated and fermented, the 10 6 -10 9 Bacterial cells of the attenuated strain YM001 of CFU / mL are mixed with sterile phosphate buffer solution as an attenuated live vaccine for the prevention and treatment of tilapia streptococcosis, and the injection concentration is 10 5 -10 8 CFU / tail, oral administration concentration is 10 8 -10 9 CFU / tail, soaking administration concentration is 10 7 -10 8 CFU / mL. The invention relates to strain identification, virulence measurement, safety and stability of reversion, immunogenicity and immune protection effect of the attenuated strain. The tilapia streptococcal disease vaccine made with the strain has good immune protection effect and safety and strong stability.
Owner:GUANGXI ACADEMY OF FISHERY SCI
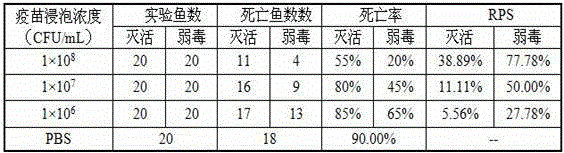
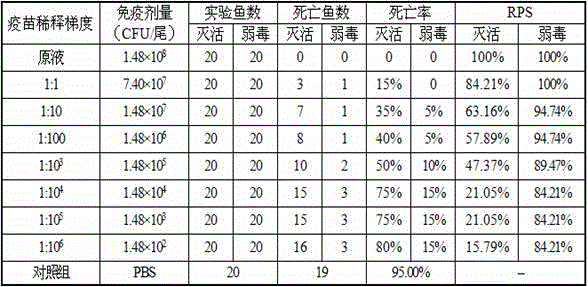
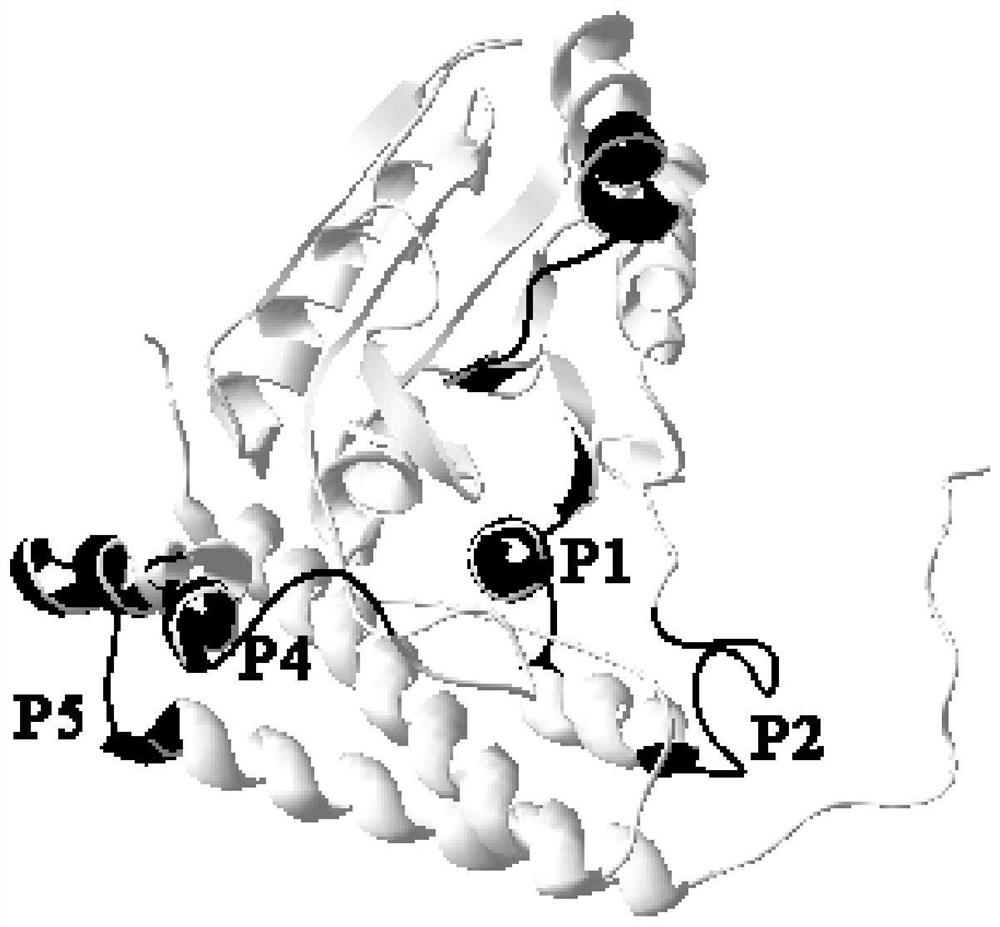
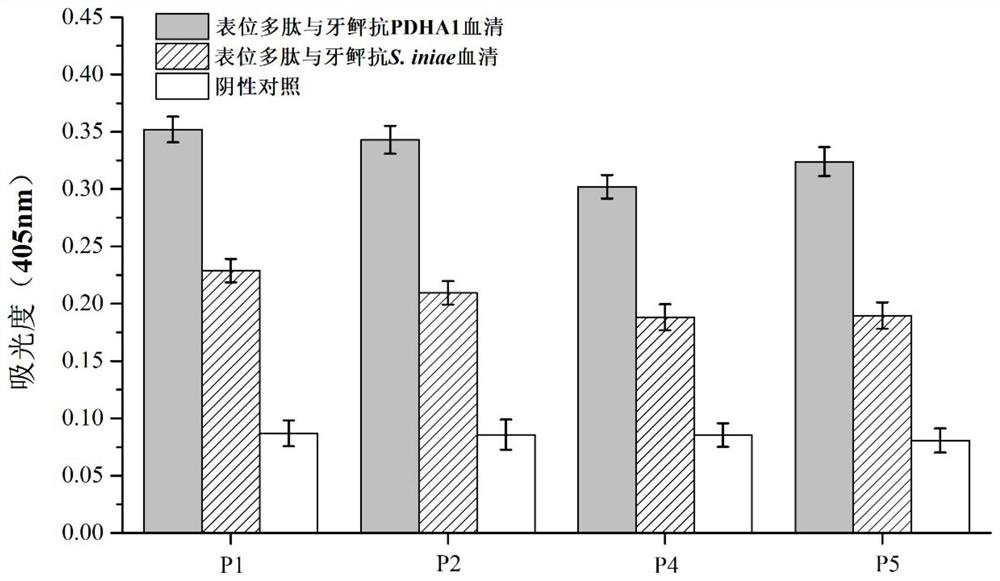
Streptococcus iniae PDHA1 multi-epitope polypeptide
ActiveCN110592033AStrong immune protectionImprove protectionAntibacterial agentsBacterial antigen ingredientsMicrobiologyStreptococcus iniae
The invention provides streptococcus iniae PDHA1 multi-epitope polypeptide. The streptococcus iniae PDHA1 multi-epitope polypeptide includes four streptococcus iniae PDHA1 protein epitope peptides which are connected through polypeptide connectors, and the amino acid sequences of the streptococcus iniae PDHA1 protein epitope peptides are respectively SEQID NO: 1-4. The invention provides a streptococcus iniae PDHA1 multi-epitope vaccine in another respect, wherein the antigen is the multi-epitope polypeptide. The prepared PDHA1 multi-epitope vaccine can provide stronger immunoprotection for paralichthys olivaceus to resist S.iniae infection, and the protection effects are obviously better than those of PDHA1 recombinant subunit vaccines and S.iniae whole bacterium inactivation vaccines.
Owner:OCEAN UNIV OF CHINA
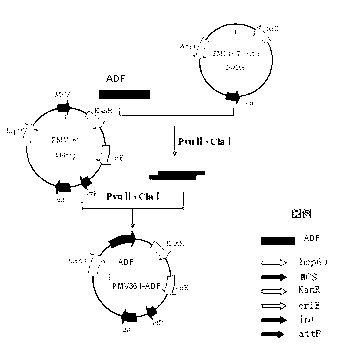
Chicken Eimeria tenella actin depolymerizing factor (ADF) recombinant bacillus calmette-guerin and preparation method thereof
InactiveCN102552893AStrong cellular immune adjuvant effectImprove stabilityAntiparasitic agentsAntibody medical ingredientsActin depolymerizationEnzyme digestion
The invention provides chicken Eimeria tenella actin depolymerizing factor (ADF) recombinant bacillus calmette-guerin and a preparation method thereof. The preparation method comprises obtaining Eimeria tenella oocyst, extracting ribose nucleic acid (RNA), reversely transcribing the RNA into complementary deoxyribose nucleic acid (cDNA), designing primers according to an open reading frame of a cloned Eimeria tenella ADF gene sequence to perform polymerase chain reaction (PCR), enabling amplification products obtained by amplifying ADF genes to be connected with a PMD-18 vector to perform clone, performing enzyme digestion on a plasmid with correct sequencing identification, recycling a target fragment, enabling the plasmid to be respectively connected with an escherichia coli-mycobacterium shuttle expression vector PMV 261 and an integrated expression vector PMV 361 which use the same enzyme to perform enzyme reaction, and then obtaining the positive chicken Eimeria tenella recombinant bacillus calmette-guerin through resistance screening and PCR identification. The live vector vaccine is good in stability, easy to transport, store and produce, free of purification, capable of being directly used for immune protection tests, removing complex processes of post-processing of proteins and greatly reducing cost, and suitable for vast rural areas.
Owner:JILIN UNIV
A multi-epitope polypeptide of flounder streptococcus pdha1
ActiveCN110592033BStrong immune protectionImprove protectionAntibacterial agentsBacterial antigen ingredientsTGE VACCINEImmune protection
The invention provides a multi-epitope polypeptide of flounder Streptococcus dolphin PDHA1, which comprises four Streptococcus iniae PDHA1 protein epitope peptides connected by polypeptide linkers, and the amino acid sequences of the Streptococcus iniae PDHA1 protein epitope peptides are respectively is SEQ ID NO: 1-4. Another aspect of the present invention provides a Streptococcus iniae PDHA1 multi-epitope vaccine, wherein the antigen is the above-mentioned multi-epitope polypeptide. The PDHA1 multi-epitope vaccine prepared by the invention can provide strong immune protection for flounder against S. iniae infection, and the protection effect is obviously better than PDHA1 recombinant subunit vaccine and S. iniae whole bacteria inactivated vaccine.
Owner:OCEAN UNIV OF CHINA
Preparation and application of an att fusion protein for preventing Staphylococcus aureus infection
ActiveCN105713096BImproving immunogenicityGood immune protectionAntibacterial agentsAntibody mimetics/scaffoldsStaphylococcus cohniiCandida famata
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com