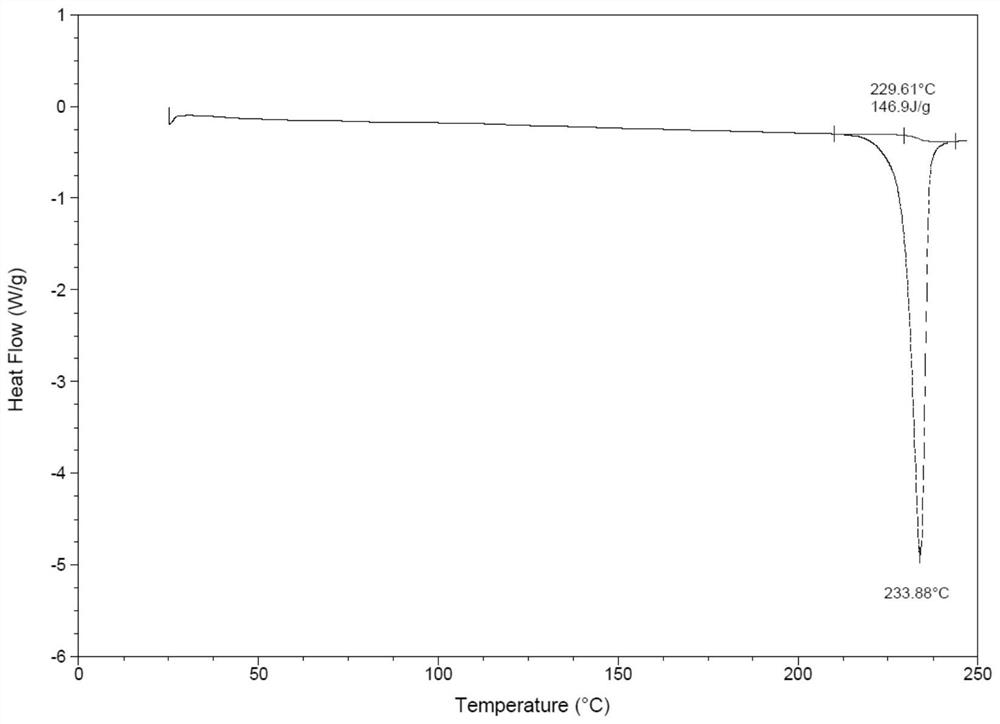
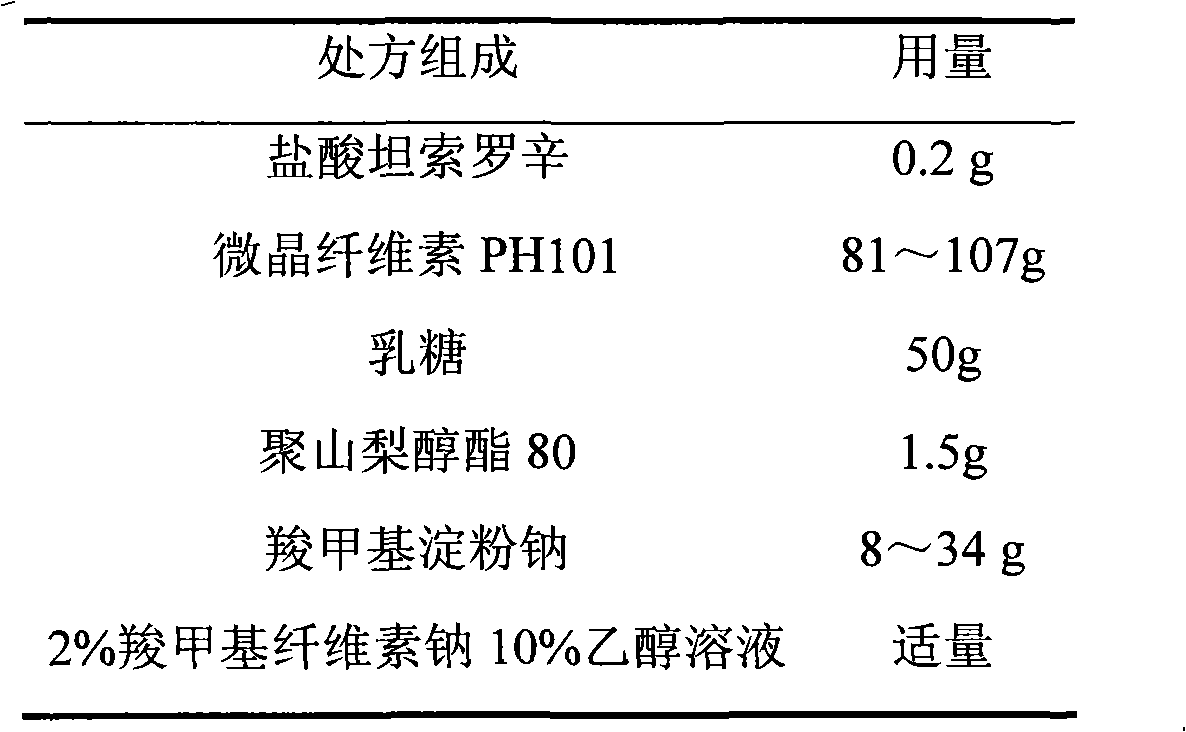
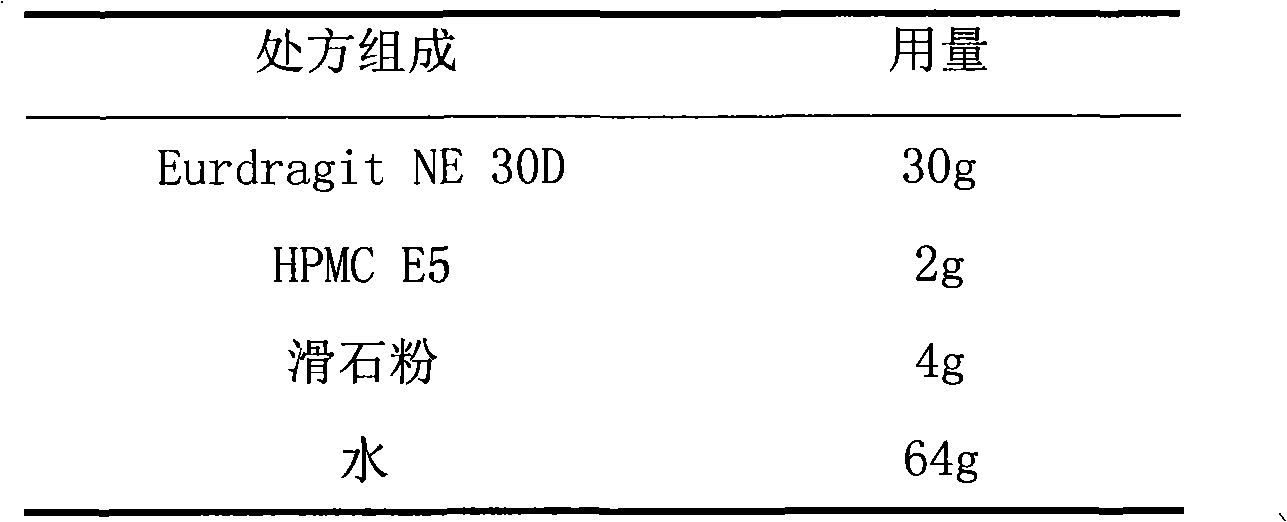
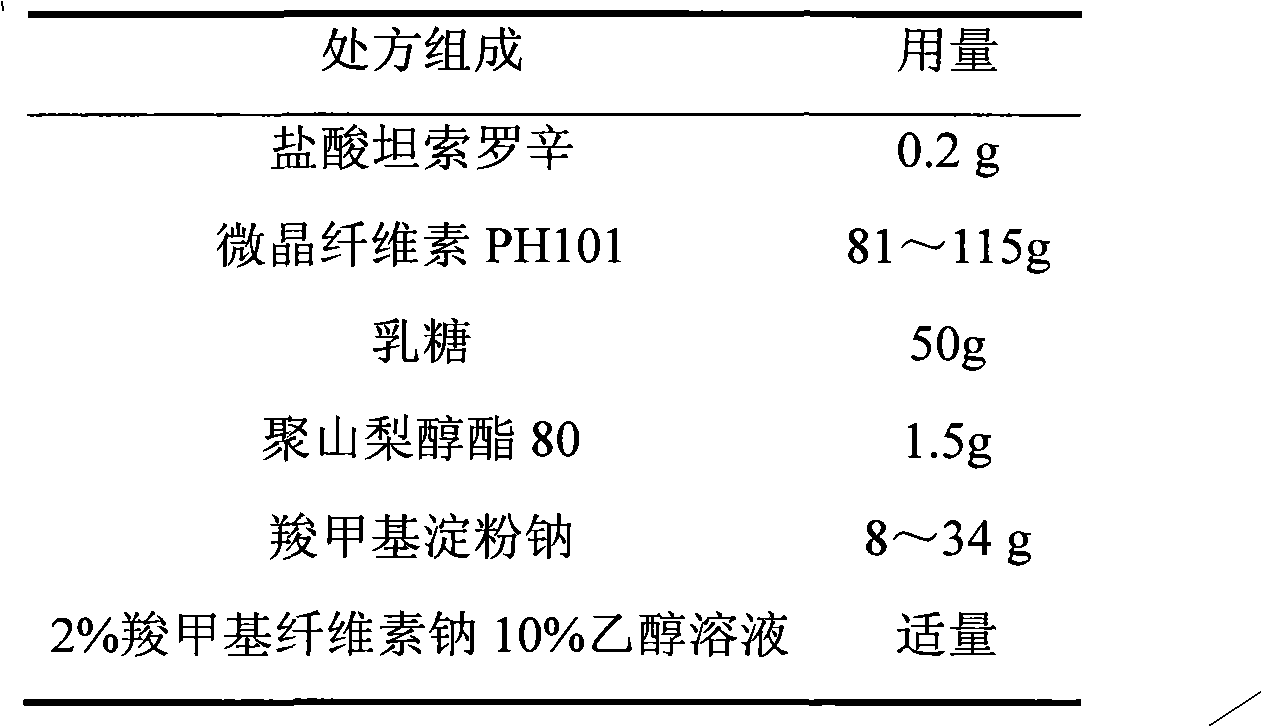
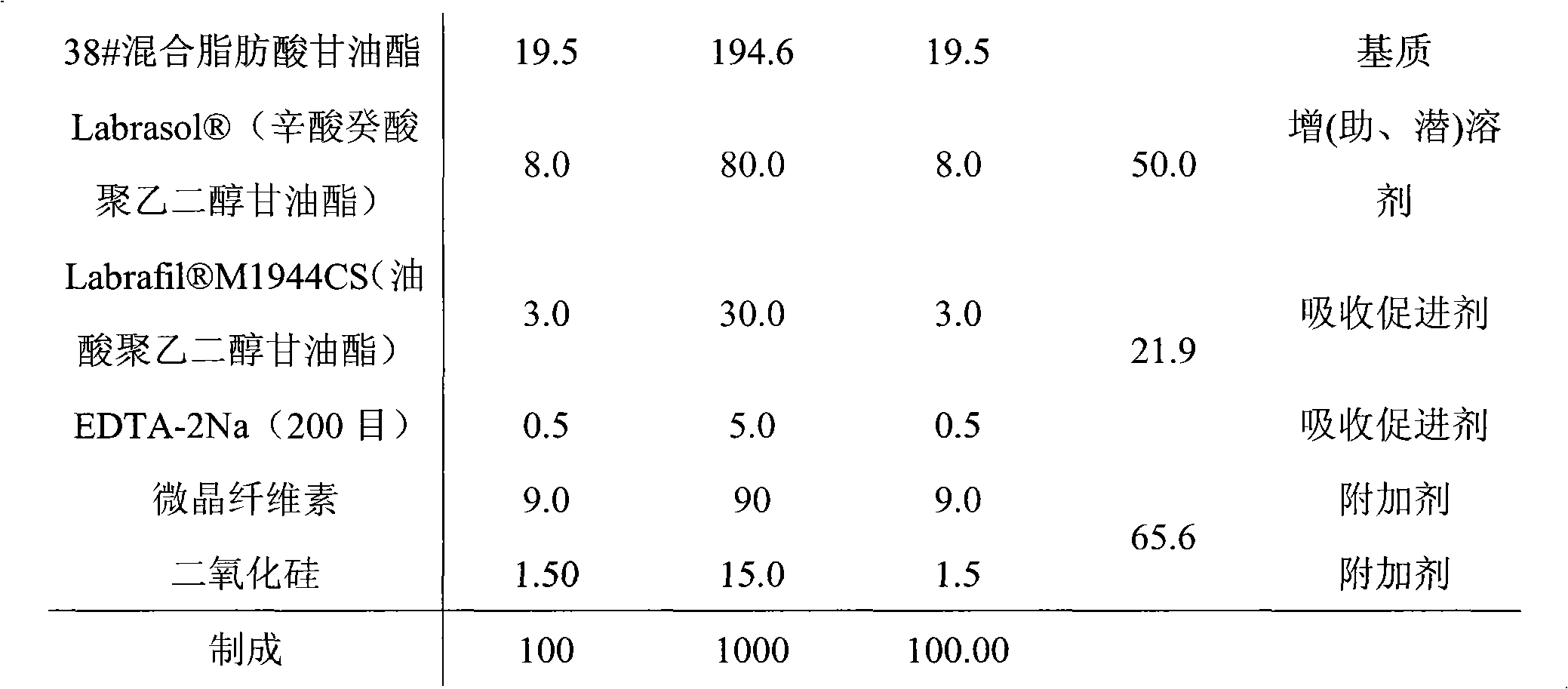Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
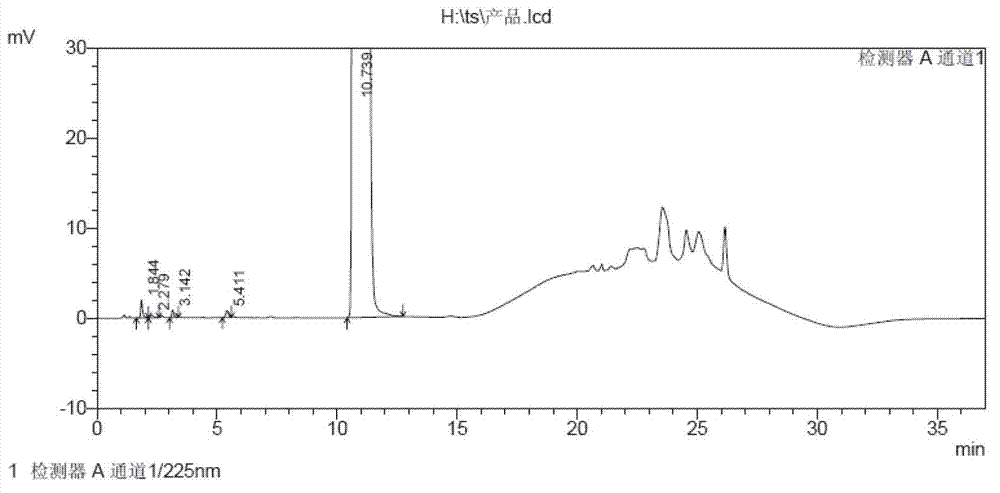
59 results about "Tamsulosin hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
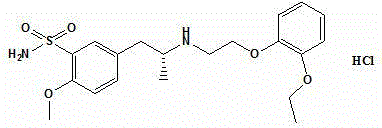
The hydrochloride salt of tamsulosin, a sulfonamide derivative with adrenergic antagonist activity. Tamsulosin selectivity binds to and blocks the activity of alpha1 adrenoreceptors in the human prostate and bladder neck; blockade of these adrenoceptors can cause smooth muscle in the prostate and bladder neck to relax, resulting in an improvement in urine flow rate. Check for http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=479148&idtype=1 active clinical trials or http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=479148&idtype=1&closed=1 closed clinical trials using this agent. (http://nciterms.nci.nih.gov.libproxy1.nus.edu.sg/NCIBrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&code=C29486 NCI Thesaurus)
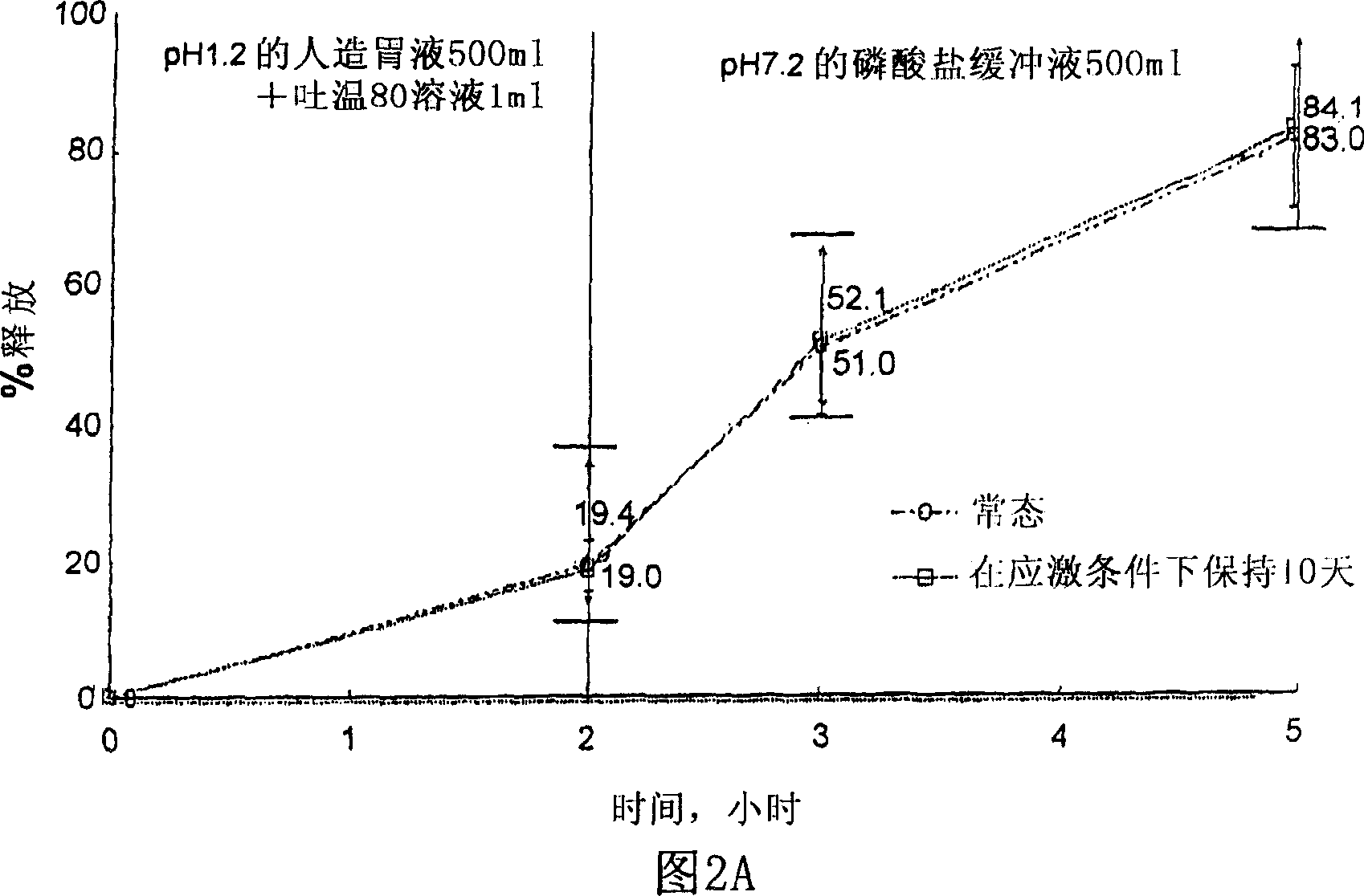
Controlled-release formulation containing tamsulosin hydrochloride
The present invention relates to a controlled-release preparation containing tamsulosin hydrochloride. The preparation of the present invention can release tamsulosin hydrochloride at zero-order rate regardless of ambient conditions of stomach and small intestine, and therefore can reduce several side effects and maintain the effect of the drug constantly. In addition, the tamsulosin hydrochloride-containing controlled-release preparation of the present invention is simple to manufacture and cost-effective, and can be made with conventional tabletting machine and coating machine used usually so that it does not need a specific equipment.
Owner:CTC BIO INC
Pharmaceutical composition having active ingredient of tamsulosin hydrochloride and finasteride
InactiveCN101108174AOrganic active ingredientsUrinary disorderHas active ingredientOrally disintegrating tablet
The invention relates to a drug compound that takes tamsulosin hydrochloride and finasteride as its active ingredients as well as its preparation method and purpose. The invention, which takes tamsulosin hydrochloride and finasteride as its active ingredients and forms drug compound by mixing pharmaceutically acceptable supplementary materials, can be applied for the treatment of benign hyperplasia of prostate. The invention takes tamsulosin hydrochloride and finasteride as its active ingredients and adds the supplementary materials with certain portion and sorts, then develop them into tablet, capsule, dispersible tablet, chewable tablet, toroches and pill via the preparation technology in the invention.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Hydrochloric tamsulosin sustained-release capsule and its preparation method
ActiveCN101125134APrecise Controlled ReleaseControl releaseOrganic active ingredientsPharmaceutical delivery mechanismSide effectOral medication
The present invention provides a tamsulosin hydrochloride sustained-release capsule. The tamsulosin hydrochloride sustained-release capsule of the present invention can avoid the sudden release of the drug tablets and the performance differences generated from the gastric emptying differences, display minor food effect or do not display food effect, and obtain the stable curve of the plasma drug concentration and longer action time simultaneously, so as to reduce the occurrence of cardiovascular side effects, greatly improve the safety, effectiveness and compliance of the medication for the patients. The tamsulosin hydrochloride sustained-release capsule of the present invention can ensure the sustained and regular release of the main ingredient tamsulosin hydrochloride after the oral administration, and the present invention is characterized by convenient administration, durable function, stable efficacy, fewer side effects and so on.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Tamsulosin hydrochloride double-layer osmotic pump controlled-releasing tablet and preparation method thereof
InactiveCN101167701AStable concentrationRelease constant speed evenlyInorganic non-active ingredientsPharmaceutical delivery mechanismExcipientMoisture
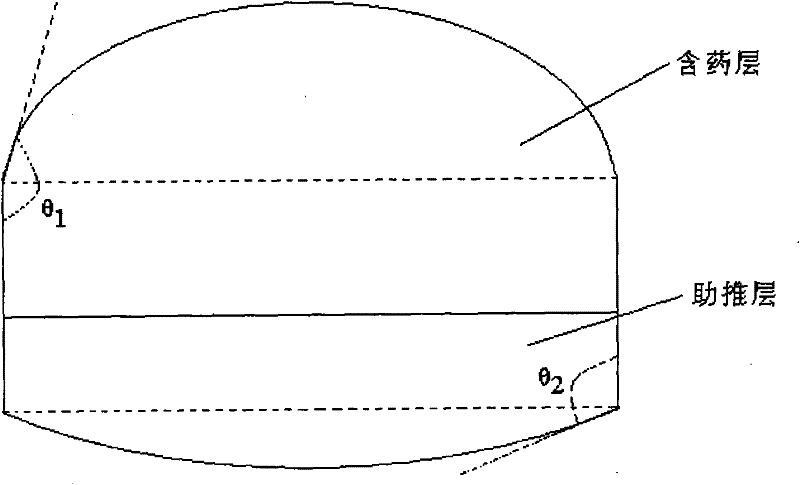
The invention provides double-layer osmotic pump tablets of tamsulosin ehydrochloride and a process for preparation. The medicament contains tamsulosin ehydrochloride and acceptable medical polymeric excipient, and is characterized in that the invention has excellent zero-level controlled releasing, pH level of environment, movements of the stomach and intestine, and food, has little effect on releasing action and food, and has no effect on the internal pharmacokinetics parameter. According to the percentage by weight, the preparation contains tamsulosin ehydrochloride 0-2%, excipient in pastille layer with the function of controlled-releasing 30-70% excipient in boosting layer with the function of controlled releasing 30-70%, and the rest percentage of other excipient. The of process for preparation the double layer permeable pump controlled-release tablets of tamsulosin ehydrochloride comprises (1) the preparation of pastille layer, (2) the preparation of boosting layer, (3) the compressing of the two layers, (4) the coating of the double layer tablets, (5) the perforating of the coated tablets, (6) the packing of moisture proof cost. The invention is clinically used for the treatment of paruria symptom like frequent micturition, diuresis at night, dysuria caused by prostatic hyperplasia.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Controlled release pellet preparation and its preparation method
InactiveCN103142492APromote absorptionGood uniformity of releaseUrinary disorderPharmaceutical non-active ingredientsControl releaseDrug release
The invention relates to a novel controlled release pellet preparation and its preparation method, and especially relates to a tamsulosin hydrochloride controlled release pellet preparation and its preparation method. The controlled release pellet adopts a drug-loaded controlled release layer to coat a blank pellet and an enteric coating to coat the drug-loaded controlled release layer in order to control the drug release, the drug release can be realized in an acidic or weakly alkaline environment, and good absorption is realized when the pellet enters a body. The pellet has the advantages of good release degree homogeneity, simple preparation technology and good repeatability. The invention further provides a novel drug controlled release pellet suitable for small-dimension drugs. The novel drug controlled release pellet still well controls the drug release when the drug load amount reduces to 10% or less of a total weight.
Owner:COSCI MED TECH CO LTD
Preparation method for tamsulosin hydrochloride
The invention relates to a preparation method of tamsulosin hydrochloride for treating urination disorder caused by prostate hyperplasia, belongs to the field of medicine, provides a low-cost synthesis process for preparing a chiral compound N-[(1R)-2-(4-methoxyphenyl)-1-methyl ethyl]-N-[(1R)-1-phenyl ethyl] amine hydrochloride and also aims to provide a preparation method of tamsulosin hydrochloride. Qualified raw materials are provided for the preparation of a tamsulosin hydrochloride preparation.
Owner:WEIHAI WEITAI PHARMA TECH DEV
Novel unsymmetrical preparation of tamsulosin hydrochloride
InactiveCN101462987AAchieve recyclingReduce consumptionSulfonic acid amide preparationSolventCarboxylic ester
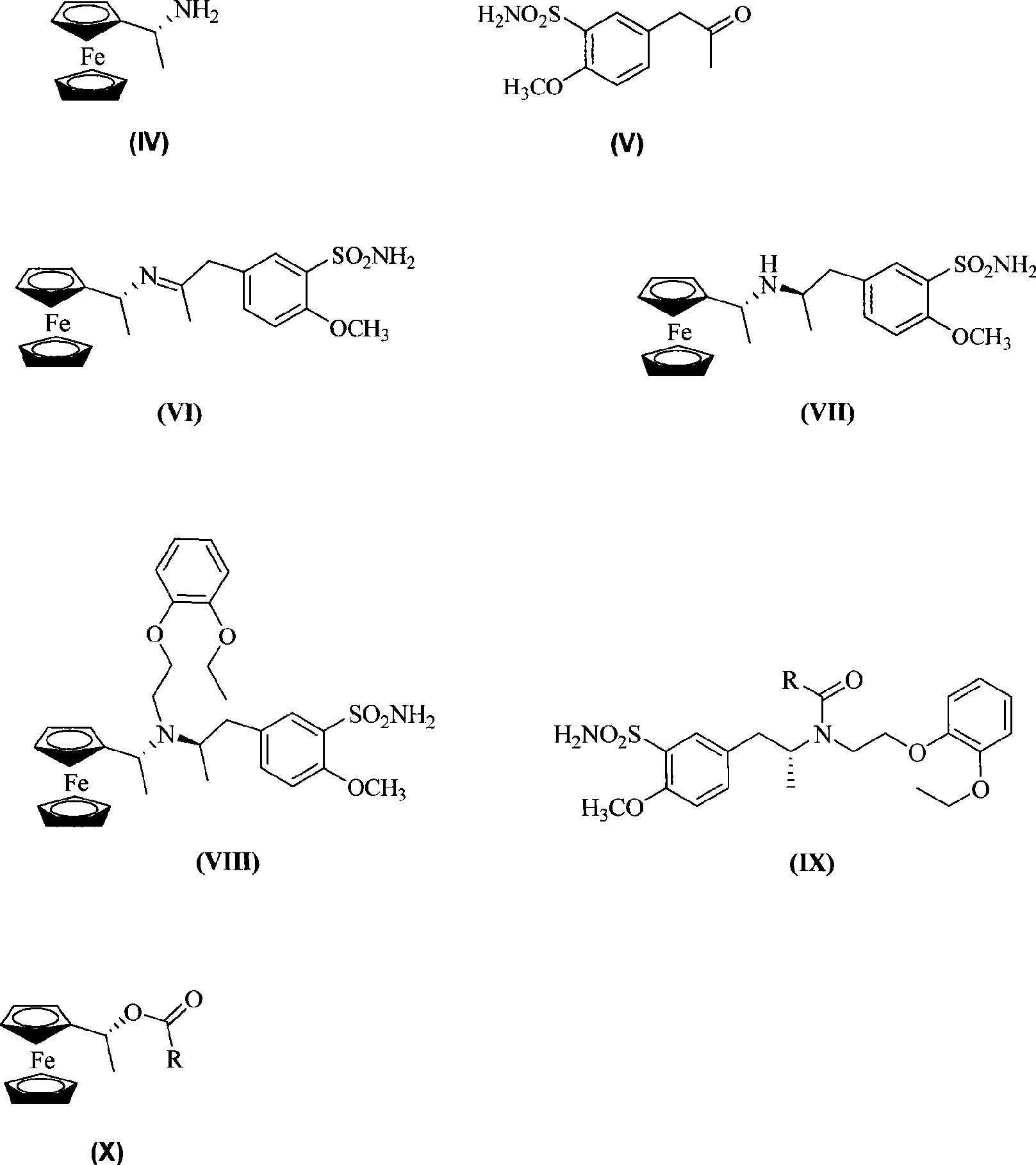
The invention relates to a method for preparing tamsulosin hydrochloride asymmetrically. (R)-ferrocenyl ethylamine and 5-acetonyl-2-methoxyl benzene sulfonamide are dissolved in solvent for condensation reaction to obtain imine compound. (R)-5-(2-(N-ferrocenyl ethylamine) propyl)-2- methoxyl benzene sulfonamide is obtained by reduction reaction of the imine compound. The (R)-5-(2-(N-ferrocenyl ethylamine) propyl)-2- methoxyl benzene sulfonamide reacts with o-2-bromine ethoxyl phenetole under the action of alkali to form (R)-5-((2-[N-(2-ethoxyl-phenoxyl) ethyl)-N-ferrocenyl ethylamine) propyl)-2-methoxyl benzene sulfonamide after treatment. The (R)-5-((2-(N-(2-ethoxyl-phenoxyl) ethyl)-N-ferrocenyl ethylamine)propyl)-2-methoxyl benzene sulfonamide generates chiral amide compound and ferrocenyl ethanol carboxylic ester under the action of anhydride, and the chiral amide compound generates the tamsulosin hydrochloride after post treatment. The preparation method has simple operation and good yield and purity, is feasible and environmental-friendly and has the prospect of industrialized production.
Owner:ZHEJIANG NORMAL UNIVERSITY
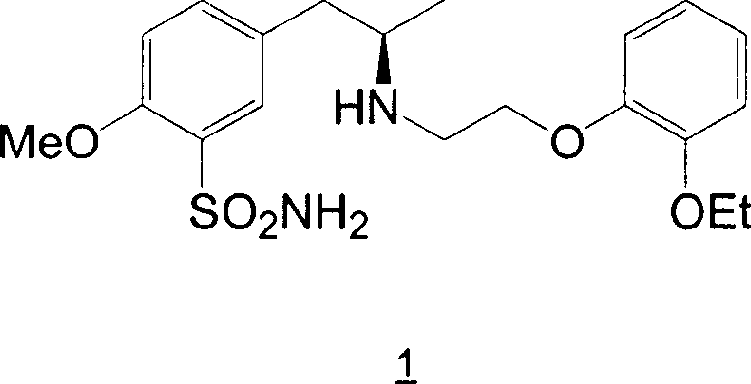
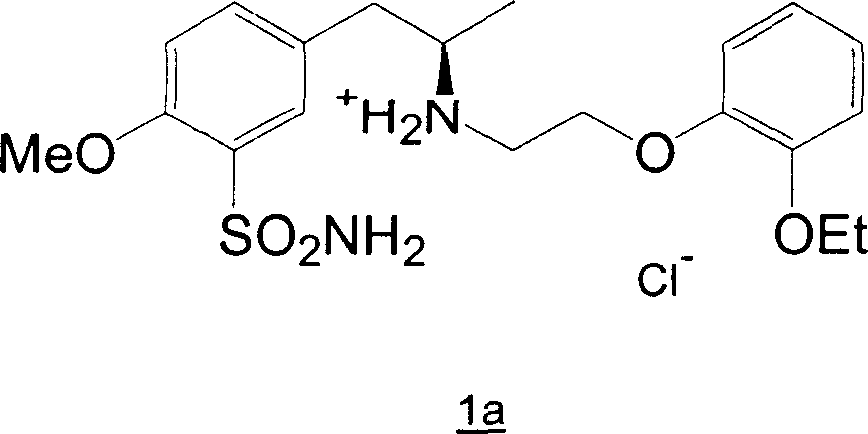
Preparation of r-5-(2-(2-ethoxyphenoxyethylamino)propyl)-2-methoxybenzenesulphonamide hydrochloride of high chemical purity
Owner:LEK PHARMA D D
Rectal administration composition containing tamsulosin
InactiveCN101889996AImprove solubilityGood curative effectSuppositories deliveryOrganic non-active ingredientsSide effectWhole body
The invention relates to a rectal administration composition containing tamsulosin, which comprises effective quantities of tamsulosin or pharmaceutically acceptable salts or derivatives thereof, solubilizer and sorbefacient, wherein the effective quantity of tamsulosin or pharmaceutically acceptable salts or derivatives thereof is metered according to the equivalence tamsulosin hydrochloride; the content of the tamsulosin or pharmaceutically acceptable salts or derivatives thereof is equivalent to 0.025-1.6 mg of tamsulosin hydrochloride; and the preparation per unit weighs 0.8-4 g. The rectal administration composition containing tamsulosin can overcome the defects of poor curative effect and great toxic or side effect in oral administration and systemic injection administration, and the defects of great side effect and poor patient dependence in partial injection administration, and can prolong the duration of drug actions, thereby providing a better treatment means for medical care personnel and patients. The production technology is simple and suitable for industrial mass production.
Owner:张立英 +1
Synthesis method of tamsulosin hydrochloride
ActiveCN103497126AEasy to synthesizeHigh puritySulfonic acid amide preparationChemical synthesisPhenylsulfonamide
The invention belongs to the technical field of chemical synthesis, and specifically relates to a synthesis method of tamsulosin hydrochloride. According to the synthesis method, benzene sulfonic amide shown as a formula (II) and bromine ether shown as a formula (III) are in a condensation reaction in an aprotic polar solvent in the presence of an acid-binding agent to generate a condensation compound intermediate shown as a formula (IV); the condensation compound intermediate is in organic solvent, in the presence of a catalyst, hydrogen is introduced into the organic solvent under certain pressure so as to hydrogenate the condensation compound intermediate, then, the R-tamsulosin free alkali shown as a formula (V) is obtained, and the R-tamsulosin free alkali further is subjected to a salt formation reaction with hydrochloric acid in an organic solvent C to produce the tamsulosin hydrochloride shown as a formula (I). In the reaction process for preparing the tamsulosin hydrochloride through the synthetic route provided by the invention, the phenomenon that bimolecular bromide and amine react with each other to generate a disubstituted by-product is avoided, and the obtained tamsulosin hydrochloride has high product purity and high yield; according to the synthesis method, the reaction conditions are moderate, and synthesis is convenient to finish.
Owner:天台宜生生化科技有限公司
Preparation method of tamsulosin hydrochloride
The invention relates to a preparation method of tamsulosin hydrochloride, which comprises the following steps: reacting R-(-)-5-(2-aminopropyl)-2-metoxybenzsulfamide hydrochloride and o-ethoxylphenoxylethyl bromide to obtain R-tamsulosin free alkali, and reacting the free alkali with hydrochloric acid to obtain the R-tamsulosin hydrochloride. The invention is characterized in that the reaction of the R-(-)-5-(2-aminopropyl)-2-metoxybenzsulfamide hydrochloride and o-ethoxylphenoxylethyl bromide is carried out in a water-containing solvent.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
Preparation method of tamsulosin hydrochloride with high optical purity
ActiveCN104926699AGuaranteed optical purityPurification process reductionOrganic chemistryOrganic compound preparationOrganosolvEthyl group
The invention discloses a preparation method of tamsulosin hydrochloride with high optical purity, and belongs to a medicine technology and a chemical field. A recrystallization method is adopted, crude products of (R)-5-(2-(2-(2-ethoxyphenoxy) ethyl amino) propyl)-2-methoxyl phenyl sulfonamide hydrochloride are refined, so that pure products of the (R)-5-(2-(2-(2-ethoxyphenoxy) ethyl amino) propyl)-2-methoxyl group sulfonamide hydrochloride of which the e.e. value is larger than 99.8% is obtained; a crystallizing solvent adopted by the recrystallization method is a mixed solvent consisting of an organic solvent and water, the organic solvent is selected from one of methanol, ethyl alcohol, acetone, acetonitrile and isopropyl alcohol, and the recrystallization temperature is under 15 DEG C. The preparation method disclosed by the invention is simple to operate, short in period, low in cost and good in repeatability, and can solve the inevitable problem of rework for treatment in the industrial production.
Owner:CHENGDU LIKAI CHIRAL TECH
Tamsulosin hydrochloride sustained-release capsule and preparation method thereof
PendingCN104873478AAvoid burst phenomenonImprove securityPharmaceutical delivery mechanismUrinary disorderSustained Release CapsuleEconomic benefits
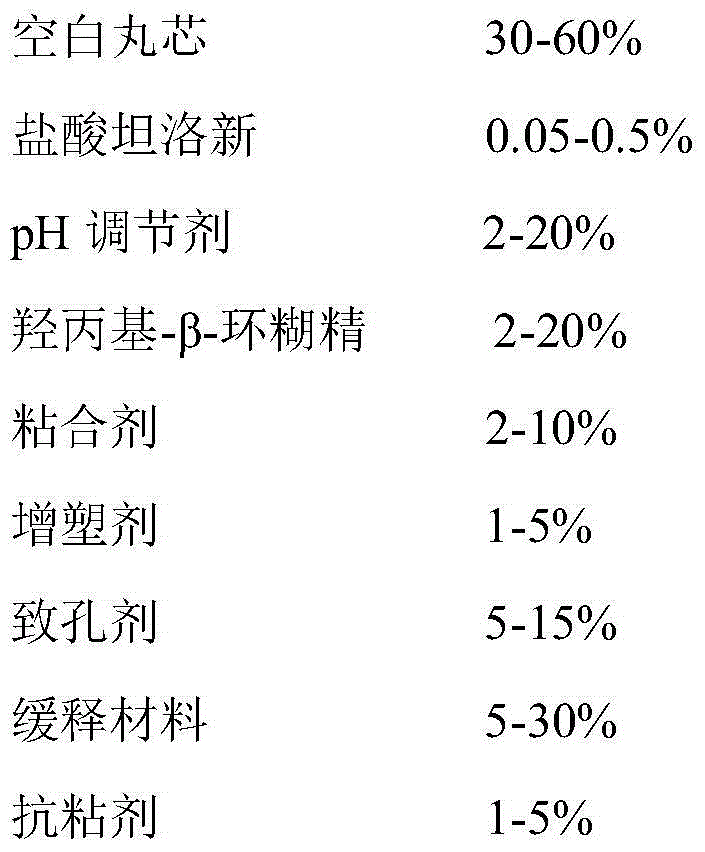
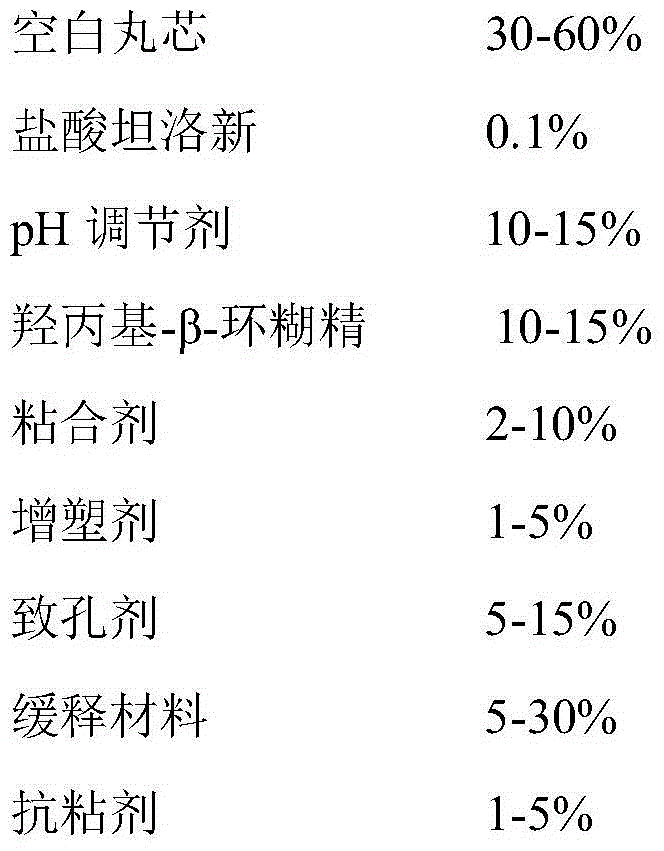
The invention provides a tamsulosin hydrochloride sustained-release capsule and a preparation method thereof. Hydroxypropyl-beta-cyclodextrin is added in a prescription, so as to form clathrate compounds on the outer part of tamsulosin hydrochloride together with the tamsulosin hydrochloride, so that the phenomenon of drug burst release in a drug release process is solved, thereby maintaining stable plasma concentration, reducing the incidence rate of adverse reactions and improving the clinical medication safety. In addition, raw materials are easy to obtain, the preparation process is simple and feasible, the yield is high, the cost is low, industrial mass production can be realized, and obvious economic benefits are achieved.
Owner:LUNAN BETTER PHARMA
Tamsulosin hydrochloride osmotic pump controlled-release tablet
The invention provides a novel tamsulosin hydrochloride osmotic pump controlled-release tablet. Ethyl cellulose and povidone are used as membrane forming materials of a semipermeable membrane, and the asymmetric tablet form is preferably selected, so that the ageing of the semipermeable membrane can be avoided, and the drug residues can be reduced. The invention also provides a method for improving the ageing resistance of the tamsulosin hydrochloride osmotic pump controlled-release tablet, wherein the method is characterized in that the ethyl cellulose-povidone are used as the membrane forming materials of the semipermeable membrane. In addition, the invention also provides applications of the ethyl cellulose-povidone composition in preparing the anti-ageing tamsulosin hydrochloride osmotic pump controlled-release tablet.
Owner:北京天衡药物研究院有限公司
Slow-release preparation containing tamsulosin hydrochloride and preparation method thereof
InactiveCN102579392AStable release rateOvercome the "peak and valley" phenomenonPharmaceutical delivery mechanismUrinary disorderFormularyBlood concentration
The invention discloses a slow-release preparation containing tamsulosin hydrochloride. The slow-release preparation is a combination comprising a coating and a plain film coated in the coating. The invention further provides a method for preparing the slow-release preparation containing the tamsulosin hydrochloride according to a formula. In the method, the preparation of the slow-release preparation can be completed by adopting a novel medicament formula and the conventional equipment. The tamsulosin hydrochloride slow-release preparation prepared with the method can be used for effectively controlling the in-vivo release of a medicament, the medicament is only required to be taken once every day, the medicament taking times are reduced, and the in-vivo blood concentration is stable, so that the in-vivo blood concentration of a patient in a medicament taking period is stable and effective, and the safety and effectiveness of the medicament are improved fundamentally.
Owner:CHONGQING KERUI PHARMA GRP
Composition for oral administration of tamsulosin hydrochloride and controlled release granule formulation comprising same
InactiveUS20070196500A1Improve stabilitySatisfactory sustained release characteristicBiocideUrinary disorderControl releaseOral medication
A composition for oral administration of tamsulosin hydrochloride and a controlled release granule formulation comprising the same exhibited excellent stability and sustained release characteristics of tamsulosin hydrochloride regardless of pH changes for an extended period of time.
Owner:HANMI PHARMA
Tamsulosin hydrochloride cotrolled-releasing tablet preparation and preparing method thereof
ActiveCN101147729AStable blood concentrationSmall individual differencesUrinary disorderAmide active ingredientsHydrophilic polymersWater insoluble
The present invention discloses a tamulosin osmotic pump type controllably-released tablet preparation and its preparation method. Said preparation is composed of double-layer table core, semipermeable film with small pres and moisture-proof film. Its double-layer table core is formed from medicine-containing layer and assistant layer, in which the medicine-containing layer contains 10-99% of ethylene pyrrolidone polymer and / or ethylene pyrrolidone copolymer, and the assistant layer contains 10-80% of water-insoluble polymer, at the same time, in the assistant layer 10-80% of hydrophilic polymer with osmotic activity and 5-50% of osmotic pressure accelerator are contained.
Owner:OCEAN STAR INT
Composition for oral administration of tamsulosin hydrochloride and controlled release granule formulation comprising same
ActiveCN1921888ASatisfactory sustained release propertiesImprove stabilityPowder deliveryUrinary disorderOral medicationControl release
A composition for oral administration of tamsulosin hydrochloride and a controlled release granule formulation comprising the same exhibited excellent stability and sustained release characteristics of tamsulosin hydrochloride regardless of pH changes for an extended period of time.
Owner:HANMI PHARMA
Tamsulosin hydrochloride sustained-release pellets and preparation method thereof
InactiveCN101695478BUrinary disorderPharmaceutical non-active ingredientsSustained release pelletsCaplet Dosage Form
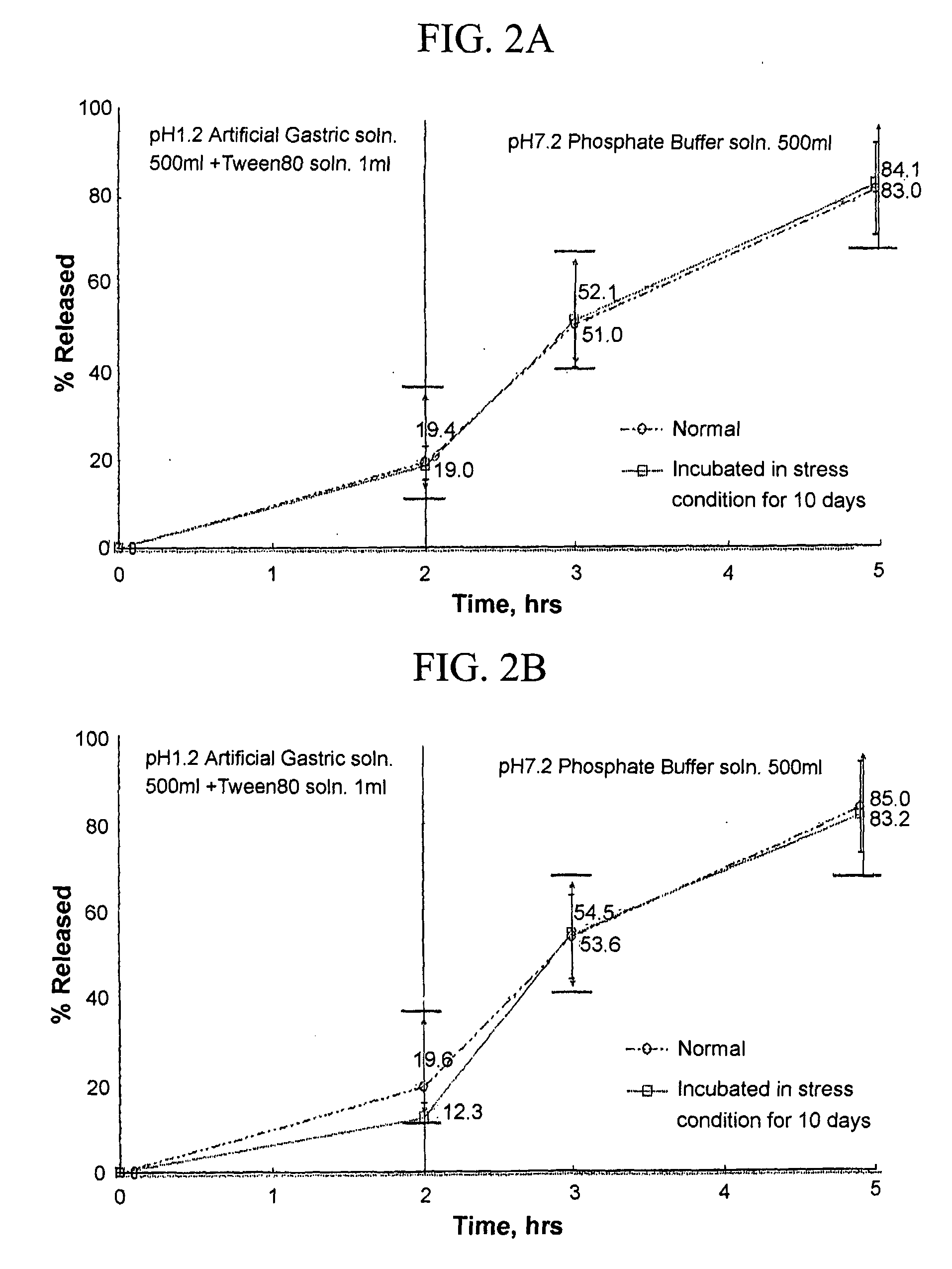
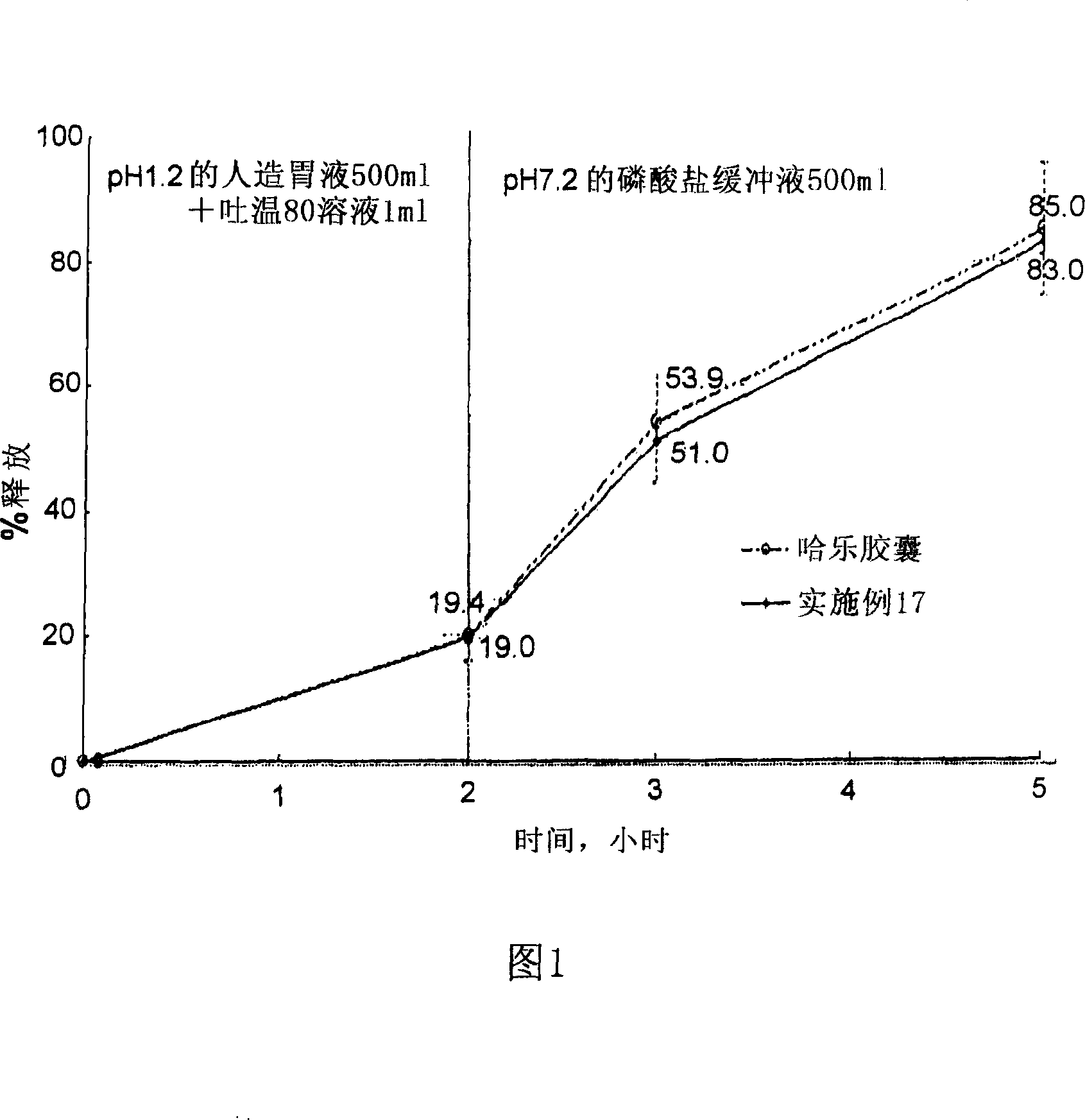
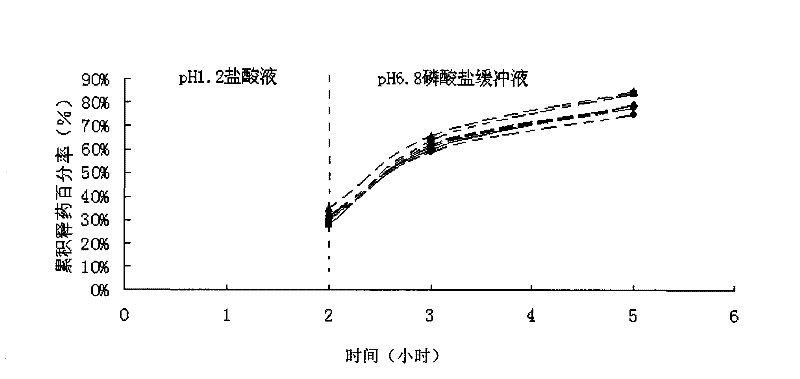
The invention discloses tamsulosin hydrochloride sustained-release pellets, which are prepared by coating medicament-containing blank pellets, wherein the blank pellets contain microcrystalline cellulose, soluble starch, lactose and croscarmellose sodium. Two layers of coating liquid are a Surelease sustained-release coating material and an enteric-coating material Kolicoat MAE 30DP respectively and the tamsulosin hydrochloride sustained-release pellets are capsules. The tamsulosin hydrochloride sustained-release pellets of the invention adopt the sustained-release coating material and the enteric-coating material to control the release of medicaments. The prepared tamsulosin hydrochloride sustained-release pellet capsules release less than 40 percent of medicaments in hydrochloric acid solution with a pH value of 1.2 in two hours and release 40 to 70 percent (about 60 percent) of medicaments in phosphate buffer solution with a pH value of 6.8 in three hours and over 70 percent (about90 percent) of medicaments in five hours, so the prepared tamsulosin hydrochloride sustained-release pellet capsules meet the requirements of quality standards. The invention discloses a preparation method of the tamsulosin hydrochloride sustained-release pellets.
Owner:JIANGSU UNIV +1
Composition of long-acting oral pills of Yansuan Tanshuluoxin and its production
InactiveCN1895241ALow dissolution rateSmall dissolution rate deviationPill deliveryUrinary disorderCarboxymethyl celluloseAlginic acid
An orally taken long-acting small pill of tamsulosin hydrochloride is proportionally composed of a core, a tamsulosin hydrochloride layer, a first release regulating layer chosen from Youteqi L30D55, Sulisi and kollicoat SR3D, a release assistant layer chosen from alginic acid, microcrystalline fiber and carboxymethyl cellulose sodium, and a second release regulating layer chosen from ethylcellulose, Youteqi L100 and Youteqi S100. Its preparing process is also disclosed.
Owner:可隆制药株式会社
Slow/controlled-release preparation of tamsulosin hydrochloride and preparation method thereof
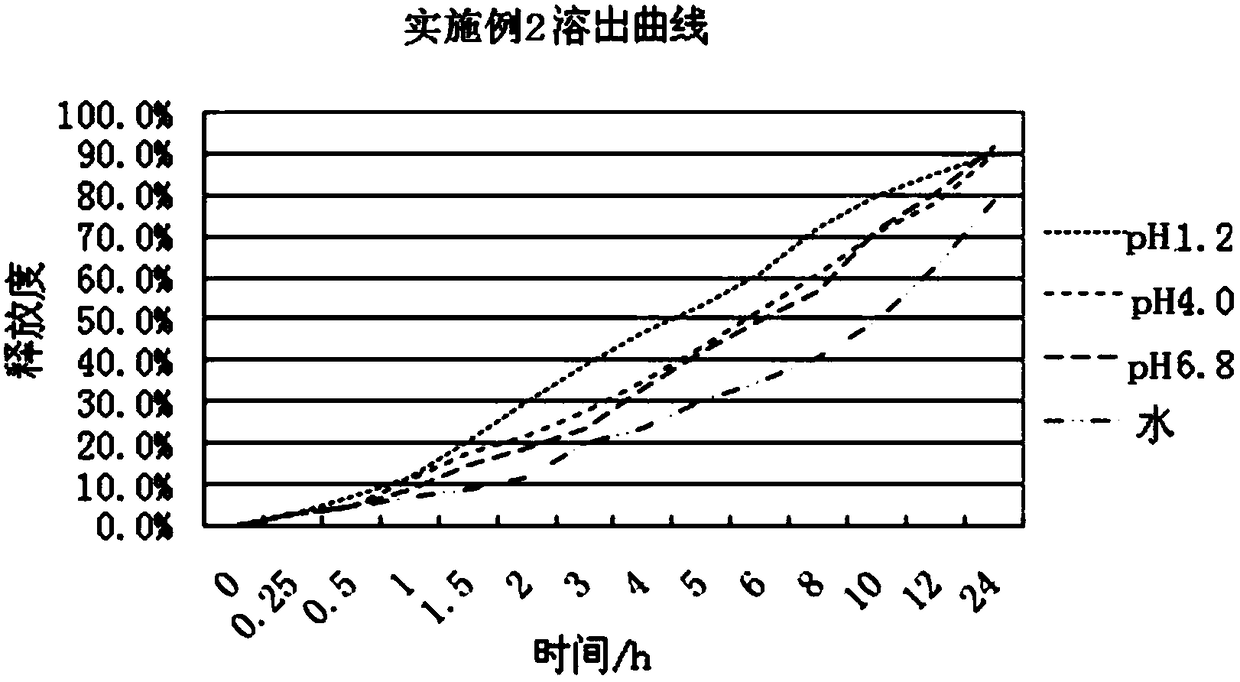
ActiveCN108096220AAffect absorptionProblems Affecting AbsorptionInorganic non-active ingredientsUrinary disorderCelluloseDissolution
The invention relates to a slow / controlled-release preparation of tamsulosin hydrochloride and a preparation method thereof. The slow / controlled-release preparation comprises (i) a pill core which ismade of the following raw material in parts by weight: 135-165 parts of a microcrystal cellulose micro-pill cores; (ii) a slow-release layer which is made of the following raw materials in parts by weight: 0.36-0.44 parts of tamsulosin hydrochloride, 145-200 parts of a slow / controlled release material and 3.8-58 parts of a pore forming agent. Dissolution degree tests show that the slow / controlled-release tamsulosin hydrochloride preparation consisting of a blank pill core and a medicine-carrying slow / controlled release coating layer has a slow-release effect within a relatively pH value range(the pH value is 1.2, 4 or 6.8), has a pH value independent slow-release property, and is capable of solving the problem that absorption of tamsulosin hydrochloride is affected as the pH value of a gastrointestinal tract is changed if a patient takes foods, therefore, the slow / controlled-release preparation of the tamsulosin hydrochloride, which is provided by the invention, can be taken both before or after a meal, and is relatively convenient to take, and absorption of the tamsulosin hydrochloride is not affected.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Oral pharmaceutical preparation having improved content uniformity, containing tamsulosin hydrochloride-containing extended release pellet
Owner:HANMI PHARMA
Tamsulosin hydrochloride sustained-release pellet and preparation method thereof
ActiveCN103919735AEvenly dispersedSmall content uniformityUrinary disorderAmide active ingredientsSustained release pelletsWater insoluble
The invention belongs to the field of medicinal preparations, and discloses a tamsulosin hydrochloride sustained-release pellet and a preparation method thereof. The tamsulosin hydrochloride sustained-release pellet comprises a drug-loading sustained-release pellet and an enteric coating layer, wherein the drug-loading sustained-release pellet comprises tamsulosin hydrochloride, an enteric coating material A, a solvent A, a water-insoluble material and a diluting agent. According to the tamsulosin hydrochloride sustained-release pellet, the tamsulosin hydrochloride can be uniformly dispersed in the drug-loading sustained-release pellet, and the content of small-dose tamsulosin hydrochloride in the prepared tamsulosin hydrochloride sustained-release pellet can be uniformly distributed; furthermore, the surface of the drug-loading sustained-release pellet is coated with the enteric coating layer, and the tamsulosin hydrochloride sustained-release pellet can be released in a sustained manner and is not influenced by pH of gastrointestinal tracts, can be released in an acidic or alkaline environment, and can be absorbed in the body. The method for preparing the tamsulosin hydrochloride sustained-release pellet is easy to operate, and is suitable for industrial production.
Owner:HYBIO PHARMA WUHAN CO LTD
Orally disintegrating sustained-release preparation containing tamsulosin hydrochloride and preparation method thereof
InactiveCN103230379AImprove complianceRapid disintegration in vitroPharmaceutical delivery mechanismPharmaceutical non-active ingredientsExtended release tabletsBlood concentration
Belonging to the field of medicines, the invention particularly relates to a preparation method of a sustained release tablet containing tamsulosin hydrochloride. The dosage form is an oral preparation that is prepared by taking tamsulosin hydrochloride as an active component and using acceptable auxiliary materials in an orally disintegrating tablet. The preparation not only has the characteristics of rapid disintegrating speed, rapid onset, and high patient compliance of orally disintegrating tablets, but also has the advantages of long action time, significantly reduced adverse reactions during instantaneous release of drugs, and stable blood concentration.
Owner:AVENTIS PHARMA HAINAN
Tamsulosin hydrochloride sustained-release pellet and preparation method thereof
InactiveCN105412020AReduce absorption rateAbsorb evenlyUrinary disorderAmide active ingredientsSustained release pelletsIrritation
The invention relates to a tamsulosin hydrochloride sustained-release pellet. The tamsulosin hydrochloride sustained-release pellet comprises a medicine-containing pellet body and a coating layer, wherein the coating layer wraps the medicine-containing pellet body, the medicine-containing pellet body comprises tamsulosin hydrochloride, a blank pellet core, a filling agent, a lubricating agent and an adhering agent, and the coating layer comprises Eudragit NE30D and talcum powder. A preparation method comprises the following steps: 1, preparing materials; 2, mixing; 3, preparing the adhering agent; 4, pelleting; 5 preparing a coating agent; 6, coating; 7, filling; and 8, packaging by aluminum-plastic for obtaining a finished product. The tamsulosin hydrochloride sustained-release pellet is suitable for the symptoms such as urination disorder caused by benign prostatic hyperplasia, and is good in drug release stability, small in irritation to the gastrointestinal tract, good in bioavailability, convenient to package, transport and store, and suitable for industrial production. The tamsulosin hydrochloride sustained-release pellet is good in absorption after oral medication, and although the tamsulosin hydrochloride sustained-release pellet can be taken on an empty stomach or after the meal, food can increase the total absorptive amount of the tamsulosin hydrochloride sustained-release pellet.
Owner:HEILONGJIANG ZHICHENG MEDICAL TECH
Tamsulosin hydrochloride sustained-release pellets and preparation method thereof
InactiveCN105287395AReduce absorption rateAbsorb evenlyUrinary disorderAmide active ingredientsSustained release pelletsDrug release rate
The invention discloses tamsulosin hydrochloride sustained-release pellets and a preparation method thereof. The tamsulosin hydrochloride sustained-release pellets comprise a coating layer, medicine-containing pellets, Eudragit NE-30D, powdered steatile, sodium dodecyl sulfate or polyethylene glycol; tamsulosin hydrochloride, blank pellets, a filler, a lubricant and an adhesive. The method employs a novel sustained release preparation and a pellets preparation, slow release means delay of drug release rate of the medicine from a dosage form, absorption rate of medicine in the body can be reduced, more stable treatment effect can be realized; pellets can increase the distribution area of the medicine on the surface of the gastrointestinal tract, irritation is reduced, bioavailability is increased, influence due to stomach evacuation factors is not generated, medicine can be uniformly absorbed in the body, individual difference is little, and simultaneous application of two advanced technologies enhances the technical advantage of the tamsulosin hydrochloride sustained-release pellets.
Owner:HARBIN SHENGJI PHARMA
Preparation method of tamsulosin hydrochloride crystal form
PendingCN112142627ASimple processHigh yieldOrganic chemistry methodsSulfonic acid amide preparationPhysical chemistryPharmaceutical drug
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of a tamsulosin hydrochloride crystal form, which comprises the following steps: adding tamsulosin into a solvent, conducting heating to a first temperature, and conducting complete dissolving to obtain a first solution; adding an acid into the first solution, and carrying out heat preservation reaction at a first temperature to obtain a second solution; cooling the second solution to a second temperature, and conducting stirring for reaction to obtain a third solution; and filtering thethird solution and conducting drying to obtain the tamsulosin hydrochloride crystal form. The preparation method is simple in process, high in yield, high in product purity and suitable for large-scale industrial production.
Owner:北京鑫开元医药科技有限公司 +1
Tamsulosin hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211790AAccelerated agingReduce permeabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsFiller ExcipientLactose
The invention relates to a tamsulosin hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the tamsulosin hydrochloride film-controlled slow-release pellet utilizes Eurdragit NE30D and HPMC E5 as film-formation materials. A pellet core of the tamsulosin hydrochloride film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose as fillers, and polysorbate 80 as a solubilizer; and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit NE30D, HPMC E5 and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE30D to HPMC E5 to talcum powder is 30: 2: 4 and a film weight increasing ratio is in a range of 19 to 36%. The tamsulosin hydrochloride film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the tamsulosin hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the tamsulosin hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Hydrochloric tamsulosin sustained-release capsule and its preparation method
ActiveCN101125134BOvercoming transfer time differencesLess irritatingOrganic active ingredientsPharmaceutical delivery mechanismSide effectFOOD EFFECT
The present invention provides a tamsulosin hydrochloride sustained-release capsule. The tamsulosin hydrochloride sustained-release capsule of the present invention can avoid the sudden release of the drug tablets and the performance differences generated from the gastric emptying differences, display minor food effect or do not display food effect, and obtain the stable curve of the plasma drug concentration and longer action time simultaneously, so as to reduce the occurrence of cardiovascular side effects, greatly improve the safety, effectiveness and compliance of the medication for the patients. The tamsulosin hydrochloride sustained-release capsule of the present invention can ensure the sustained and regular release of the main ingredient tamsulosin hydrochloride after the oral administration, and the present invention is characterized by convenient administration, durable function, stable efficacy, fewer side effects and so on.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
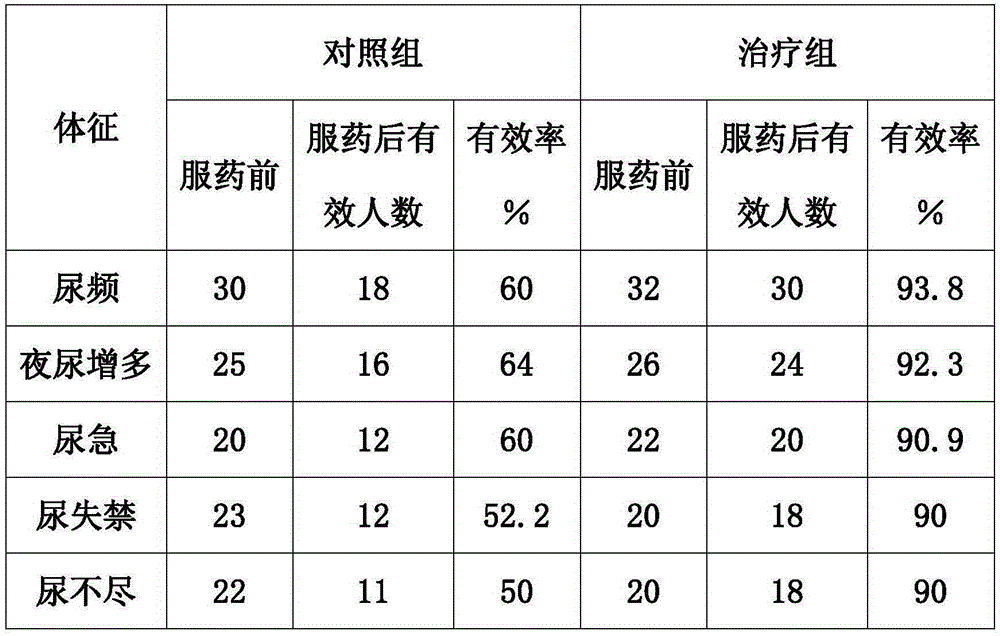
Suppository for treating prostatitis and preparation method thereof
InactiveCN102688358ARelieve symptomsSuppositories deliveryPharmaceutical non-active ingredientsGynecologyAnus
The invention discloses a suppository for treating prostatitis and a preparation method thereof. The suppository is prepared from the following raw material drugs in parts by weight: 0.3 to 0.5 part of tamsulosin Hydrochloride, 5 to 15 parts of golden cypress, 5 to 15 parts of common andrographis herb, 0.4 to 1 part of dragon blood, and 100 parts of 36 type mixed fatty acid glyceride. The suppository is administrated from the anus, and is pushed into rectum for half finger; the dosage of the suppository for an adult is 5 to 8g per time and the suppository is taken 1 to 2 times everyday, 30 continuously administrated days are taken as a course of treatment, and the total effective rate in 30 cases with chronic prostatitis treated with the suppository for one course of treatment is 94.8 percent.
Owner:CHONGQING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com