Novel unsymmetrical preparation of tamsulosin hydrochloride
A tamsulosin hydrochloride, asymmetric technology, applied in the field of optically active drug synthesis, can solve the problems of large chiral raw materials, waste, waste of raw materials, etc., achieve good industrial production prospects, good diastereoselectivity, and reduce environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
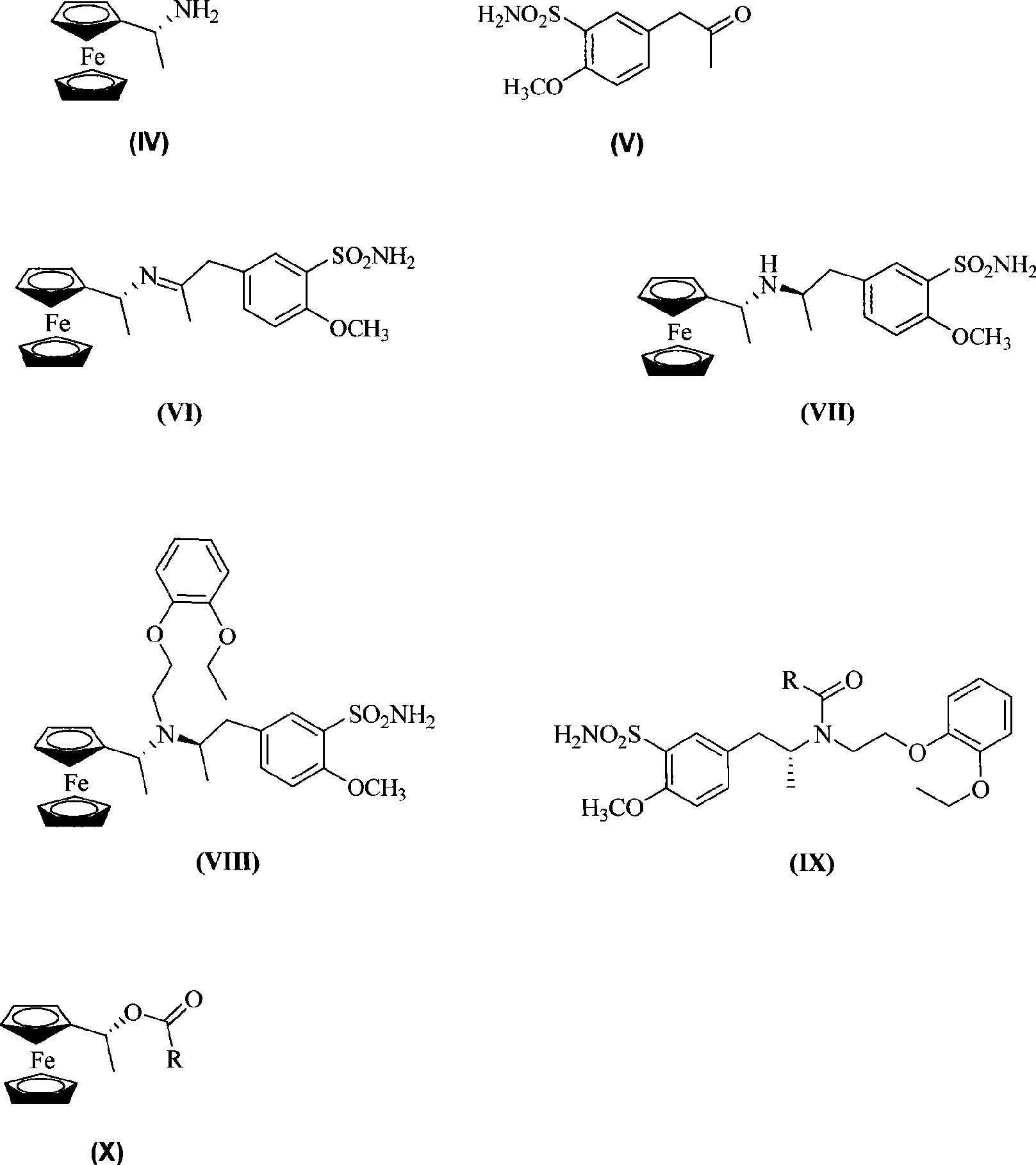
[0030] Under the protection of nitrogen, 3.95g (16.25mmol) of 5-acetonyl-2-methoxybenzenesulfonamide was dissolved in 150ml of methanol, and 1.49g (6.5mmol) of (R)-ferrocenyl ethyl The methanol mixed solution of the amine was stirred for 6 hours to form an imine compound, which was directly poured into a high-pressure reaction axe, added Raney-Ni, heated at 50°C, and hydrogenated at 2.2MP atmospheric pressure for 48 hours. After carefully venting the excess hydrogen, filter off Raney-Ni, concentrate to remove methanol, dilute with ethyl acetate, add 20ml of aqueous solution and stir for 30 minutes, extract three times with ethyl acetate, anhydrous Na 2 SO 4 Drying, concentration and removal of solvent, the crude product was purified by silica gel column chromatography to obtain 1.6g orange solid namely: (R)-5-[2-(N-ferroceneethylamine)propyl]-2-methoxy Benzenesulfonamide. The eluent is ethyl acetate:methanol (volume ratio)=5:2. Yield: 53%, m.p.92-95°C, (c=1.0, CH 3 OH). ...
Embodiment 2
[0044] Under nitrogen protection, 3.66g (15.0mmol) of 5-acetonyl-2-methoxybenzenesulfonamide was dissolved in dry 150ml THF, and then slowly added dropwise 2.12g (8.6mmol) of (R)-diocene The THF mixed solution of iron-based ethylamine was heated to reflux and stirred for 4.5 hours to form an imine compound, and then 640mg (34.4mmol) of NaBH was added 4 , reacted at room temperature for 4h, removed THF, extracted three times with ethyl acetate, anhydrous Na 2 SO 4 Drying, concentration to remove the solvent, the crude product was purified by silica gel column chromatography to obtain 1.76g orange solid namely: (R)-5-[2-(N-ferroceneethylamine)propyl]-2-methoxy Benzenesulfonyl, the eluent is ethyl acetate: methanol (volume ratio) = 5:2. Yield: 45%, m.p.92.5-95°C. (c=1.0, CH 3 OH).
[0045] (R)-5-[2-(N-ferroceneethylamine)propyl]-2-methoxybenzenesulfonamide nuclear magnetic spectrum is: 1 HNMR (400MHz, CDCl 3 )δ: 0.97(d, J=6.4Hz, 3H), 1.30(d, J=6.4Hz, 3H), 2.57(m, 1H), 2...
Embodiment 3
[0058] Under the protection of nitrogen, dissolve 2.4g (10.0mmol) of 5-acetonyl-2-methoxybenzenesulfonamide in dry 100ml THF, and slowly add 2.3g (10.0mmol) (R)-ferrocene The THF mixed solution of ethylamine was stirred for 3 hours to form an imine compound, which was directly poured into a high-pressure reaction axe, added with Pd / C, heated at 50°C, and hydrogenated at 2.2MP atmospheric pressure for 30 hours. After carefully venting excess hydrogen, filter off Pd / C, concentrate to remove THF, dilute with ethyl acetate, add 10ml of aqueous solution and stir for 30 minutes, extract three times with ethyl acetate, anhydrous Na 2 SO 4 Drying, concentration and removal of solvent, the crude product was purified by silica gel column chromatography to obtain 2.2g orange solid namely: (R)-5-[2-(N-ferroceneethylamine)propyl]-2-methoxy Benzenesulfonamide. The eluent is ethyl acetate:methanol (volume ratio)=5:2. Yield: 48.2%, m.p.92.5-95°C. (c=1.0, CH 3 OH).
[0059] (R)-5-[2-(N-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com