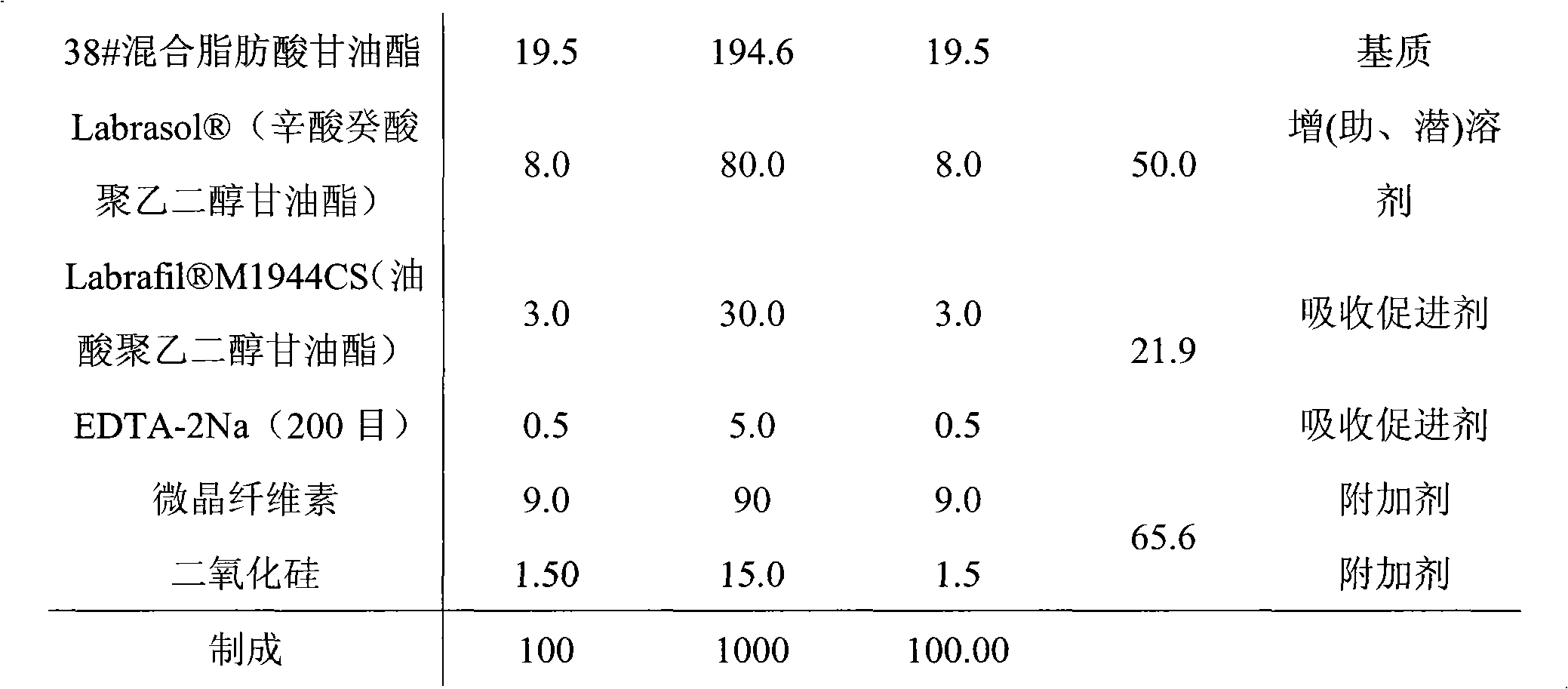Rectal administration composition containing tamsulosin
A technology of rectal administration and tamsulosin, applied in the application of urination disorder drugs, the field of preparation and treatment of urination disorders, can solve the problems that drugs are difficult to penetrate into prostatic fluid, difficult to achieve therapeutic effects, poor body fluid circulation, etc. High compliance, long duration of drug efficacy, and the effect of avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of suppository A
[0037] 1. Prescription A (amount of 100 suppositories)
[0038]
[0039]
[0040] 2. Preparation method of suppository A
[0041] 1) Take the main drug tamsulosin hydrochloride in prescription A and go through a 200-mesh sieve, and other solid materials through a 100-mesh sieve;
[0042] 2) The substrate is melted, filtered and sterilized at 45~60℃;
[0043] 3) Weigh the above-mentioned melted matrix of the prescription amount and heat it at 45~55℃, add the prescription amount of other excipients under stirring and mix well, continue to add the prescription amount of the main drug under stirring, and mix well under the insulation condition to obtain mixture;
[0044] 4) Injection molding of the above mixture at 40~45℃, and the prepared suppository is cooled at 5~20℃ for 30 minutes;
[0045] 5) Seal the obtained suppository, inspect, package and store the finished product.
Embodiment 2
[0046] Example 2 Preparation of suppository B
[0047] 1. Prescription B (amount of 100 suppositories)
[0048]
[0049] 2. Preparation method of suppository B
[0050] The same as in Example 1.
Embodiment 3
[0051] Example 3 Preparation of suppository C
[0052] 1. Prescription C (amount of 100 suppositories)
[0053]
[0054] 2. Preparation method of suppository C
[0055] The same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com