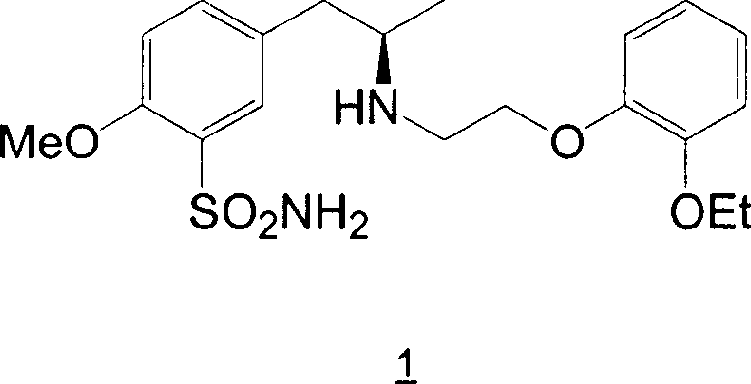
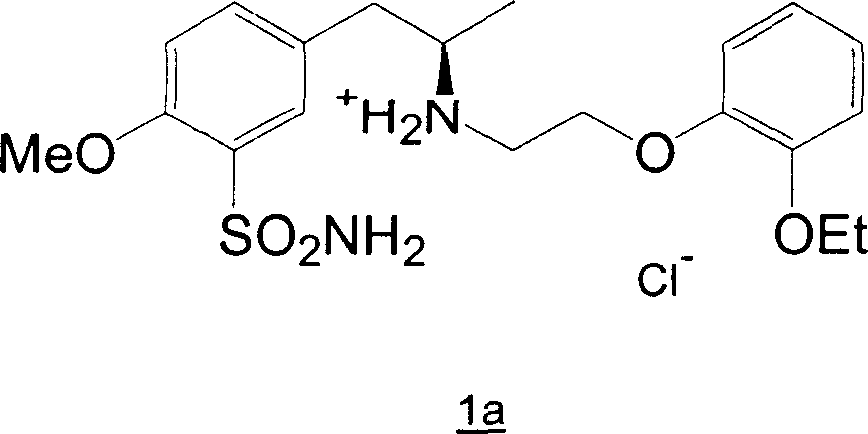
Preparation of r-5-(2-(2-ethoxyphenoxyethylamino)propyl)-2-methoxybenzenesulphonamide hydrochloride of high chemical purity
A technology of methoxybenzenesulfonamide and ethoxyphenoxy, which is applied in the field of chemical synthesis and can solve the problems such as the possibility of excess of compounds that are not discussed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The method for preparing tamsulosin of the present invention can provide a crude product with high purity and high yield. The product isolated directly from the reaction transformation may contain about 75%-90% tamsulosin hydrochloride. Surprisingly, it is found that the degree of occurrence of the expected over-alkylation is very limited, so in the tamsulosin hydrochloride crude product provided by the preparation method of the present invention, any over-alkylated product, such as N-(2-(2 -Ethoxyphenoxy)ethyl)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)-1-propyl)-2-methoxybenzenesulfonamide ( 5 ) or 5-(2-(di-(2-(2-ethoxyphenoxy)ethyl)amino)-1-propyl)-2-methoxybenzenesulfonamide ( 4 ) or the remaining 1-(2-bromoethoxy)-2-ethoxybenzene ( 3 ) content is not more than 6%.
[0023]
[0024] Tamsulosin hydrochloride can be obtained by treating tamsulosin base with HCl in ethanol.
[0025] The tamsulosin hydrochloride crude product of the present invention may contain no mo...
Embodiment 1
[0043] 10g (41mmol) 5-((R)-2-amino-1-propyl)-2-methoxybenzenesulfonamide, 19g (77mmol) 2-(2-ethoxyphenoxy)ethyl bromide and 170ml of methanol were heated to reflux for 43 hours. Methanol was evaporated under vacuum at 60 °C using a rotary evaporator. 170 ml of water and 130 ml of ethyl acetate were added to the residue, and while cooling and stirring, 16 g of a 50% aqueous sodium hydroxide solution were added. After separation of the two phases, the aqueous phase was extracted twice with 100 ml of ethyl acetate. The combined extracts were washed with 130 ml of water and evaporated in vacuo at 60°C using a rotary evaporator. The residue was dissolved in 100 ml of ethanol, and while cooling and stirring, 7 ml of ethanolic hydrogen chloride (300 mg HCl / ml) was added. Under cooling (0 °C), the mixture was stirred for 4 hours and the resulting crude (-)-(R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)- 1-Propyl)-2-methoxybenzenesulfonamide hydrochloride (TH), washed with 20 ml of cold ...
Embodiment 2
[0046] 200g (0.82mol) 5-((R)-2-amino-1-propyl)-2-methoxybenzenesulfonamide, 350g (1.43mol) 2-(2-ethoxyphenoxy) ethyl Bromide and 3.4 liters of methanol were heated at reflux for 45 hours. Methanol was evaporated under vacuum at 60 °C using a rotary evaporator. 3.4 liters of water and 2.6 liters of ethyl acetate were added to the residue, and while cooling and stirring, 650 g of a 50% aqueous sodium hydroxide solution was added. After separation of the two phases, the aqueous phase was extracted twice with 2 liters of ethyl acetate. The combined extracts were washed twice with 2.6 liters of water and evaporated in vacuo at 60°C using a rotary evaporator. The residue was dissolved in 2 liters of ethanol and 140 ml of ethanolic hydrogen chloride (300 mg HCl / ml) was added while cooling and stirring. Under cooling (0 °C), the mixture was stirred for 4 hours and the resulting crude (-)-(R)-5-(2-(2-(2-ethoxyphenoxy)ethylamino)- 1-Propyl)-2-methoxybenzenesulfonamide hydrochloride ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com