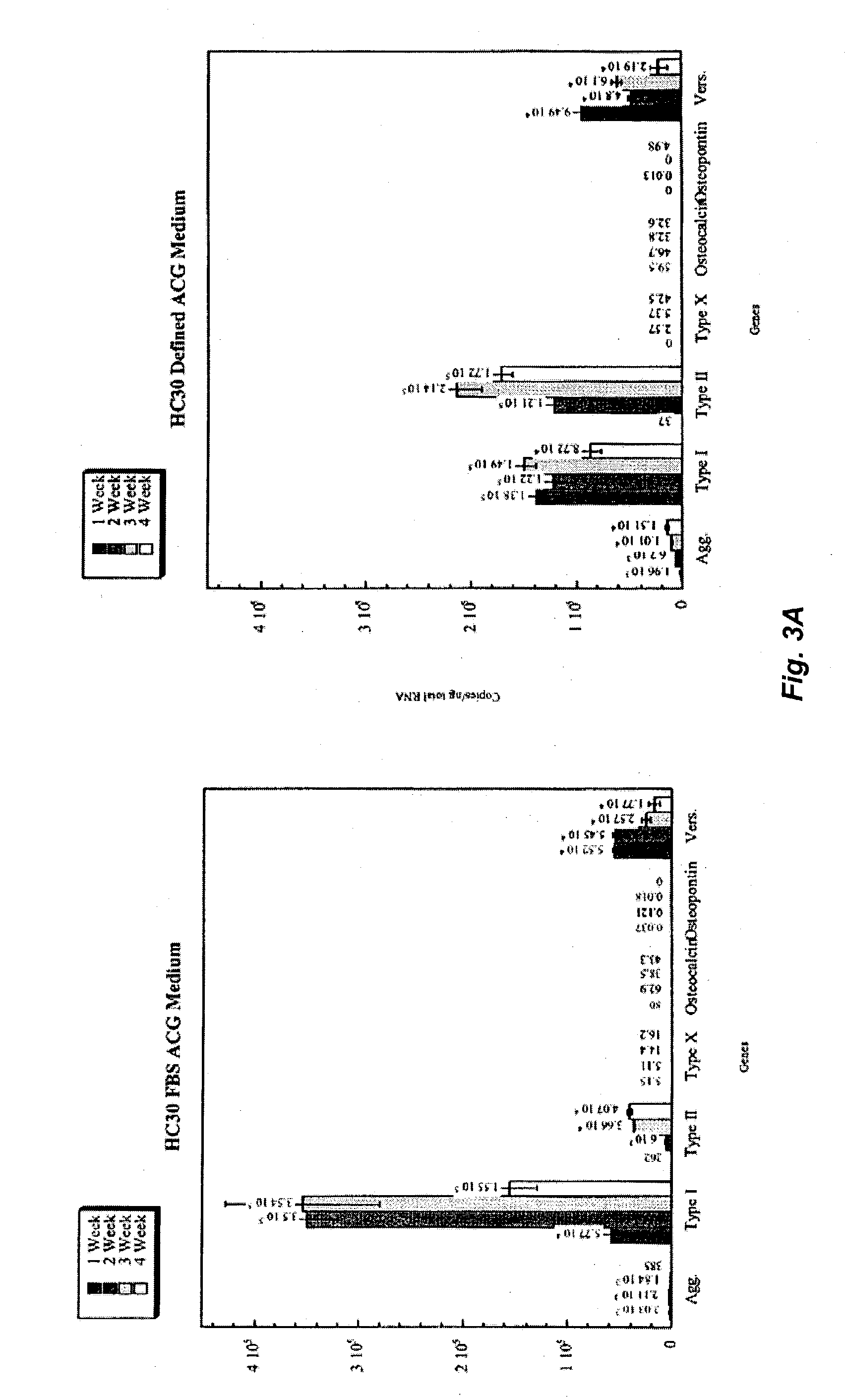
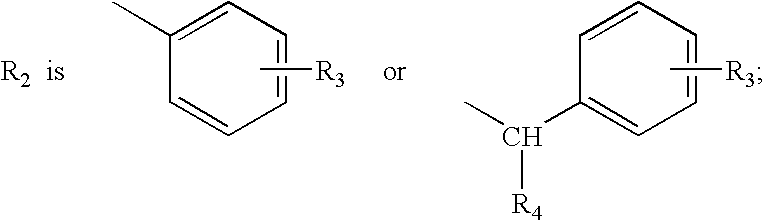
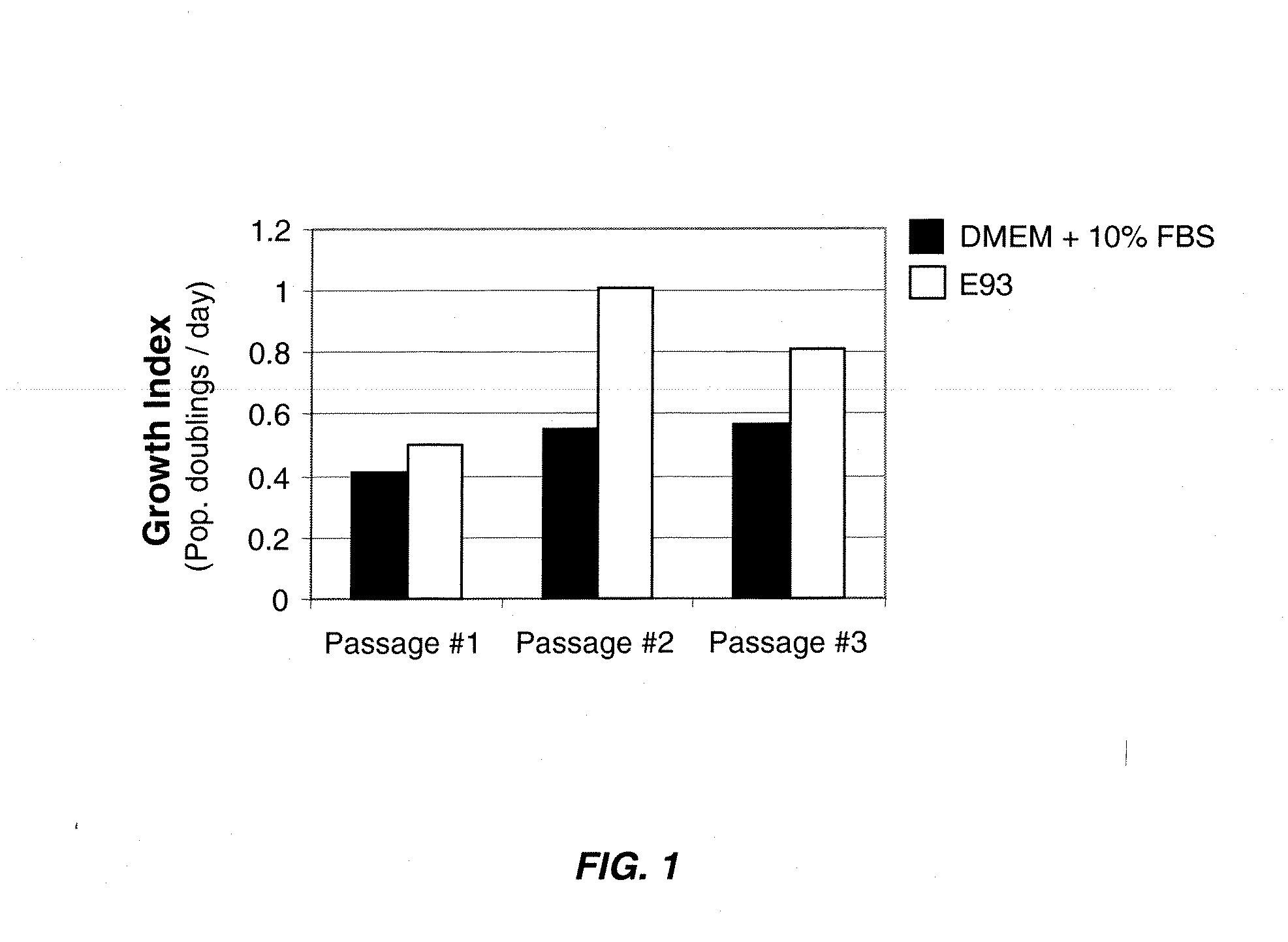
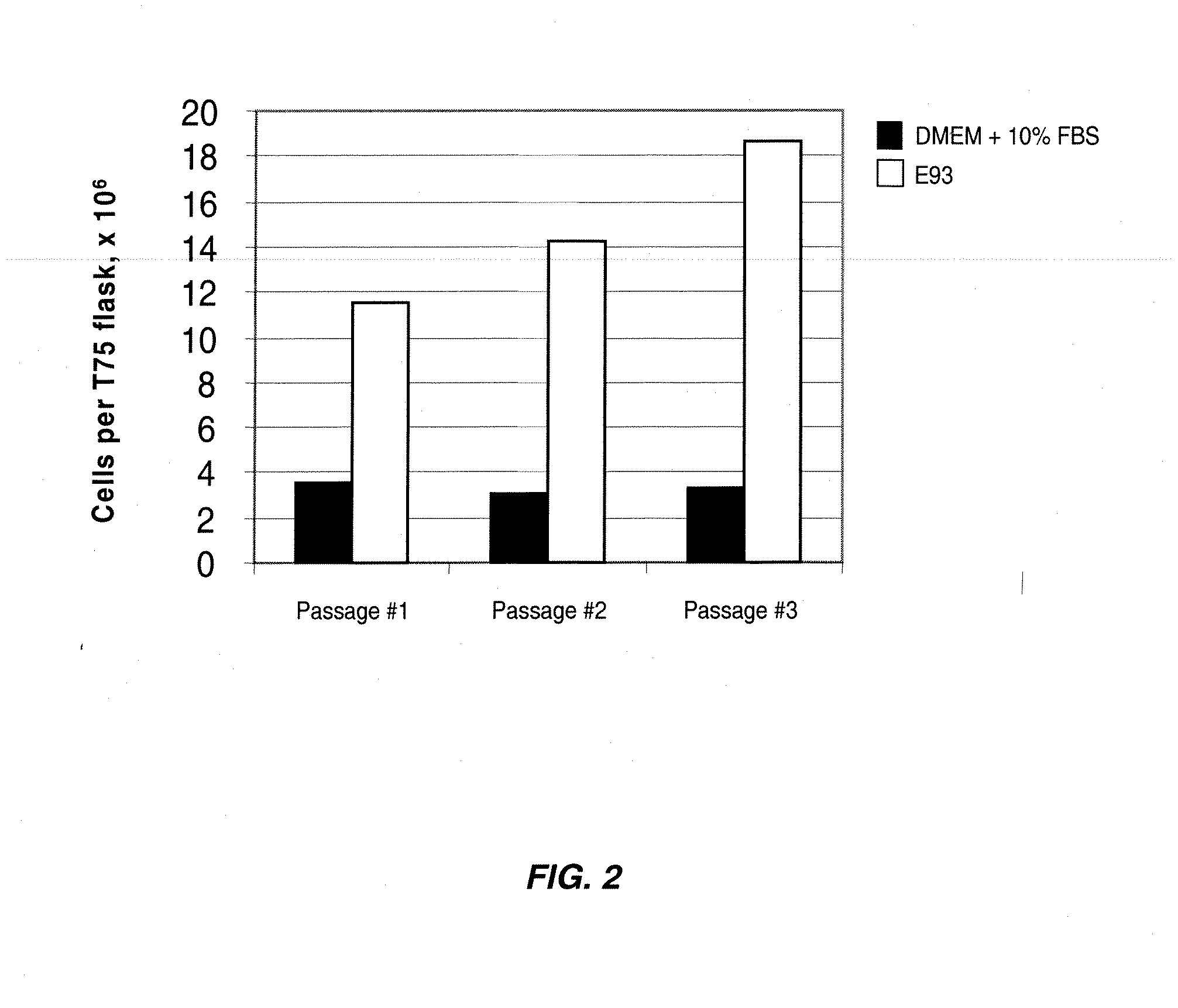
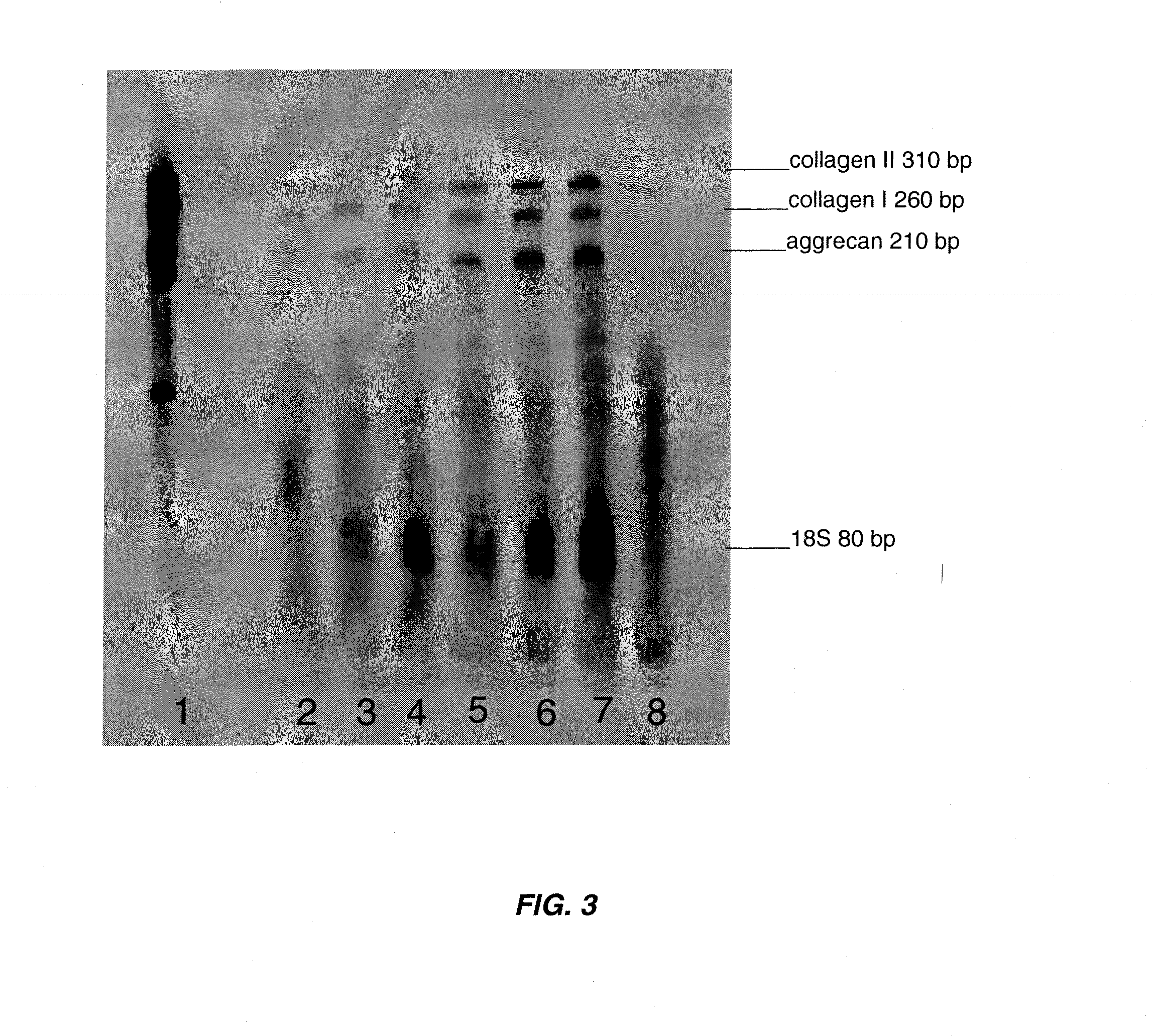
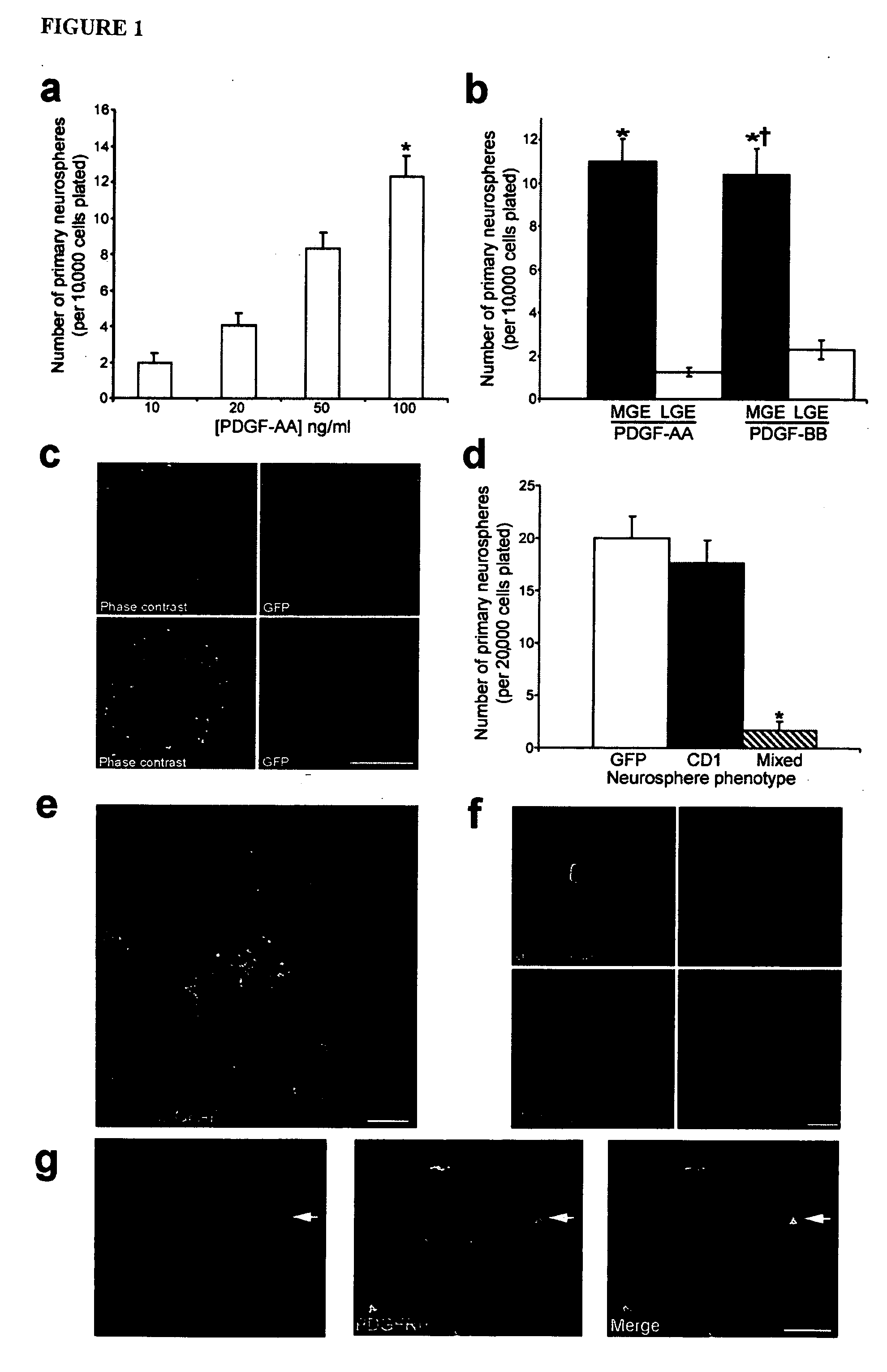
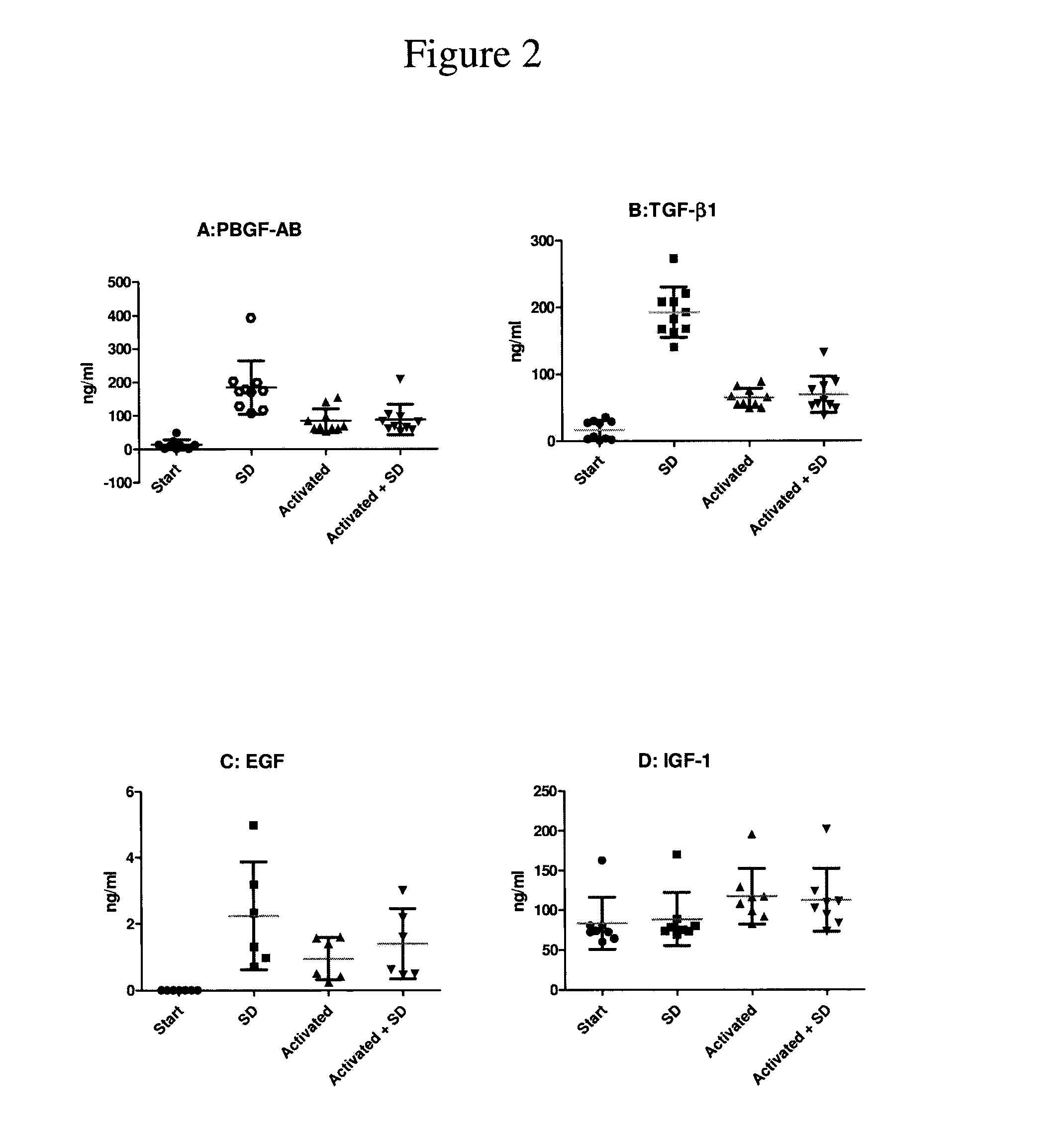
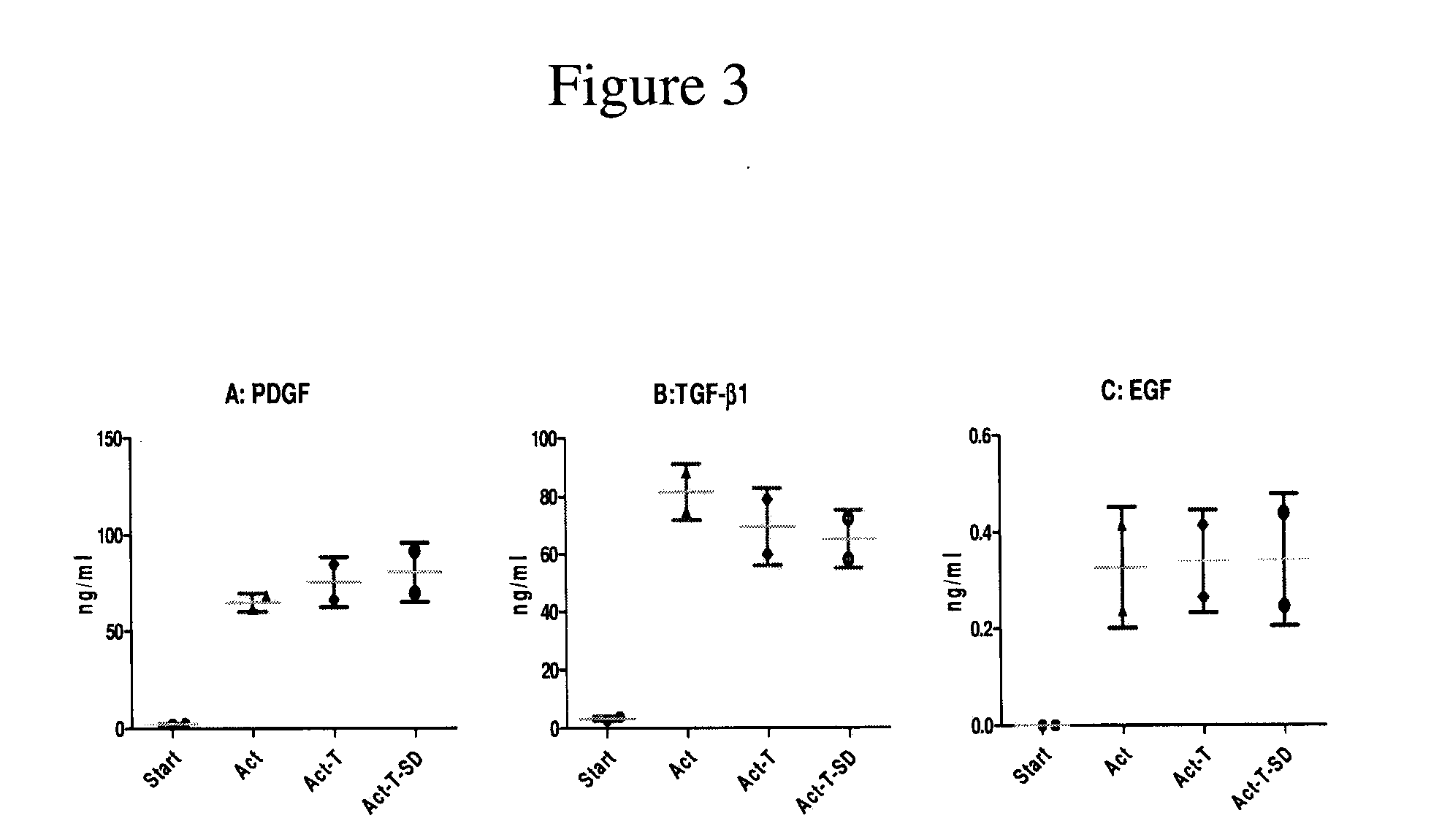
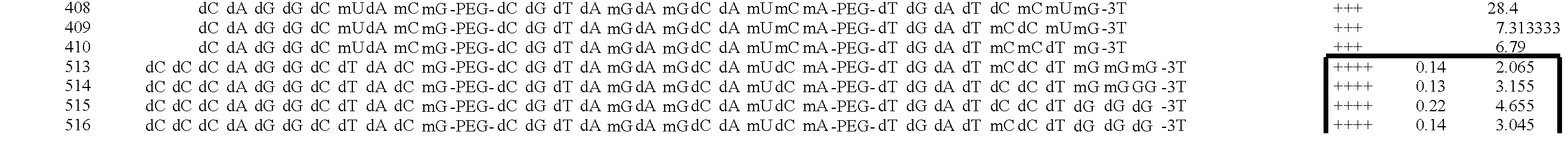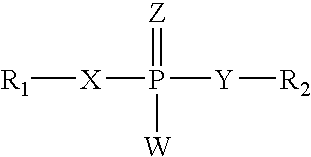Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
149 results about "Platelet-derived growth factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
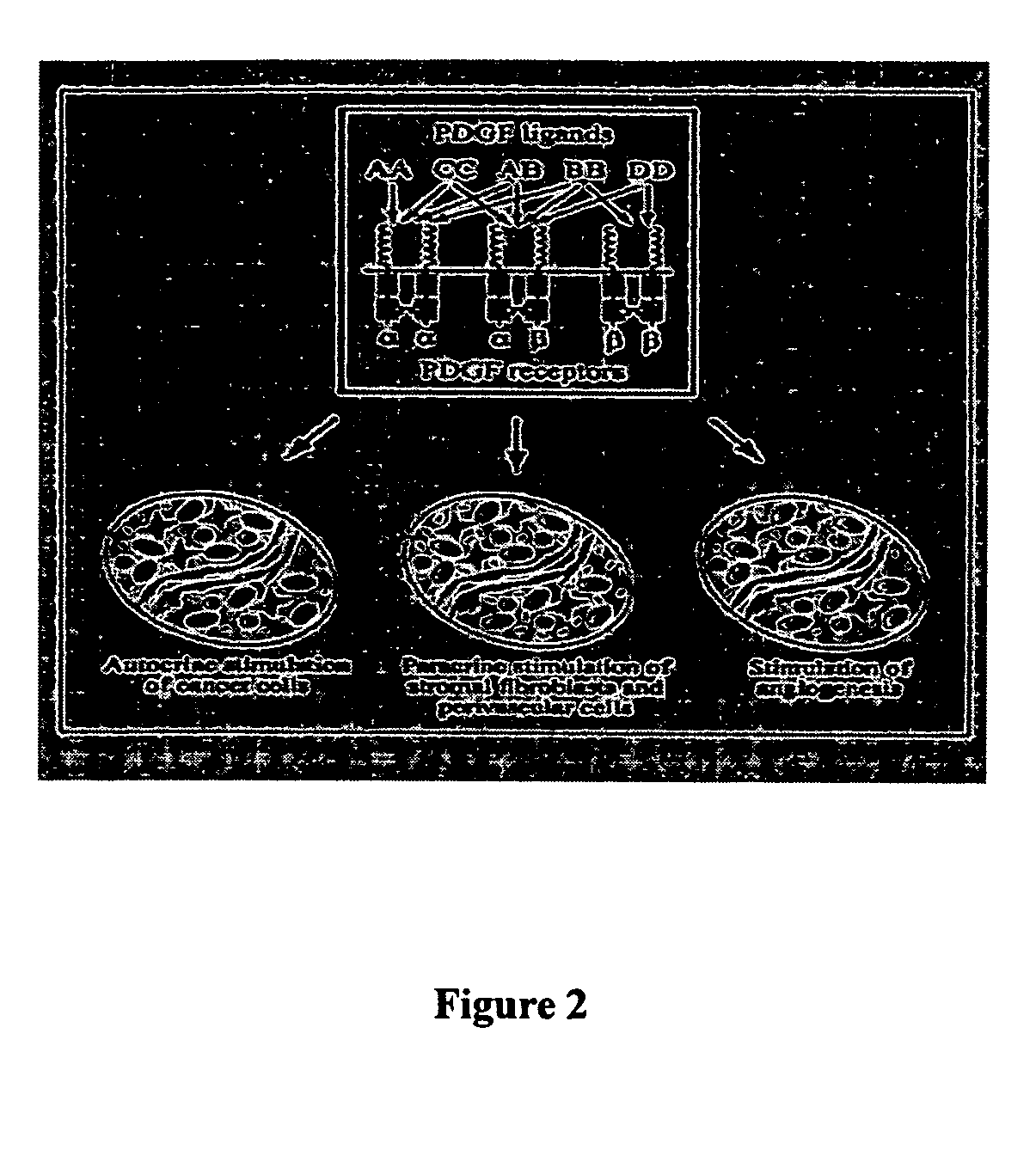
Platelet-derived growth factor (PDGF) is one among numerous growth factors that regulate cell growth and division. In particular, PDGF plays a significant role in blood vessel formation, the growth of blood vessels from already-existing blood vessel tissue, mitogenesis, i.e. proliferation, of mesenchymal cells such as fibroblasts, osteoblasts, tenocytes, vascular smooth muscle cells and mesenchymal stem cells as well as chemotaxis, the directed migration, of mesenchymal cells. Platelet-derived growth factor is a dimeric glycoprotein that can be composed of two A subunits (PDGF-AA), two B subunits (PDGF-BB), or one of each (PDGF-AB).
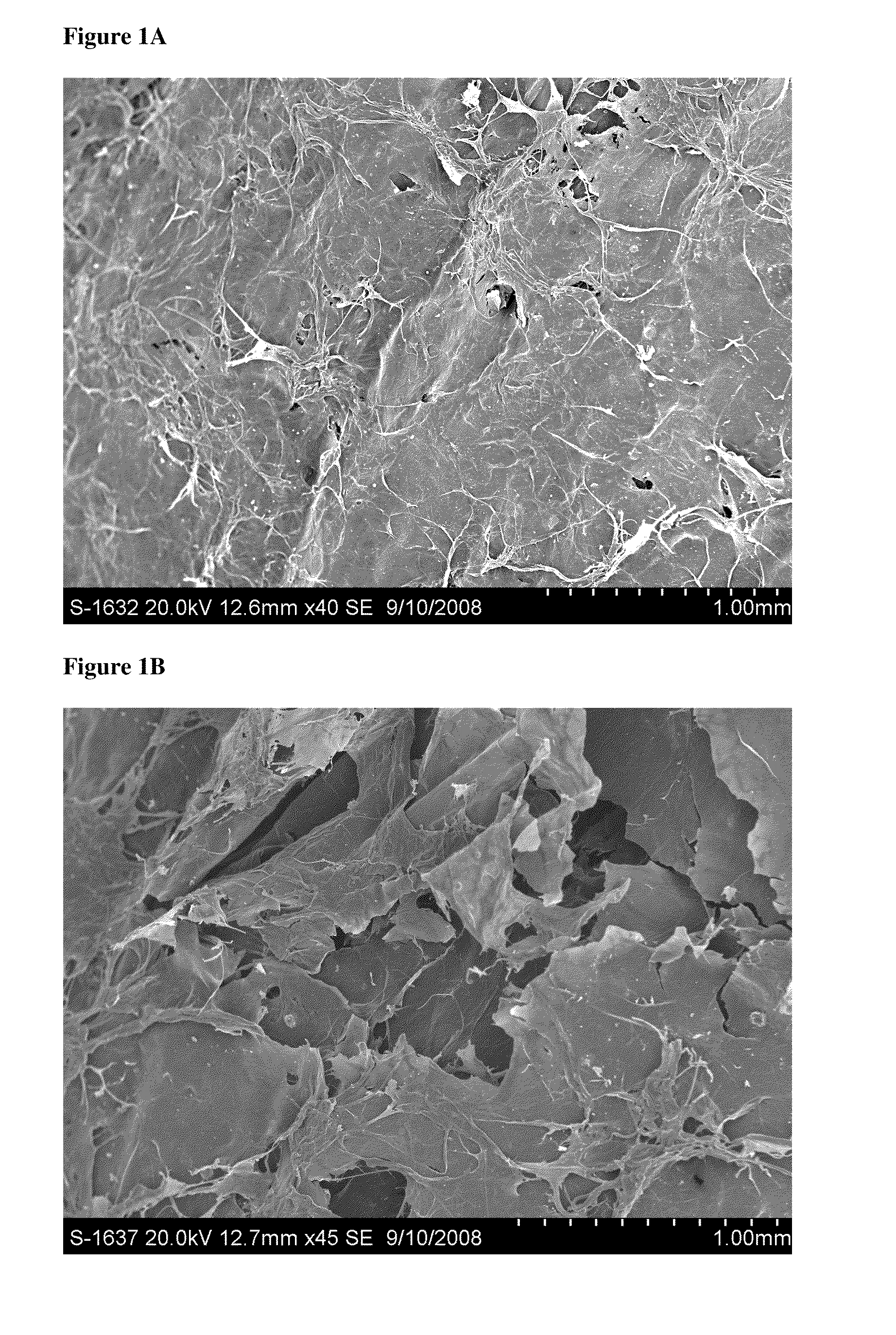
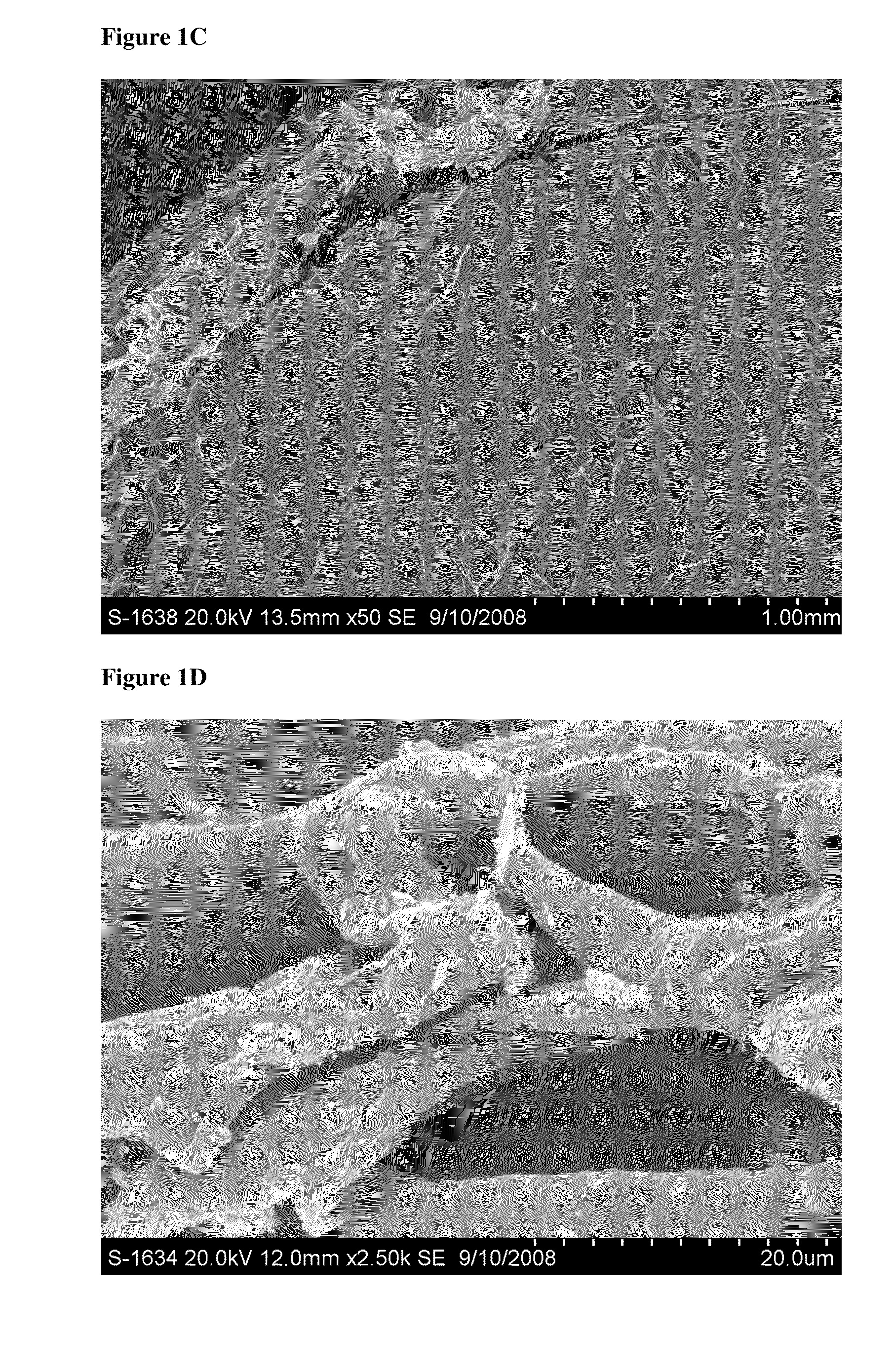
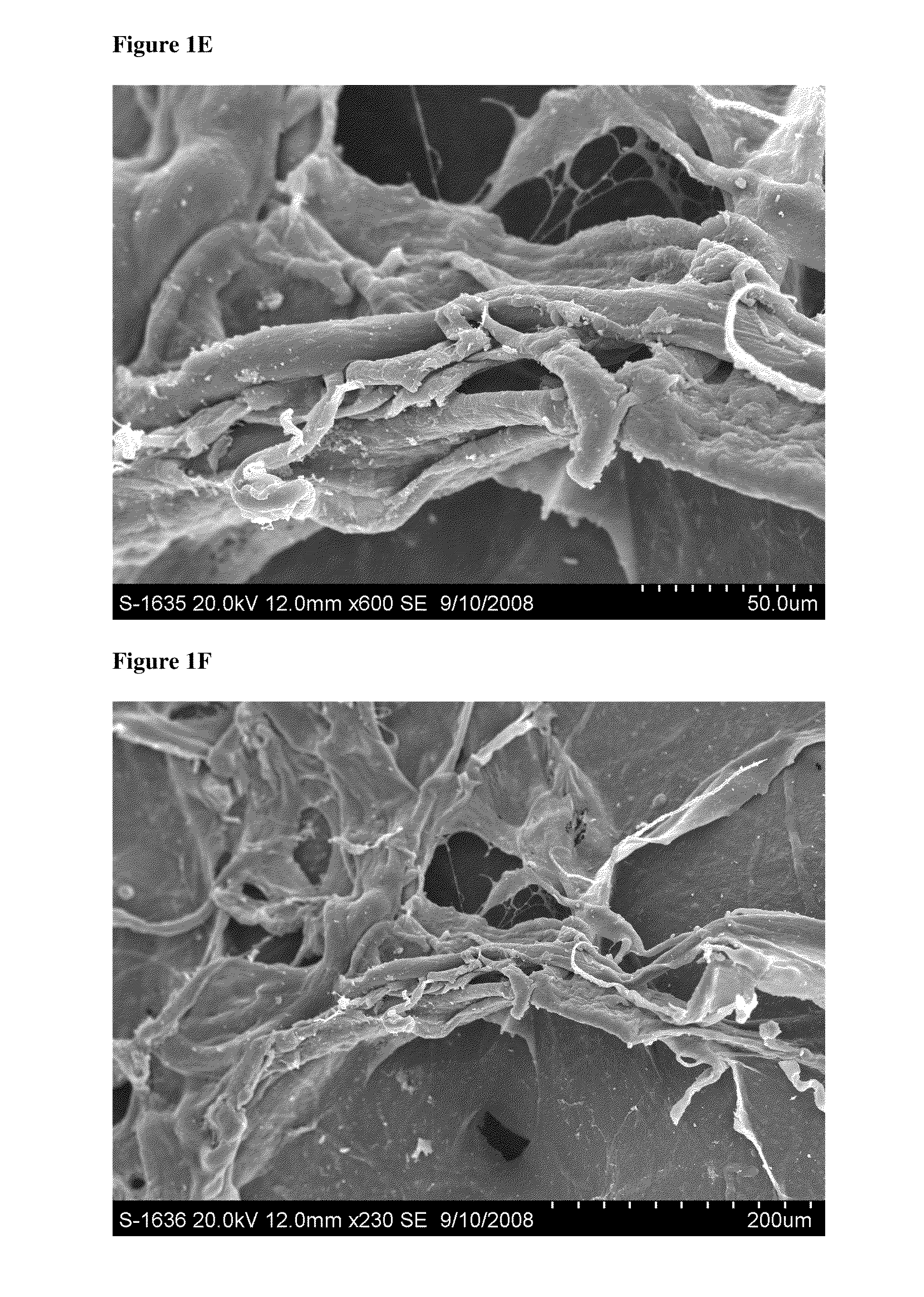
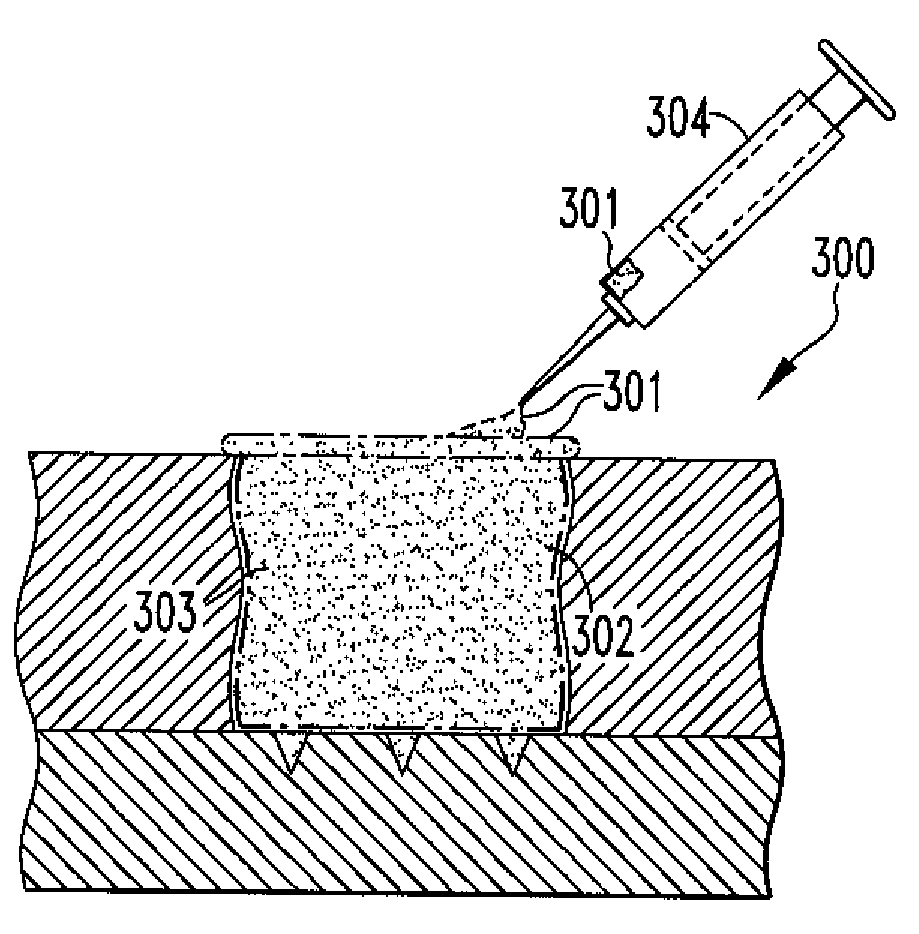
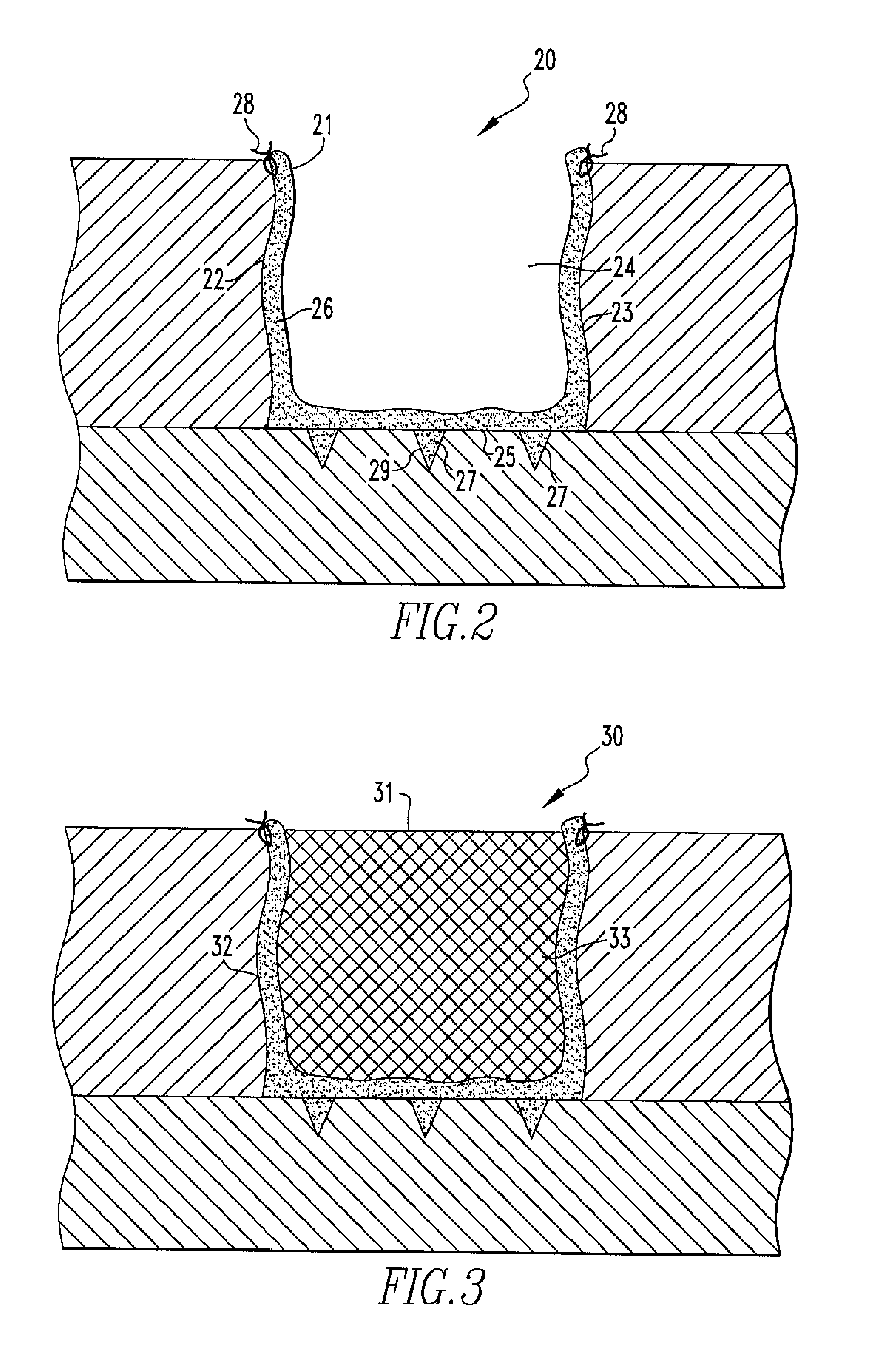
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Serum-free media for chondrocytes and methods of use thereof
InactiveUS7169610B2Safe effective inexpensiveSafe and effective and inexpensiveCulture processArtificial cell constructsLipid formationSerum free media
The present invention provides defined serum-free cell culture media useful in culturing fibroblasts, especially articular chondrocytes, that avoids problems inherent in the use of serum-containing media. The defined media comprise platelet-derived growth factor (PDGF), and chemically defined lipids, or combinations of these compounds. In another aspect, the present invention also provides tissue culture methods that comprise incubating chondrocytes in the defined serum free media. The methods enhance attachment and proliferative expansion of chondrocytes seeded at low density while maintaining their redifferentiation potential.
Owner:GENZYME CORP
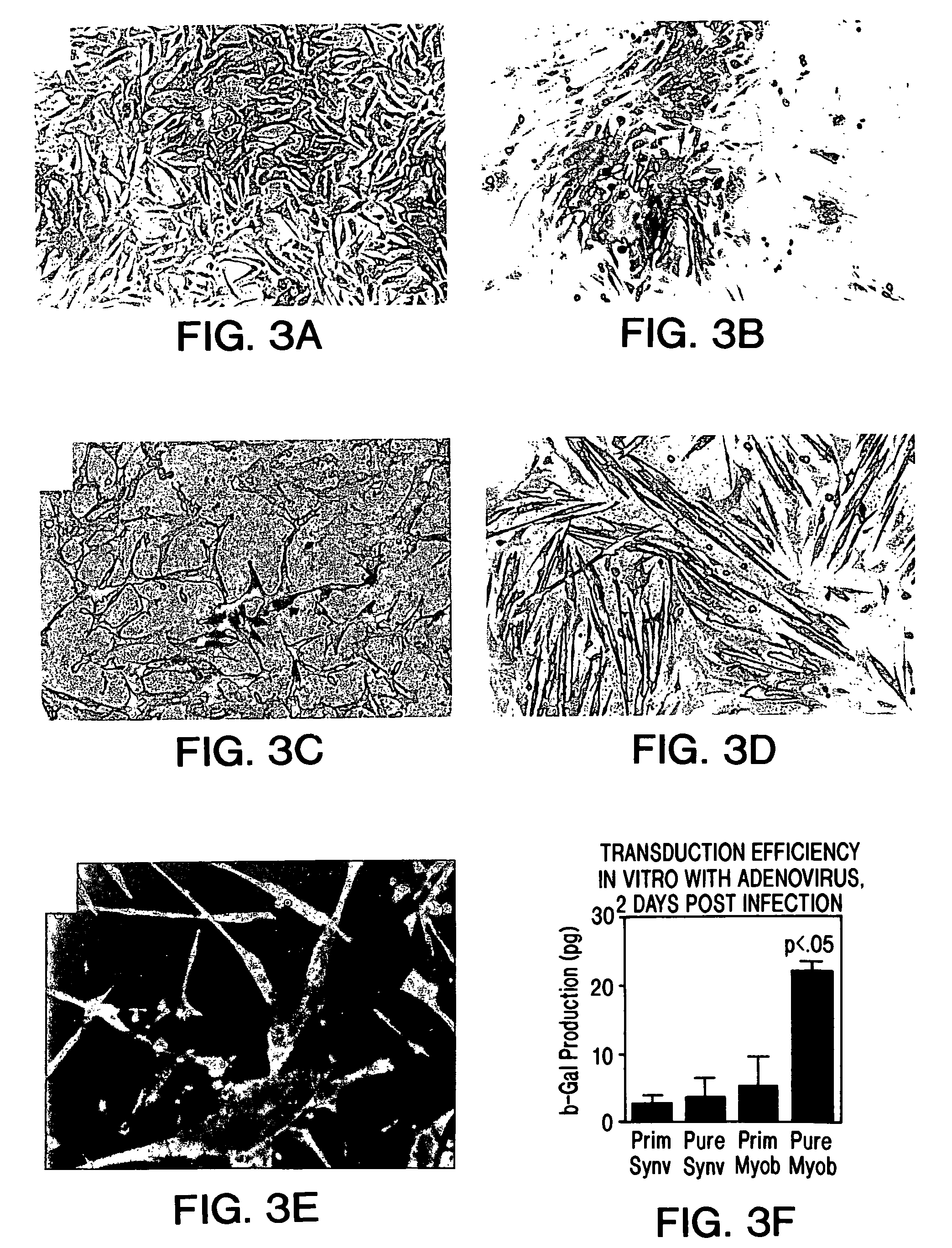
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
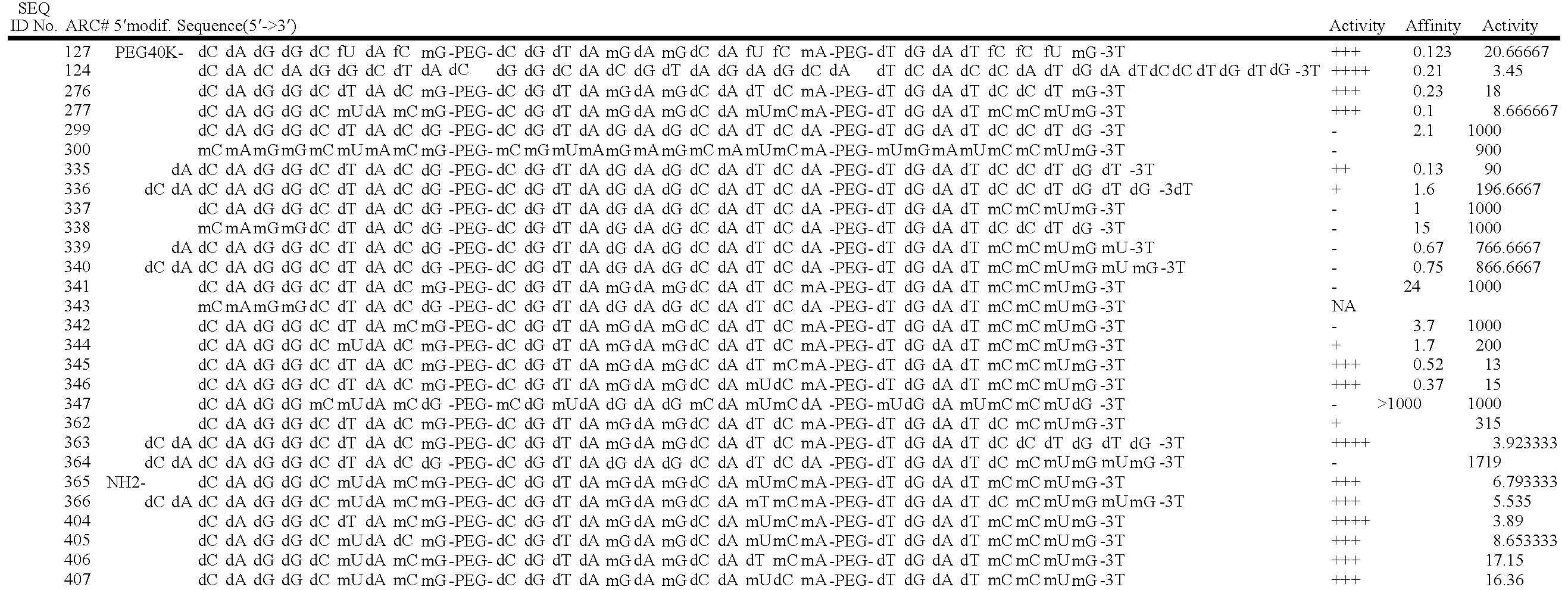
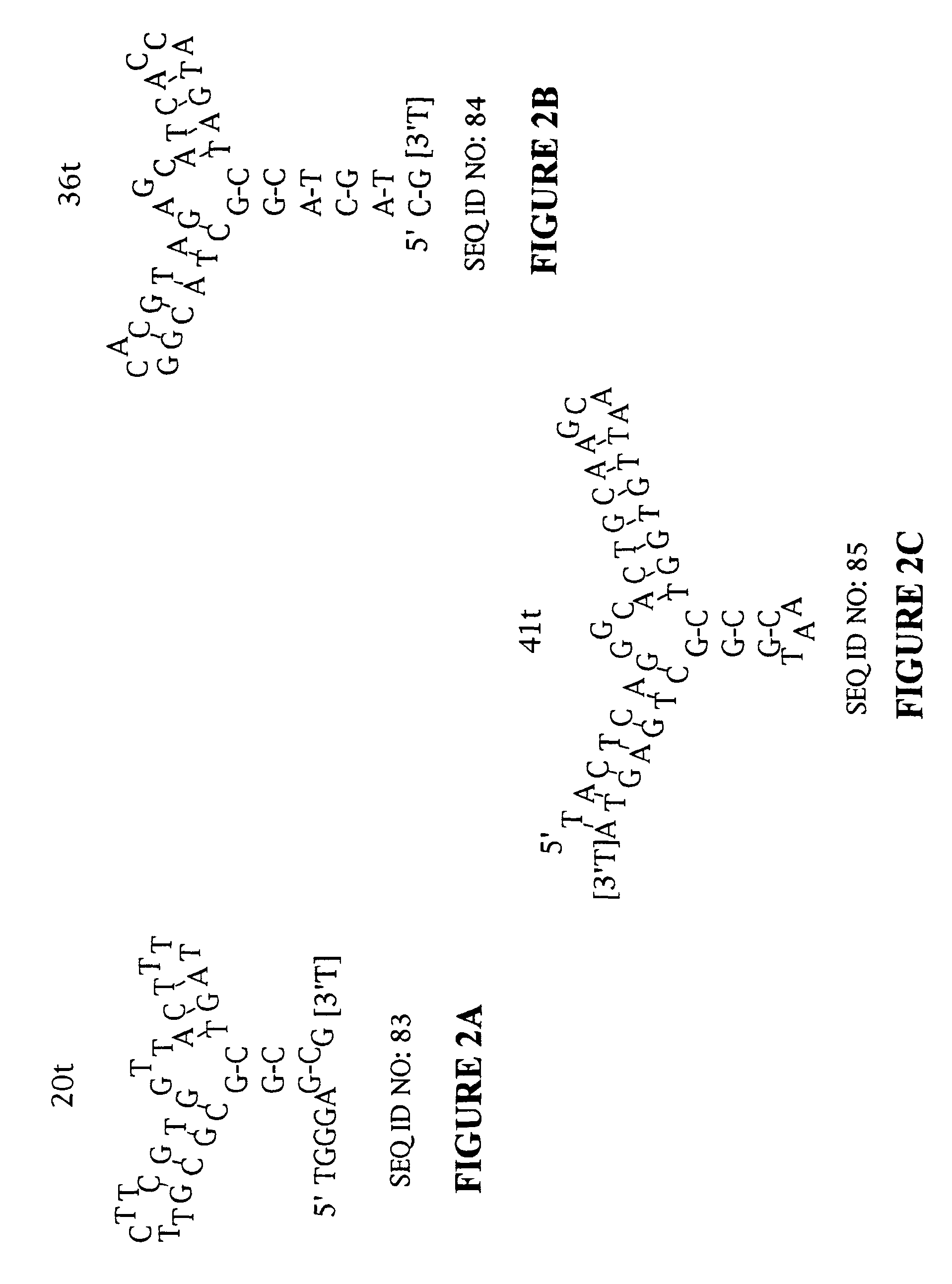
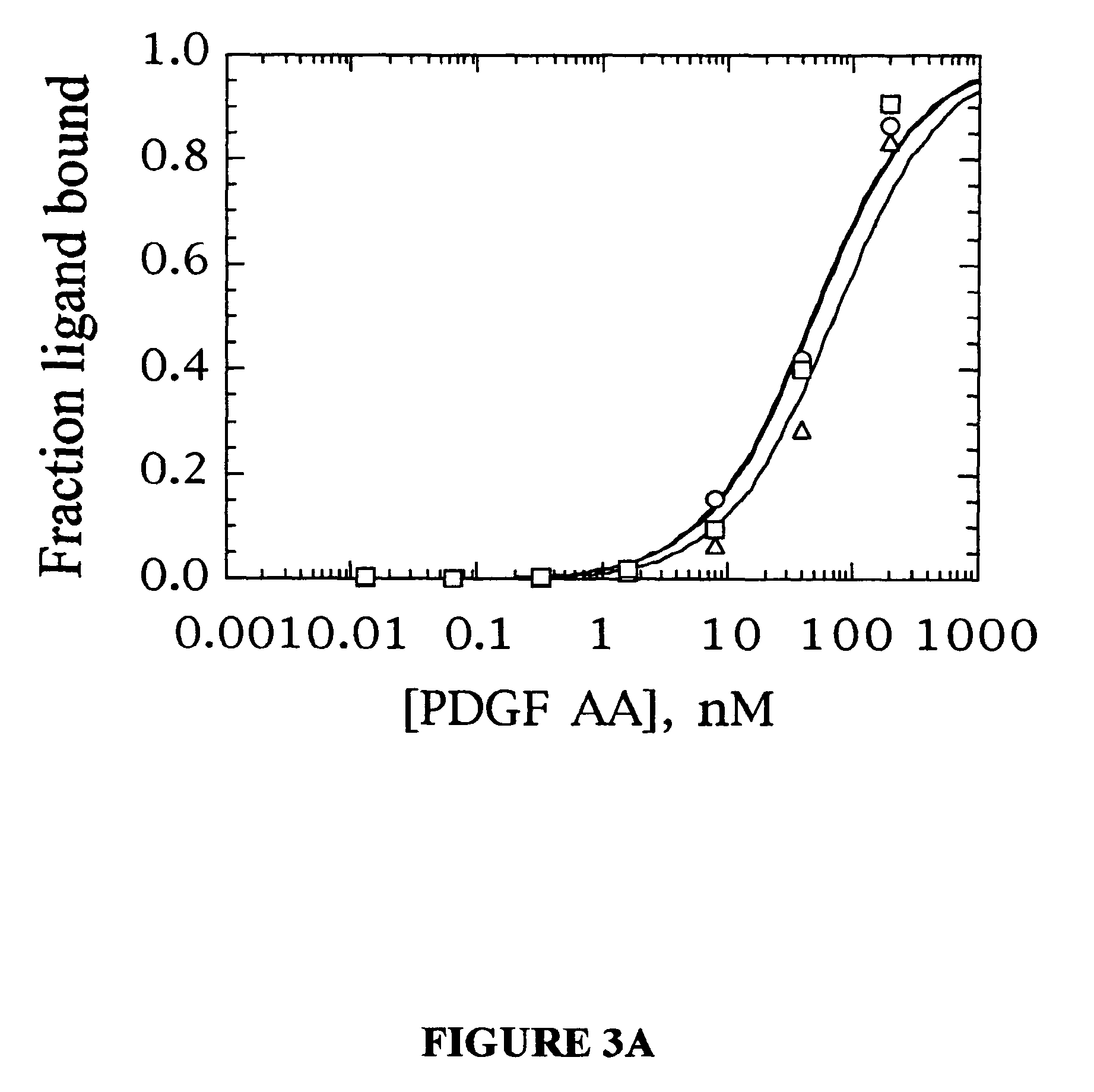
Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics
Materials and methods are provided for producing and using aptamers useful as oncology therapeutics capable of binding to PDGF, PDGF isoforms, PDGF receptor, VEGF, and VEGF receptor or any combination thereof with great affinity and specificity. The compositions of the present invention are particularly useful in solid tumor therapy and can be used alone or in combination with known cytotoxic agents for the treatment of solid tumors. Also disclosed are aptamers having one or more CpG motifs embedded therein or appended thereto.
Owner:ARCHEMIX CORP
Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics
Materials and methods are provided for producing and using aptamers useful as oncology therapeutics capable of binding to PDGF, PDGF isoforms, PDGF receptor, VEGF, and VEGF receptor or any combination thereof with great affinity and specificity. The compositions of the present invention are particularly useful in solid tumor therapy and can be used alone or in combination with known cytotoxic agents for the treatment of solid tumors. Also disclosed are aptamers having one or more CpG motifs embedded therein or appended thereto.
Owner:ARCHEMIX CORP
Platelet-derived growth factor compositions and methods for the treatment of osteochondral defects
InactiveUS20100247651A1Increasing cell cell growthIncrease the number of cellsPowder deliveryPeptide/protein ingredientsMedicinePlatelet
The present invention provides compositions and methods for treating an osteochondral defect. In one embodiment, provided is a composition for treating an osteochondral defect comprising a biphasic biocompatible matrix and platelet derived growth factor (PDGF), wherein the biphasic biocompatible matrix comprises a scaffolding material and wherein the scaffolding material forms a porous structure comprising an osseous phase and a cartilage phase. In another embodiment, also provided is a method for treating an osteochondral defect in an individual comprising administering to the individual an effective amount of a composition comprising a biphasic biocompatible matrix and PDGF to at least one site of the osteochondral defect, wherein the biphasic biocompatible matrix comprises a scaffolding material and wherein the scaffolding material forms a porous structure comprising an osseous phase and a cartilage phase.
Owner:BIOMIMETIC THERAPEUTICS INC
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
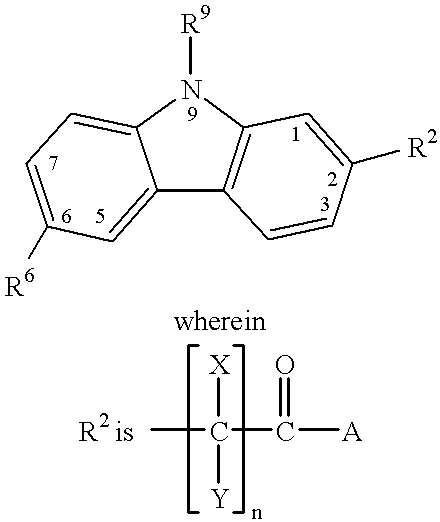
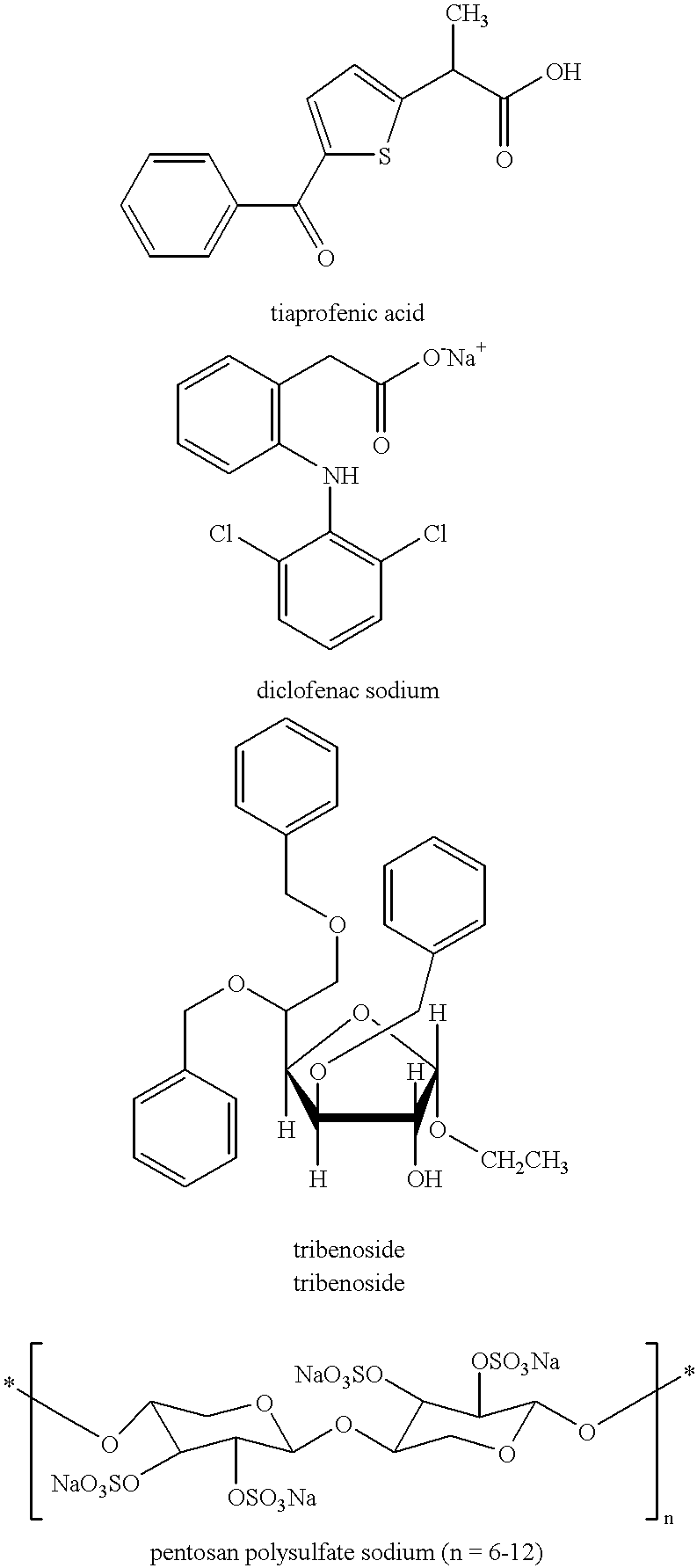
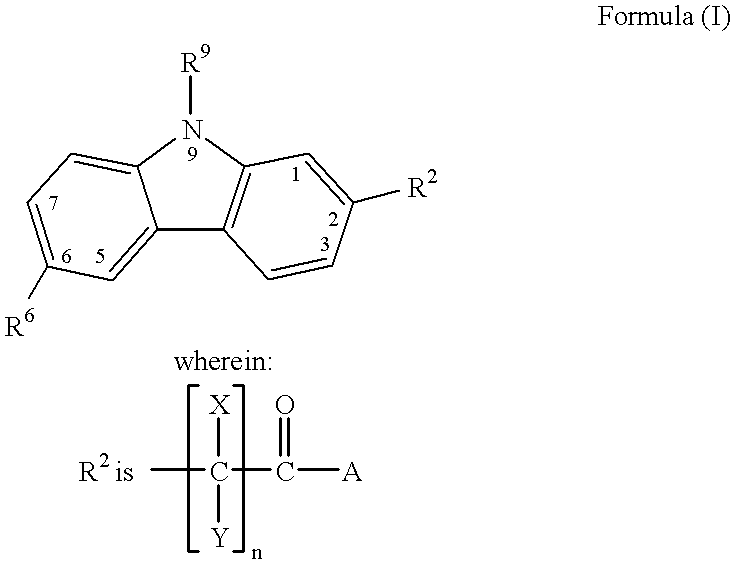
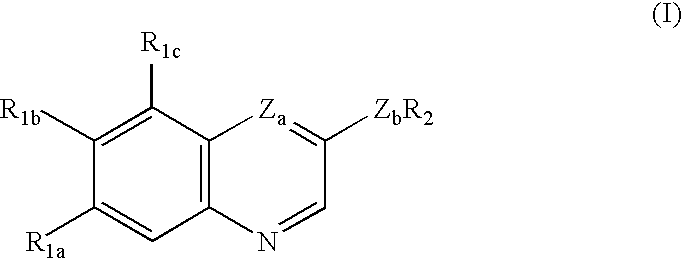
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
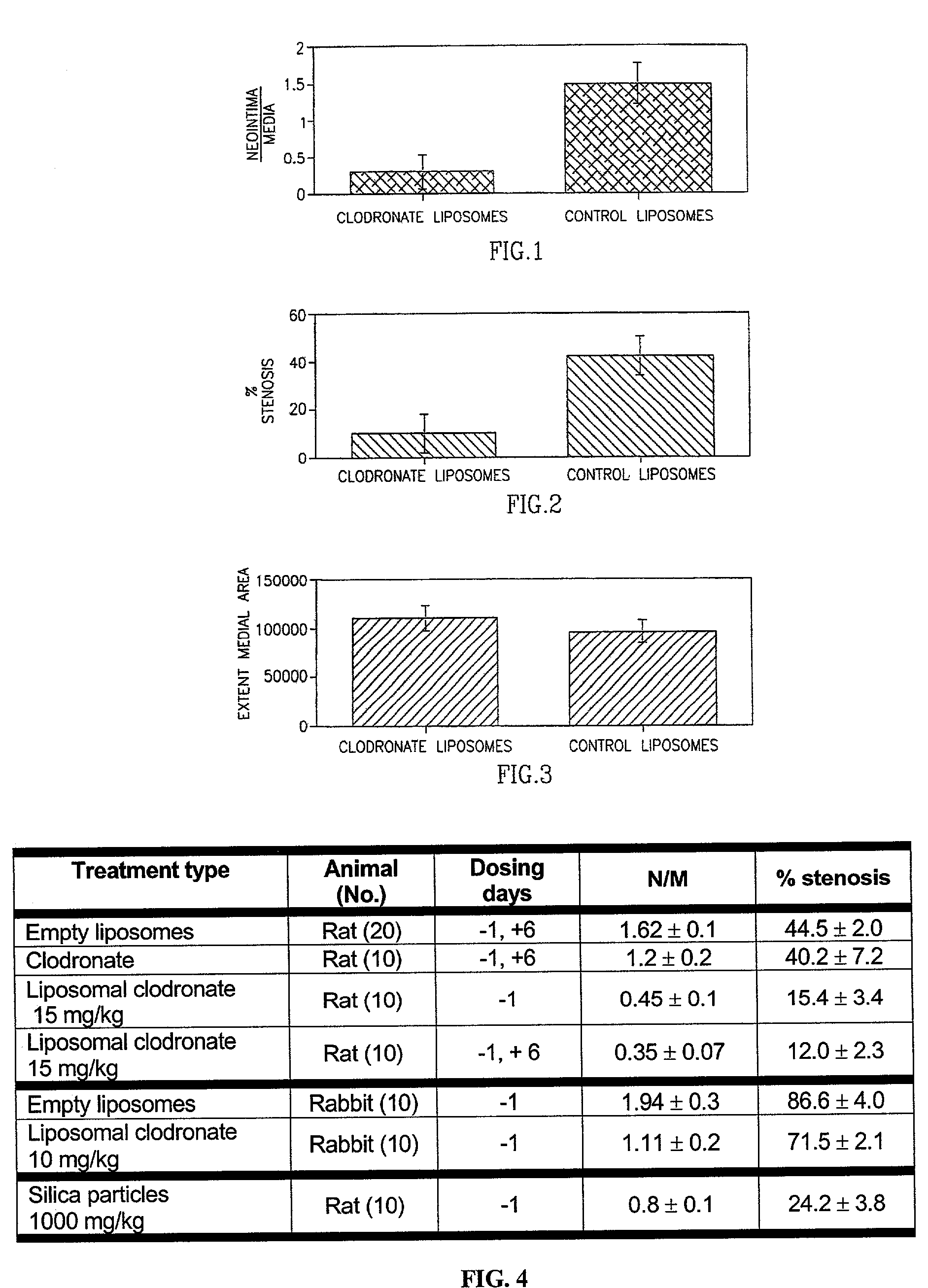
Method of inhibiting restenosis using bisphosphonates
InactiveUS7008645B2Affect activityEfficient transportPowder deliveryBiocidePlatelet-Derived Growth Factor BetaParticulates
A method of inhibiting the activity or production of cytokines or growth factors associated with vascular restenosis, by administering to an individual an effective amount of an active ingredient comprising a bisphosphonate particle or a bisphosphonate particulate. The bisphosphonate may be encapsulated, embedded or adsorbed within the particle, dispersed uniformly in the polymer matrix, adsorbed on the particle surface, or in combination of any of these forms. The particles include liposomes or inert polymeric particles, such as microcapsules, nanocapsules, nanoparticles, nanospheres, or microparticles. The particulates include any suspended or dispersed form of the bisphosphonate which is not encapsulated, entrapped, or adsorbed within a polymeric particle. The particulates include suspended or dispersed colloids, aggregates, flocculates, insoluble salts and insoluble complexes of the active ingredient. The cytokines and growth factors include, but are not limited to interleukin 1-β, matrix metalloproteinase-2, and platelet-derived growth factor β (PDGFβ).
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
Preparation method of essence containing human mesenchymal stem cell factors
InactiveCN106344493APromote growthPromote proliferationCosmetic preparationsToilet preparationsFreeze-dryingVascular endothelium
The invention discloses a preparation method of an essence containing human mesenchymal stem cell factors. The preparation method of the essence containing the human mesenchymal stem cell factors comprises the steps: preparation of freeze-dried powder of the human mesenchymal stem cell factors, preparation of a solvent and mixing. The preparation method obtains a high-purity and high-activity stem cell active factor concentrated solution by a cryoconcentration technique and the concentrated solution is freeze-dried to obtainfreeze-dried powder at low temperature in vacuum, so that the factor activity can be kept for a long time; and the solvent contains multiple skin nourishing and moisturizing components and a stem cell lysis solution, can well dissolve the cell factor freeze-dried powder and keeps the cell factor activity. The stem cell essence disclosed by the invention contains vascular endothelial cell growth factors, platelet derived growth factors, epidermal growth factors and other components, can promote skin regeneration, can play roles in moisturizing, tendering, whitening and repairing the skin, and has good application prospects in fields of medical beauty treatment, health protection and the like.
Owner:GENESIS STEMCELL REGENERATIVE MEDICINE ENG CO LTD
Quinoline and quinoxaline compounds which inhibit platelet-derived growth factor and/or p56lck tyrosine kinases
This invention is directed to quinoline / quinoxaline compounds which inhibit platelet-derived growth factor tyrosine kinase and / or Lck tyrosine kinase, to pharmaceutical compositions comprising these compounds, and to the use of these compounds for treating a patient suffering from or subject to disorders / conditions involving cellular differentiation, proliferation, extracellular matrix production or mediator release and / or T cell activation and proliferation.
Owner:AVENTIS PHARMA INC
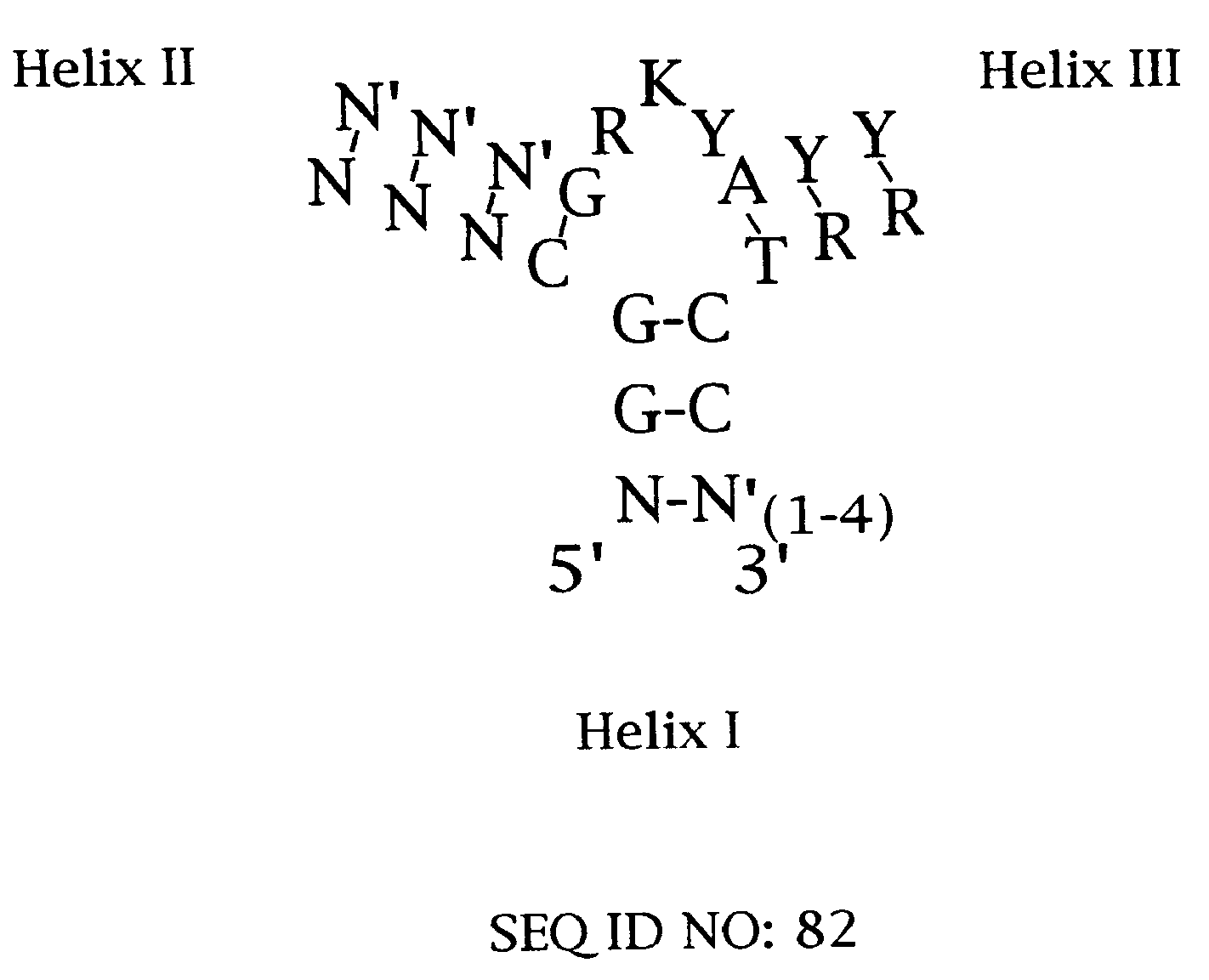
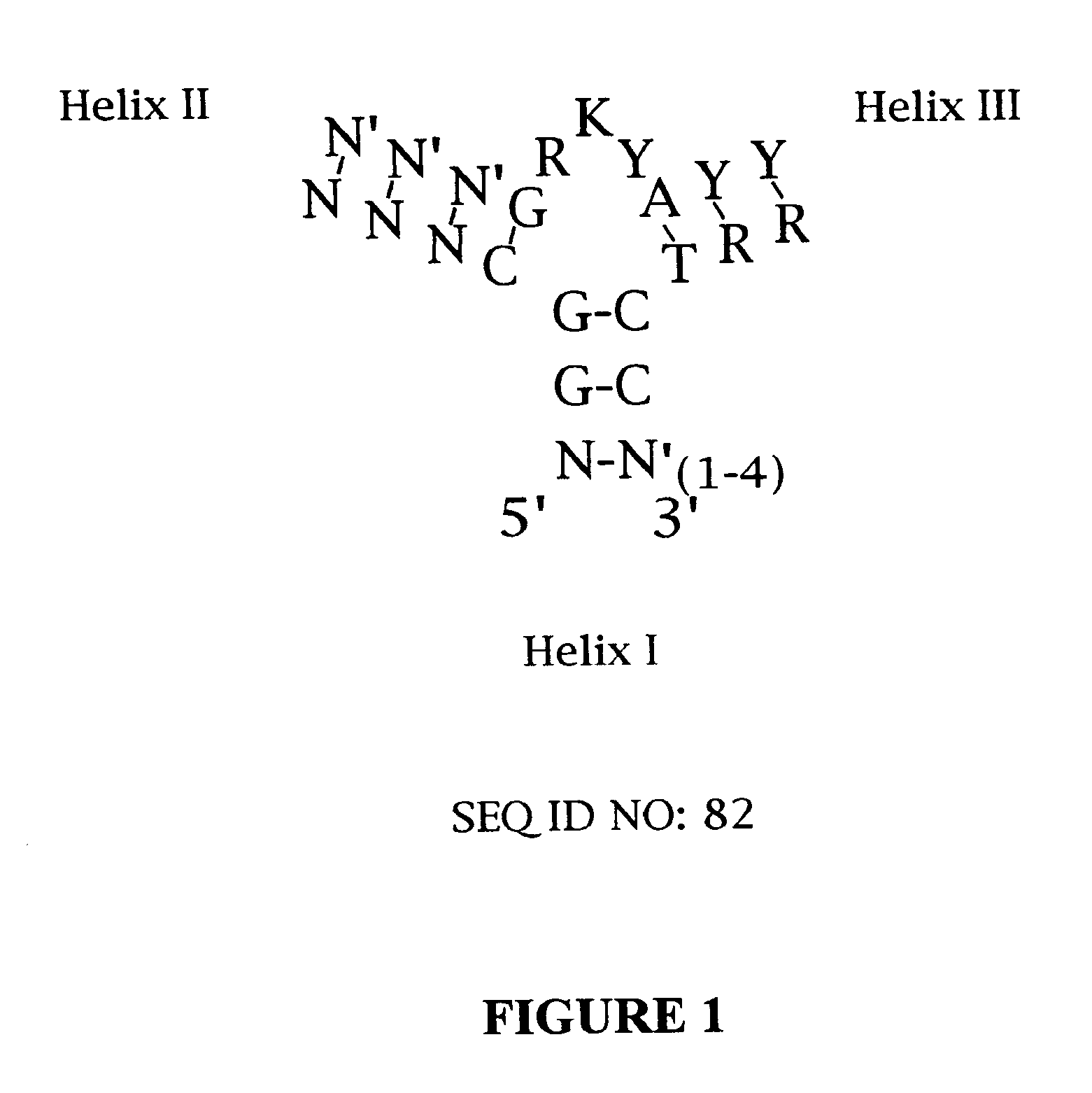
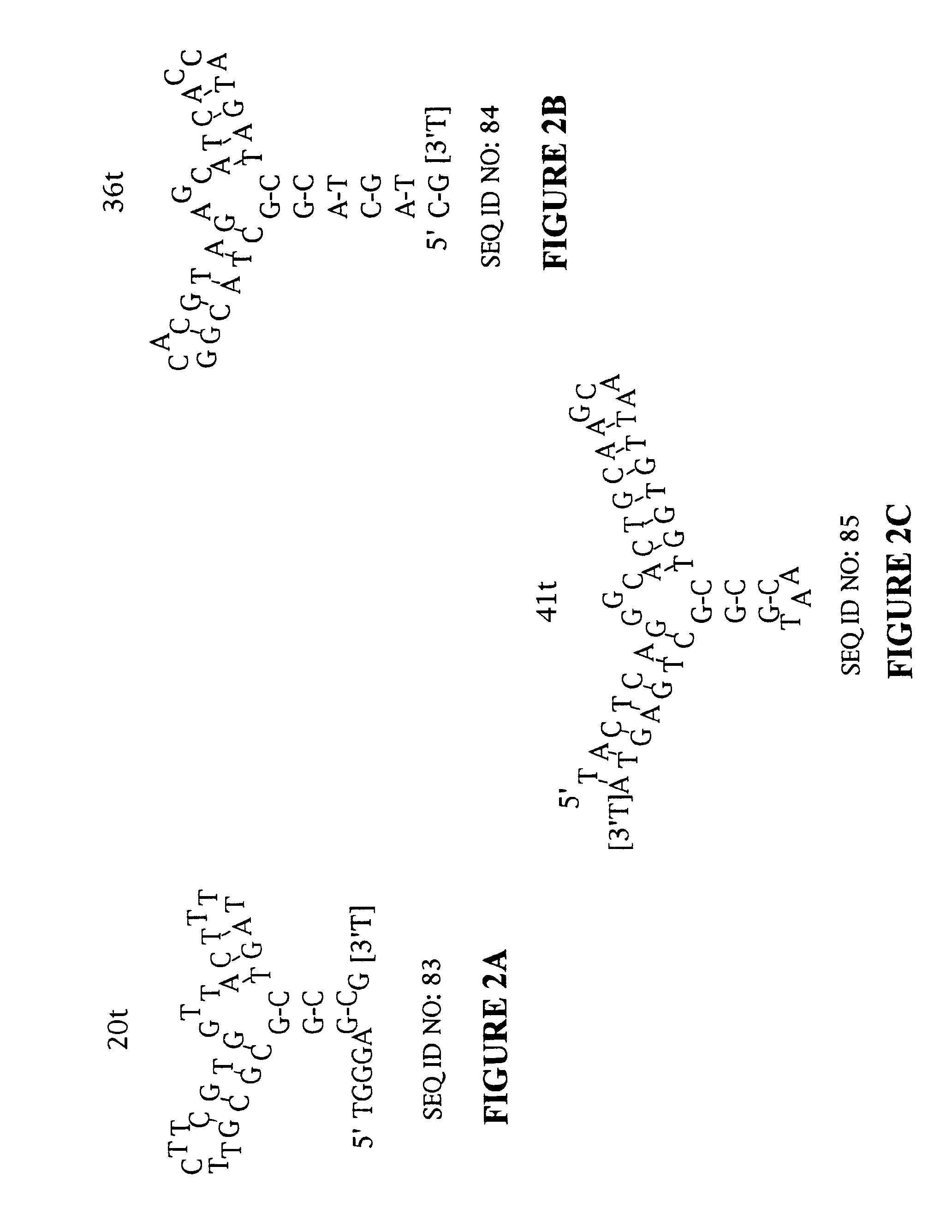
Designed ankyrin repeat proteins binding to platelet-derived growth factor
New designed ankyrin repeat proteins with binding specificity for PDGF-BB are described, as well as nucleic acids encoding such PDGF binding proteins, pharmaceutical compositions comprising such proteins and the use of such proteins in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
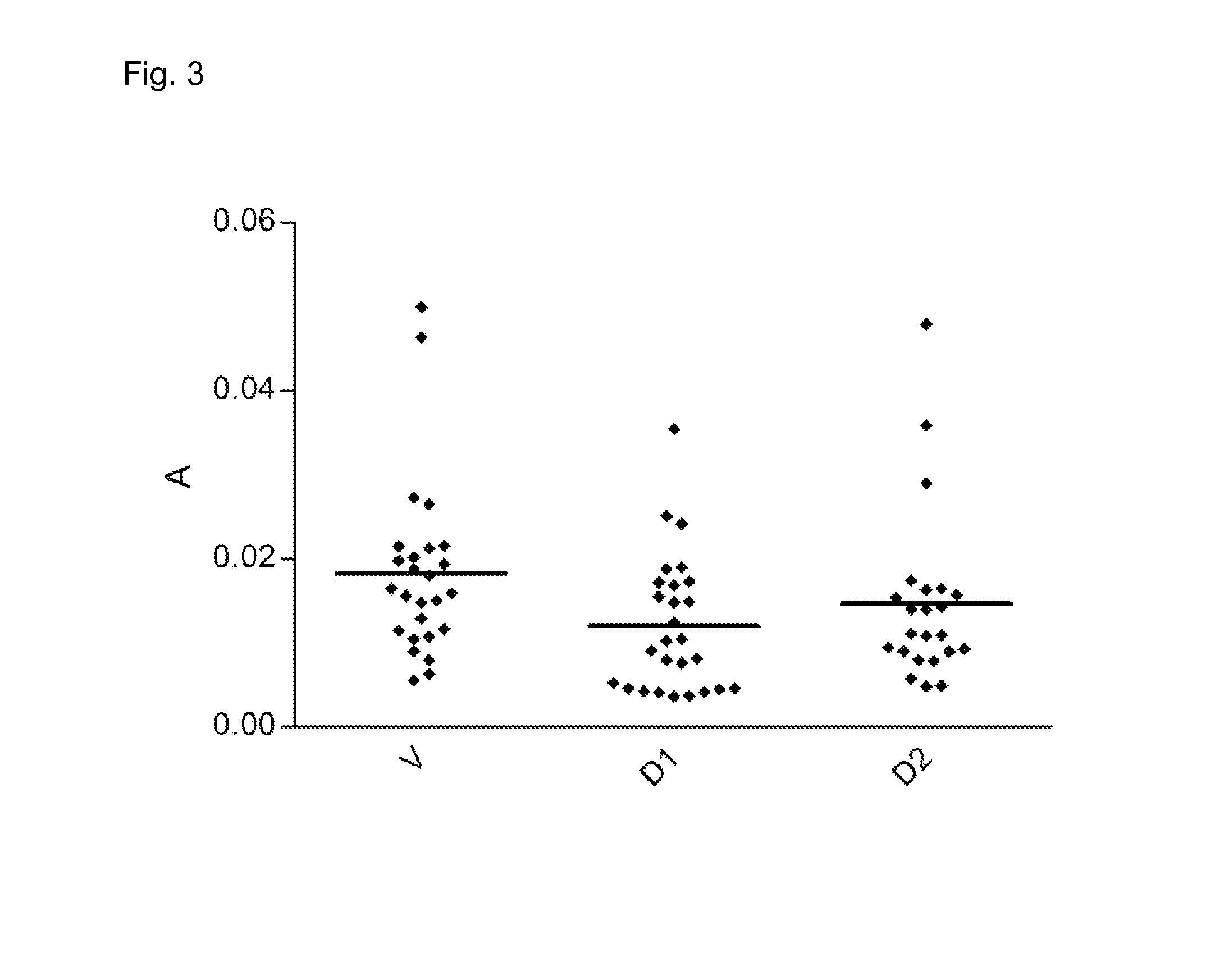
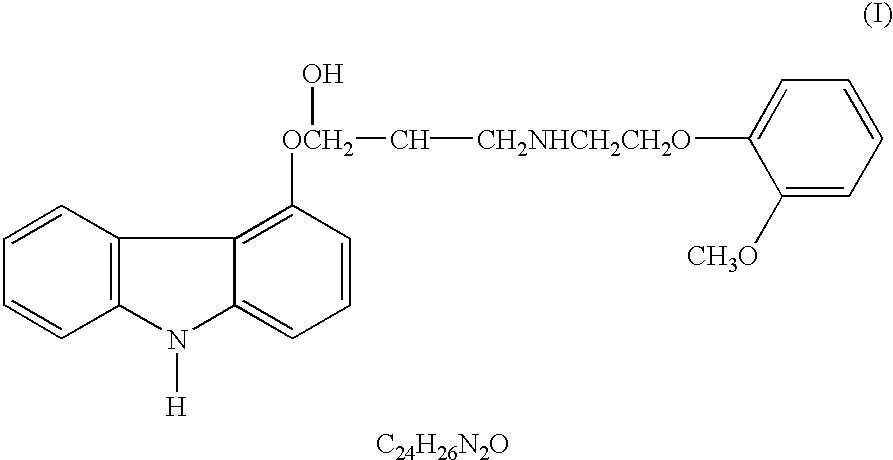
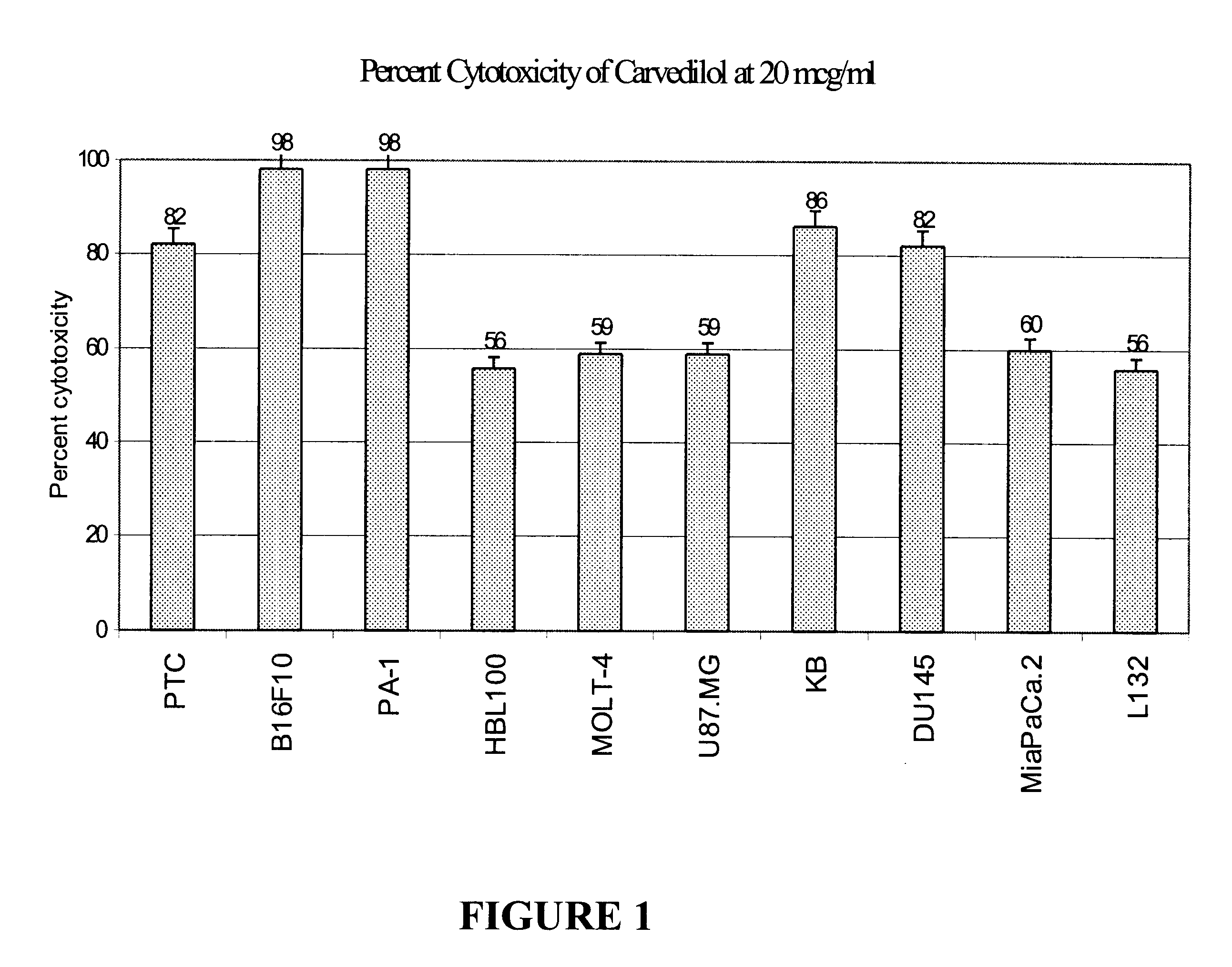
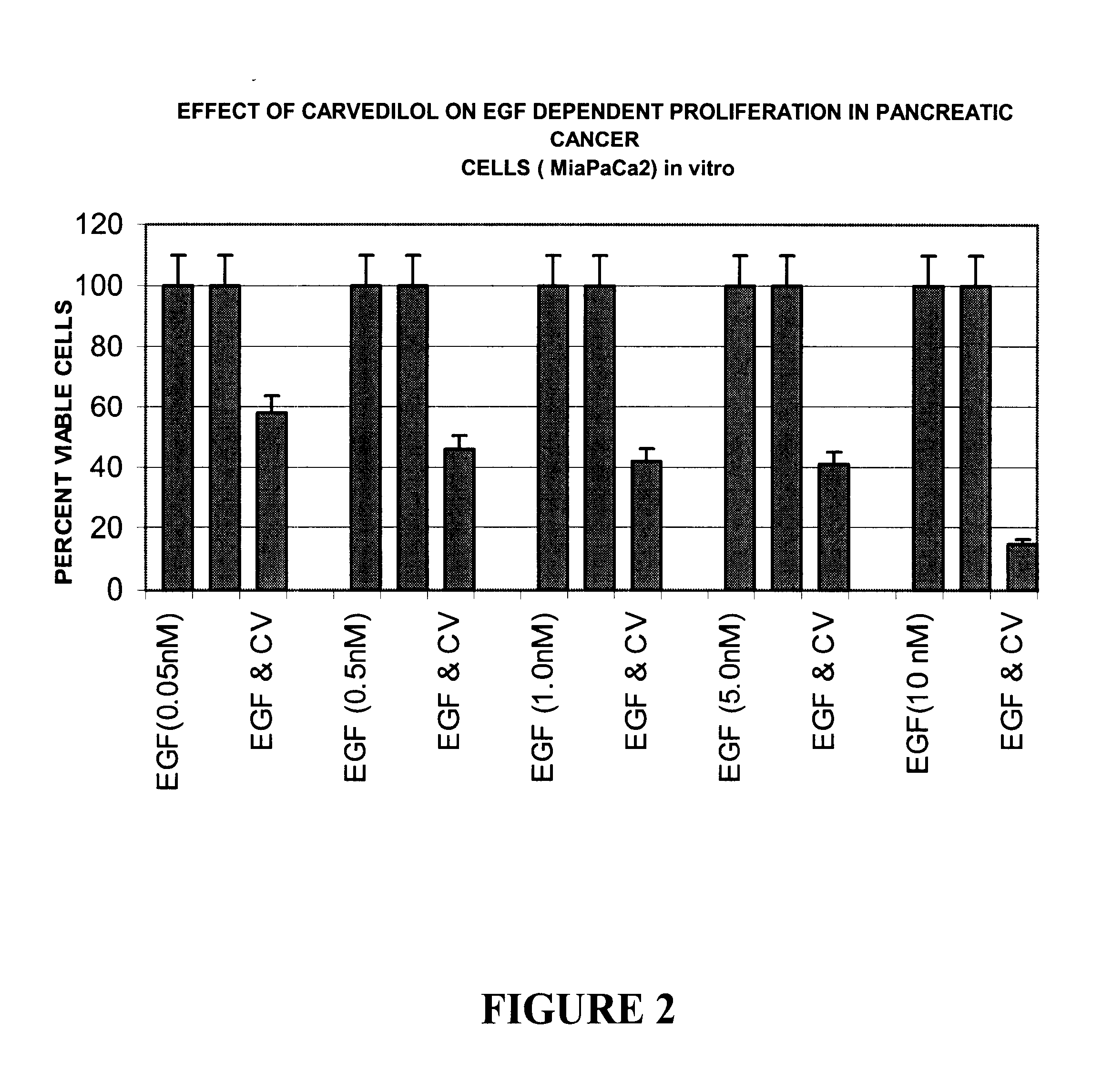
Anti-cancer activity of carvedilol and its isomers
InactiveUS6632832B1Inhibits COX activityPrevent and inhibit tumor growthBiocideArtificial cell constructsMelanomaProstate cancer
The present invention provides for pharmaceutical compositions comprising carvedilol for treatment of cancer. More particularly the invention relates to the use of carvedilol for treatment of cancers of the colon, ovary, breast, prostate, pancreas, lung, melanoma, glioblastoma, oral cancer and leukemias. Although not bound to any theory, the anticancer activity of carvedilol appears to be attributed to the inhibition of Epidermal Growth Factor and Platelet derived growth factor dependent proliferation of cancer cells. Further, carvedilol exerts anticancer effect by inhibition of the Protein kinase C (PKC) activity and that of the cyclooxygenase 2 enzyme. The invention also relates to the anticancer activity of the optically pure isomers S(-) and R(+) of carvedilol and the use of carvedilol and its isomers in pharmaceutical compositions for the treatment of cancer.< / PTEXT>
Owner:DABUR RESEARCH FOUNDATION
Collagen sustained-release carrier material for promoting repair of various traumas in oral and maxillofacial regions and method for preparing same
InactiveCN102120033AEasy to moistenFully absorbedPeptide/protein ingredientsAbsorbent padsOral ulcersLiposome
The invention discloses a compound growth factor collagen sustained-release carrier material for promoting repair of various traumas in oral and maxillofacial regions and a method for preparing the same. In order to overcome the defect of poor curative effect existing in the conventional medicaments for treating the traumas of various tissues such as an oral cavity and a face, a bioactive collagen sustained-release material is prepared by combining collagen modified by liposome, chitosan, glycosaminoglycan and the like with one or more of a basic fibroblast growth factor (bFGF), a platelet derived growth factor (PDGF-BB), a vascular endothelial growth factor (VEGF) and a keratinocyte growth factor (KGF). The bioactive collagen sustained-release material is characterized in that: each gram of bioactive collagen sustained-release material contains not less than 10ng of growth factors, and the bioactive collagen sustained-release material has the obvious effect of treating various facial tissue traumas such as tooth extraction wound, oral ulcer, facial nerve injury, mandibular defect and the like. The collagen sustained-release carrier material can continuously release an effective amount of the growth factors to the wound for a long time, fully exert the inducing effect of the growth factors and obviously shorten the time for wound healing so as to fulfill the aim of treatment.
Owner:WENZHOU MEDICAL UNIV
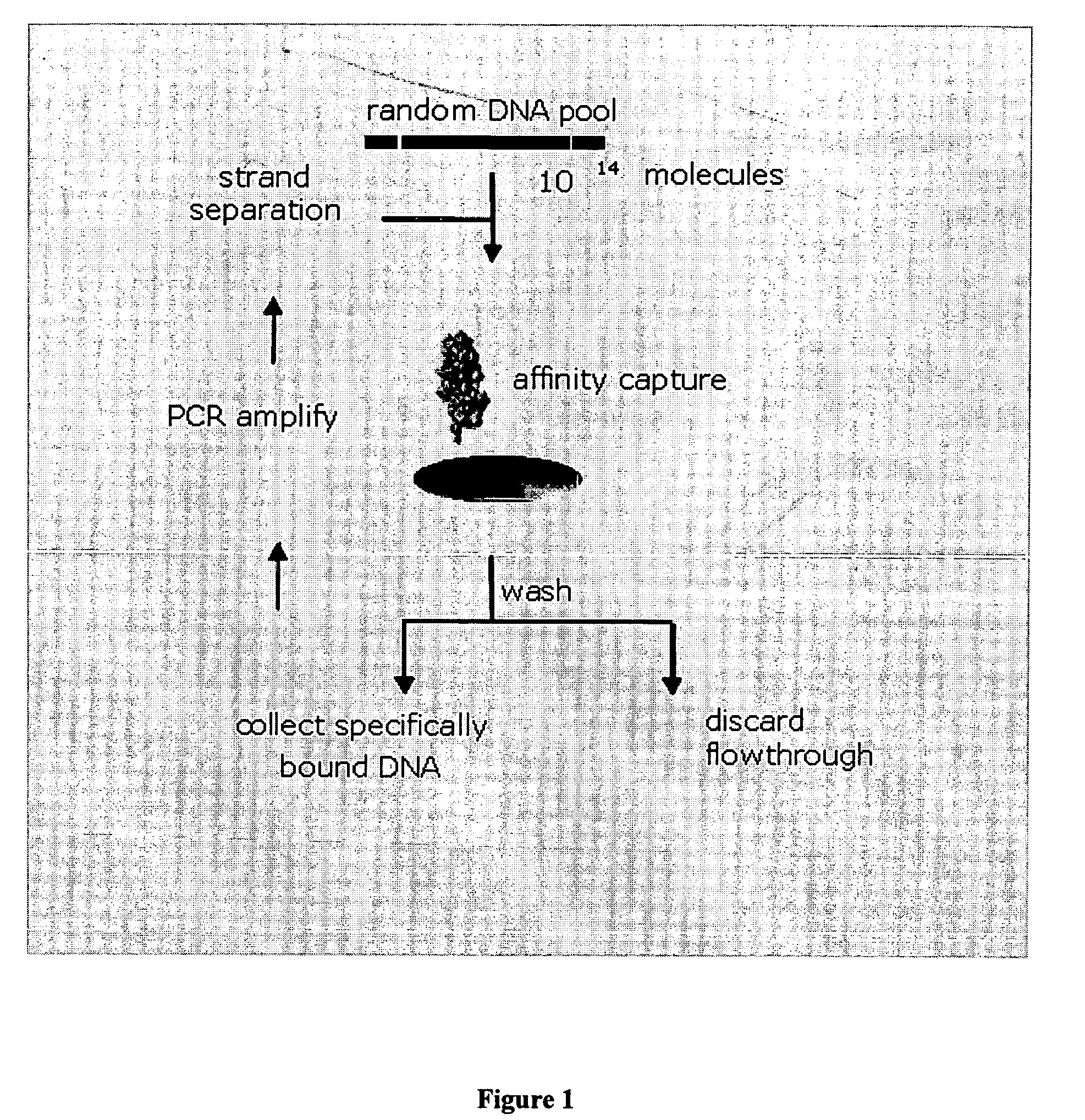
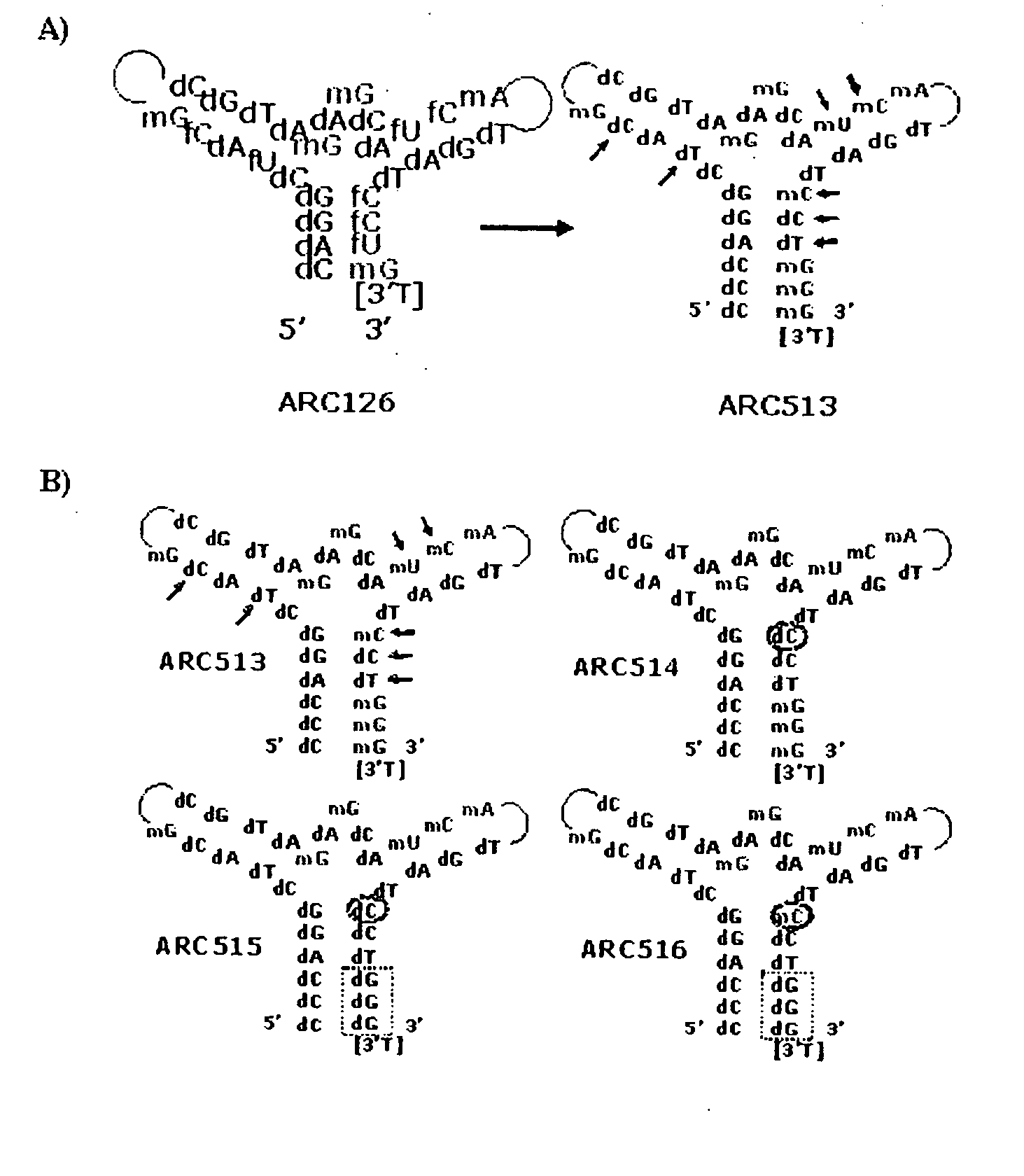
Platelet Derived Growth Factor (PDGF) Nucleic Acid Ligand Complexes
InactiveUS20080207883A1Convenient treatmentInhibit angiogenesisOrganic active ingredientsPeptide/protein ingredientsImmunogenicityLipophilicity
This invention discloses a method for preparing a complex comprised of a PDGF Nucleic Acid Ligand and a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound by identifying a PDGF Nucleic Acid Ligand by SELEX methodology and associating the PDGF Nucleic Acid Ligand with a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound. The invention further discloses Complexes comprising one or more PDGF Nucleic Acid Ligands in association with a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound. The invention further includes a Lipid construct comprising a PDGF Nucleic Acid Ligand or Complex and methods for making the same.
Owner:GILEAD SCI INC
RNA interference mediated inhibition of platelet derived growth factor (PDGF) and platelet derived growth factor receptor (PDGFR) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050233344A1Improve bioavailabilityMinimize the possibilityCompounds screening/testingSpecial deliveryCompound (substance)Platelet
This invention relates to compounds, compositions, and methods useful for modulating platelet derived growth factor (PDGF) and / or platelet derived growth factor receptor (PDGFr) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of platelet derived growth factor (PDGF) and / or platelet derived growth factor receptor (PDGFr) gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of platelet derived growth factor (PDGF) and / or platelet derived growth factor receptor (PDGFr) genes, such as PDGF and / or PDGFr.
Owner:SIRNA THERAPEUTICS INC
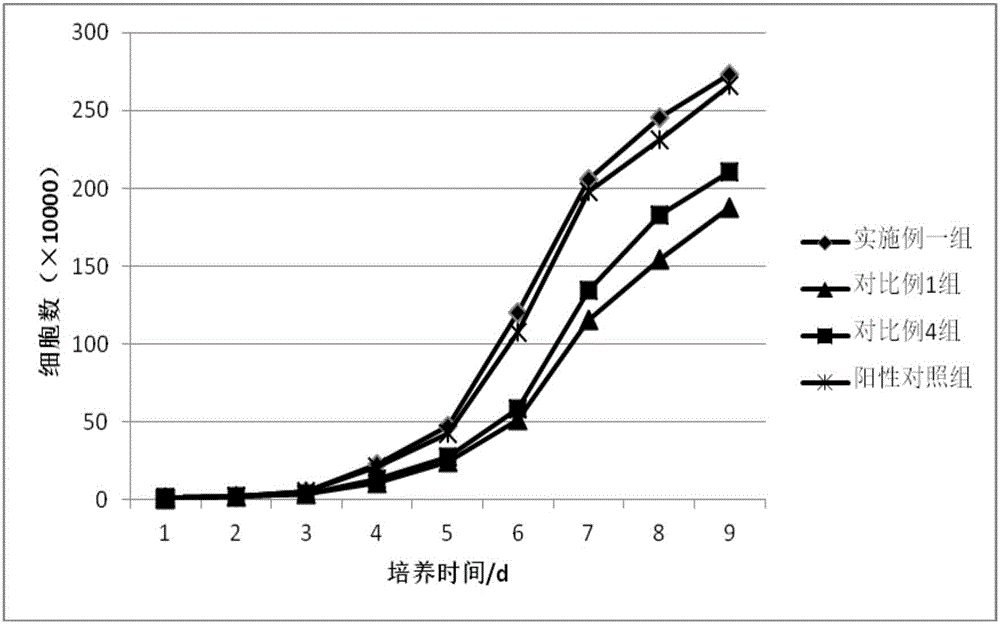
Method for isolated culture of human fat mesenchyma stem cell and special culture medium thereof
ActiveCN101314766AThe method of isolation and culture is simpleImprove efficiencySkeletal/connective tissue cellsAntigenMuscle injury
The invention discloses a method for separately culturing a human adipose mesenchymal stem cell and a dedicated culture medium thereof. The culture medium used for separately culturing the human adipose mesenchymal stem cell comprises an animal cell basic culture medium, fetal calf serum, an epidermal growth factor and a platelet-derived growth factor. The final concentration of the fetal calf serum is 1-200 mL / L, the final concentration of the epidermal growth factor is 1-100 ng / ml, and the final concentration of the platelet-derived growth factor is 1-100 ng / ml. The adipose mesenchymal stem cell of the invention has CD31-, CD34-, CD45- and HLA-DR-, as well as the phenotype of CD29+, CD44+, CD105+ and Flk-1+. The specificity cell surface marker and the relevant antihelion molecule of a skeletal muscle cell and a vascular endothelia cell can be expressed after inducement is performed in vitro. Muscle fiber, vascular endothelin and functional muscle satellite cells can be differentiated in a muscle injury model mouse body caused by medicine and the expression of dystrophin protein on the ducheme muscular dystrophy (DMD) model mouse (mdx) myolemma can be partially recovered, so as to release the pathological symptom of the model mouse.
Owner:微能生命科技集团有限公司
Serum-free media and their uses for chondrocyte expansion
InactiveUS20070292949A1Enhance cell attachmentIncreased proliferationCulture processSkeletal disorderInterleukin 6Lipid formation
The present invention provides defined serum-free cell culture media useful in culturing fibroblasts, especially articular chondrocytes, that avoid problems inherent in the use of serum-containing media. The defined media comprise platelet-derived growth factor (PDGF), chemically defined lipids, oncostatin M (OSM), interleukin-6 (IL-6), leukemia inhibitory factor (LIF), or combinations of these compounds. In another aspect, the present invention also provides tissue culture methods that comprise incubating chondrocytes in the defined serum-free media. The methods enhance attachment and proliferative expansion of chondrocytes seeded at low density while maintaining their redifferentiation potential.
Owner:GENZYME CORP
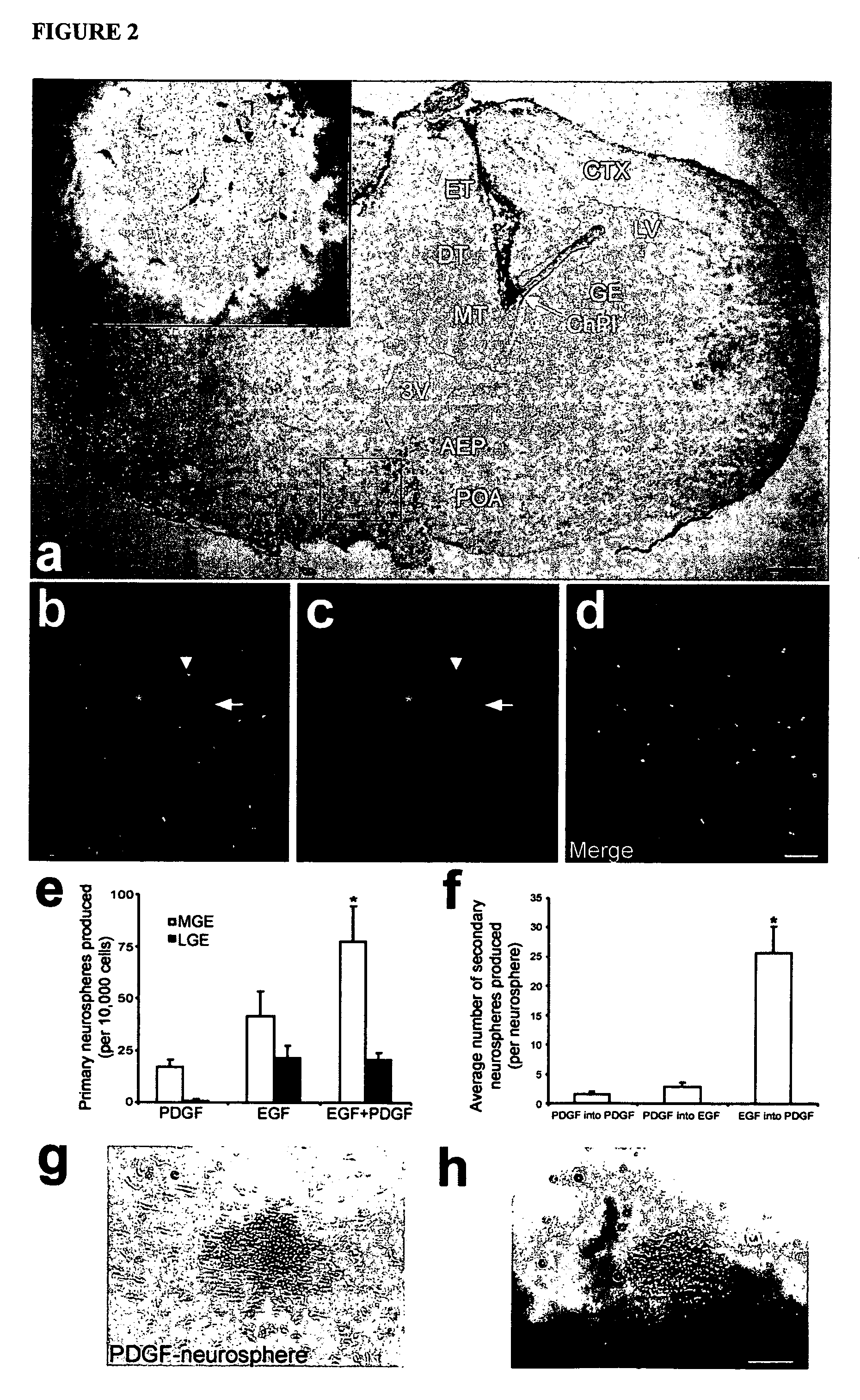
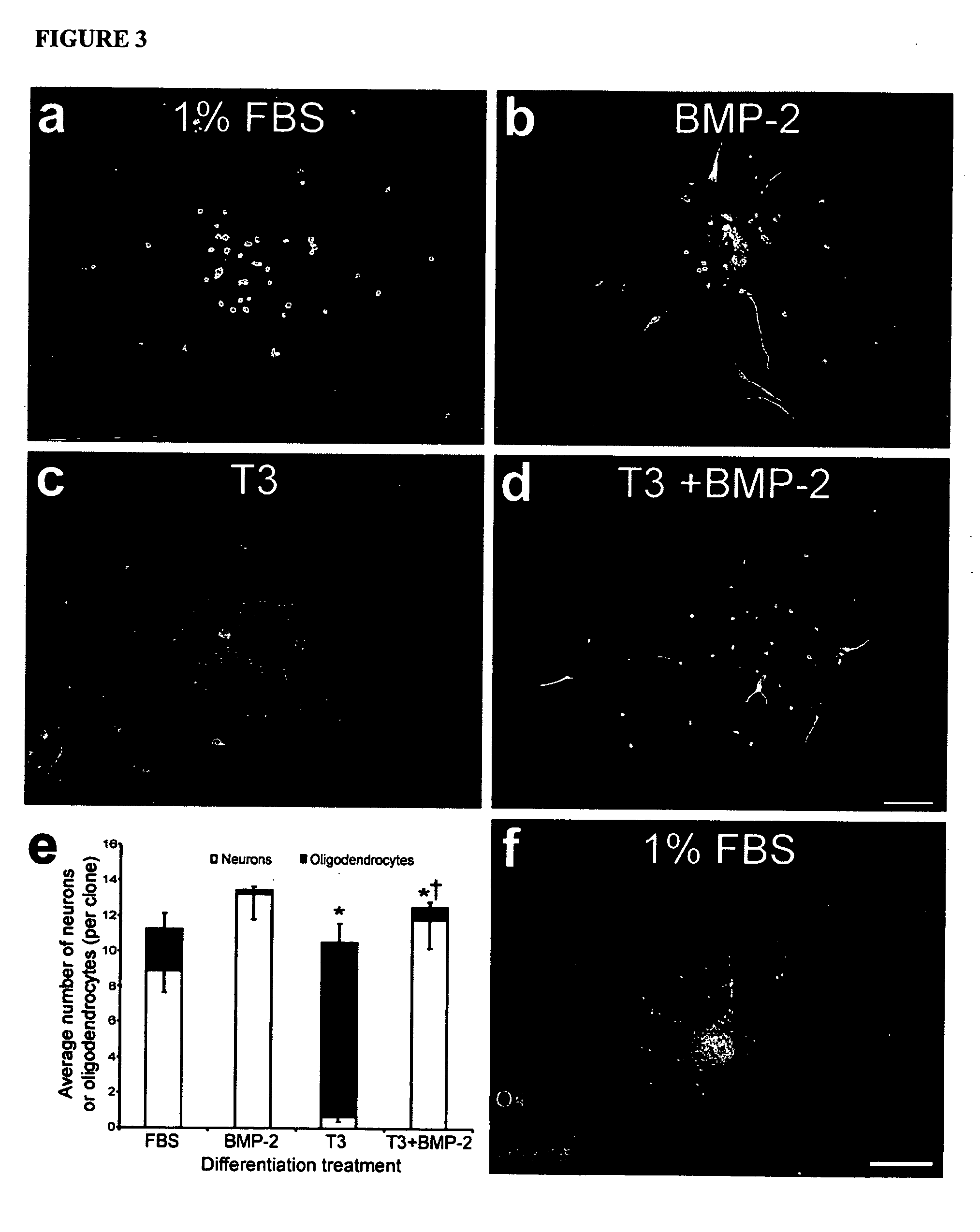
Platelet-derived growth factor-responsive neural precursor cells and progeny thereof
This invention provides platelet-derived growth factor-responsive neural precursor (PRP) cells and methods of producing such cells in vivo or in vitro. These cells can further be used to generate neurons, oligodendrocytes and / or astrocytes.
Owner:STEM CELL THERAPEUTICS
Method for making a topical composition comprising growth factors derived from human umbilical cord blood platelets
The present invention is directed to a method of making a stable composition comprising growth factors, which may include platelet derived growth factors and transforming growth factors. The method comprises providing human umbilical cord blood plasma containing platelets, but substantially free of whole blood cells, and lysing the platelets to extrude growth factors into the plasma. The plasma may be obtained from multiple donors and pooled to form a homologous plasma mixture. In another embodiment, the growth factors are encapsulated in a liposome formed by a lipid bilayer, wherein the resulting composition remains stable and viable for at least 30 months. The present invention is also directed to the composition produced by the methods of the present invention and the process of applying the composition to a skin defect or wound.
Owner:NOVO SOLUTIONS MD L L C
Methods of Regenerating Cartilage
The present disclosure relates to methods of regenerating cartilage. In an embodiment, a method includes initiating a release of precursor cells, including bone marrow cells and progenitor cells, into a cartilage defect; and applying a population of exogenous cells to the cartilage defect. The exogenous cells, selected from a group including chondrocytes, synoviocytes, fat pad cells, chondroprogenitor cells, mesenchymal stem cells, and any combination thereof, induce the precursor cells to form cartilage tissue through a release of factors by the exogenous cells. The factors stimulate the precursor cells to form cartilage cells. The cartilage cells then form cartilage tissue. The factors are selected from a group including transforming growth factors, fibroblast growth factors, platelet-derived growth factors, insulin-like growth factors, epidermal growth factors, interleukins, and any combination thereof. Other methods of regenerating cartilage are also disclosed.
Owner:SMITH & NEPHEW INC
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
Serum-free culture medium for umbilical cord mesenchymal stem cells, as well as preparation method and application thereof
ActiveCN106635978AExcellent adhesionIncrease speedSkeletal/connective tissue cellsAdipogenesisStem cell culture
The invention provides a serum-free culture medium for umbilical cord mesenchymal stem cells. The serum-free culture medium is characterized by comprising a DMEM low-sugar culture medium, transferrin, serum albumin, insulin, platelet-derived growth factors, epidermal growth factors, transforming growth factors, beta-mercaptoethanol, dihydromyricetin and catechin. The invention belongs to the technical field of stem cell culture. The serum-free culture medium for umbilical cord mesenchymal stem cells, provided by the invention, can obviously promote the adherence performance and multiplication rate of the umbilical cord mesenchymal stem cells, is beneficial to the multiplication of the umbilical cord mesenchymal stem cells and the maintenance of features of the stem cells, is excellent in adipogenesis and osteogenic induction differentiation potential, is relatively simple in component of the culture medium and is relatively low in cost.
Owner:GUANGDONG COOWAY BIOTECH CO LTD +1
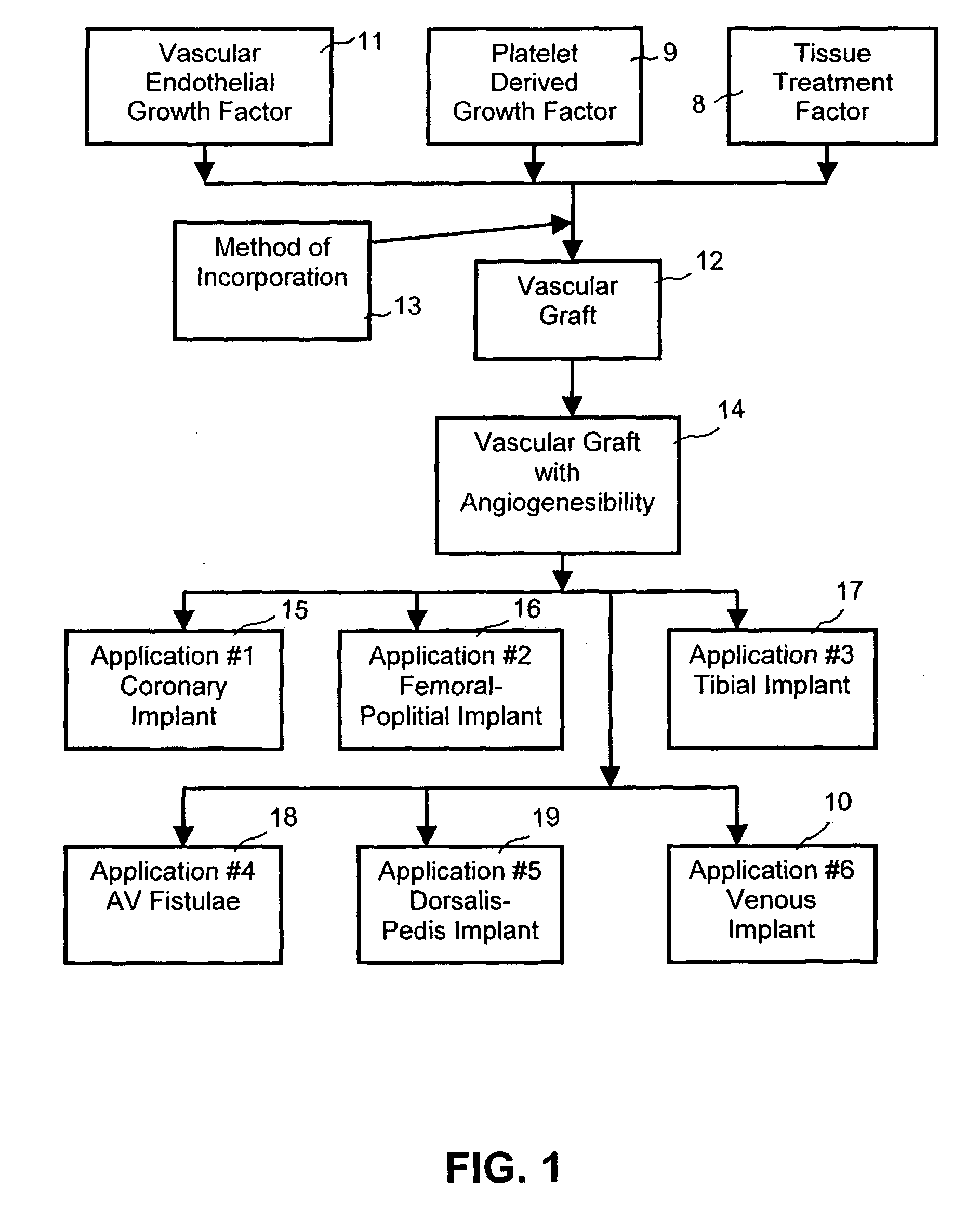
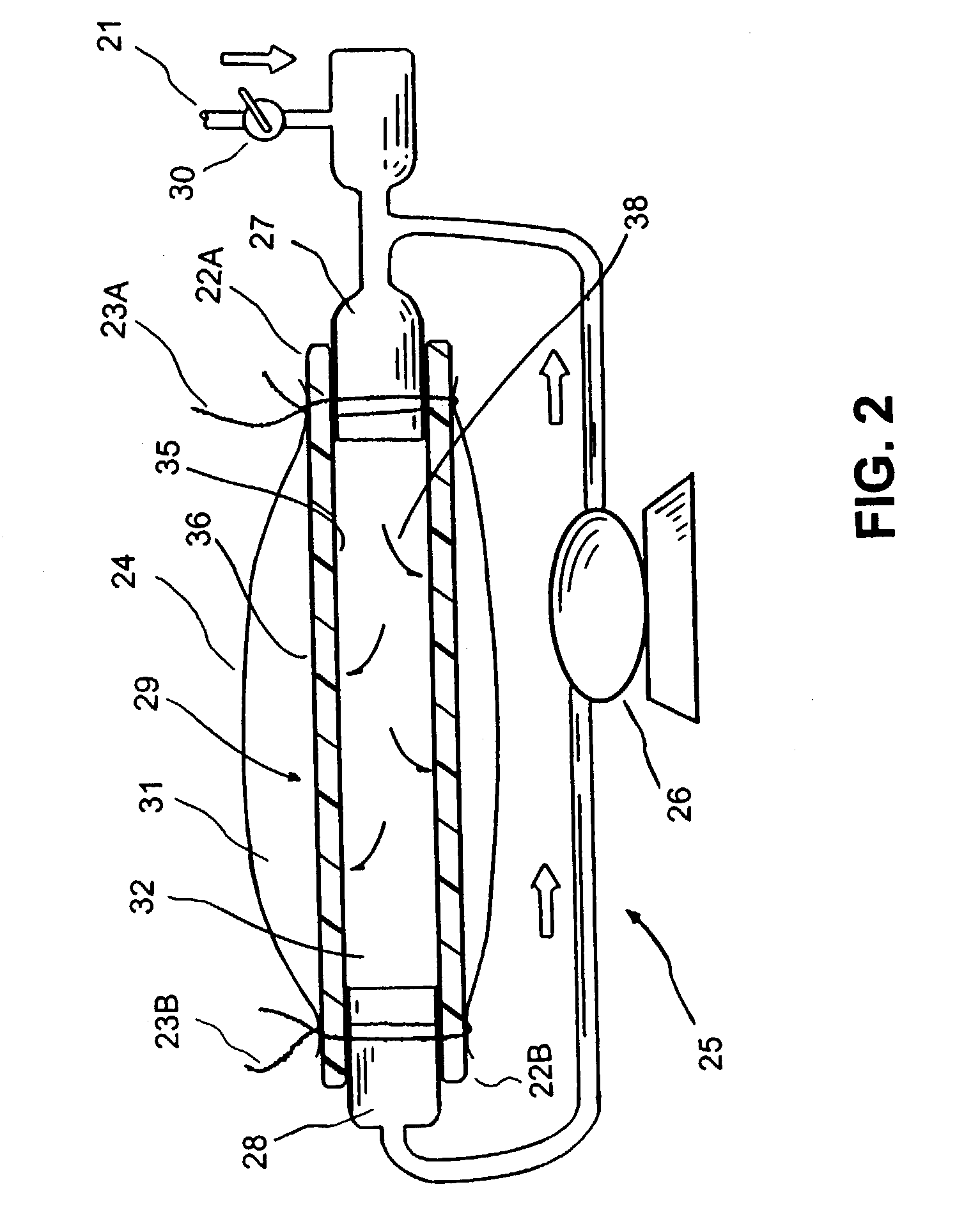
Device for treating diabetes and methods thereof
InactiveUS7060684B1Improve angiogenesisMore propensityPeptide/protein ingredientsPharmaceutical delivery mechanismHigh concentrationFactor ii
A composite supported vascular graft comprising Vascular Endothelial Growth Factor (VEGF) and / or Platelet Derived Growth Factor (PDGF) for enhanced site-specific angiogenesis and methods thereof are disclosed. At least one VEGF, PDGF or angiogenesis factor is incorporated into the composite vascular graft to facilitate enhanced angiogenesis so as the cells are stimulated to migrate to environments having higher concentration of growth factors and start mitosis.
Owner:QUIJANO RODOLFO C +1
Platelet-derived growth factor compositions and methods for the treatment of osteochondral defects
InactiveUS20130122095A1Promote growthIncrease the number ofPowder deliveryPeptide/protein ingredientsFactor iiPlatelet
The present invention provides compositions and methods for treating an osteochondral defect. In one embodiment, provided is a composition for treating an osteochondral defect comprising a biphasic biocompatible matrix and platelet derived growth factor (PDGF), wherein the biphasic biocompatible matrix comprises a scaffolding material and wherein the scaffolding material forms a porous structure comprising an osseous phase and a cartilage phase. In another embodiment, also provided is a method for treating an osteochondral defect in an individual comprising administering to the individual an effective amount of a composition comprising a biphasic biocompatible matrix and PDGF to at least one site of the osteochondral defect, wherein the biphasic biocompatible matrix comprises a scaffolding material and wherein the scaffolding material forms a porous structure comprising an osseous phase and a cartilage phase.
Owner:BIOMIMETIC THERAPEUTICS INC
Methods of generating mesenchymal stem cells which secrete neurotrophic factors
A method of generating MSCs which secrete neurotrophic factors (NTFs) comprising incubating a population of undifferentiated mesenchymal stem cells (MSCs) in a differentiating medium comprising basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF), heregulin and cAMP.
Owner:BRAINSTORM CELL THERAPEUTICS LTD
Chinese medicinal detoxifying myo-regeneration paste for treating diabetic foot ulcer and preparation method thereof
InactiveCN101926872AIncrease contentPromote gene expressionHeavy metal active ingredientsAnthropod material medical ingredientsSide effectPutrefaction
The invention provides Chinese medicinal detoxifying myo-regeneration paste for treating diabetic foot ulcer, which belongs to the fields of traditional Chinese medical science and traditional Chinese medicines. The paste is prepared from the following raw materials in percentage by weight: 6 to 11 percent of rhubarb, 6 to 11 percent of golden thread, 6 to 11 percent of amur corktree bark, 14 to 19 percent of plaster, 12 to 17 percent of sinkiang arnebia root, 14 to 20 percent of Chinese angelica, 7 to 11 percent of angelica dahurica, 2 to 5 percent of calomel, 7 to 11 percent of dragon's blood and 23 to 30 percent of bee wax. Animal experiments and clinical researches prove that the Chinese medicinal detoxifying myo-regeneration paste provided by the invention has the effects of activating blood, dissolving stasis, removing putrefaction, regenerating myo, clearing away heat and toxic material and the like, has the advantages of keeping the surface of a wound wet, increasing the content of cell factors, namely, a vascular endothelial growth factor (VEGF) and a platelet-derived growth factor (PDGF) and promoting relevant gene expressions of muscles and blood vessels, along with definite curative effect, simple and convenient operation, economy, safety and no toxic or side effect and special significance in treating diabetic foot ulcer.
Owner:韩会民 +1
Platelet derived growth factor (PDGF) nucleic acid ligand complexes
This invention discloses a method for preparing a complex comprised of a PDGF Nucleic Acid Ligand and a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound by identifying a PDGF Nucleic Acid Ligand by SELEX methodology and associating the PDGF Nucleic Acid Ligand with a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound. The invention further discloses Complexes comprising one or more PDGF Nucleic Acid Ligands in association with a Non-Immunogenic, High Molecular Weight Compound or Lipophilic Compound. The invention further includes a Lipid construct comprising a PDGF Nucleic Acid Ligand or Complex and methods for making the same.
Owner:GILEAD SCI INC
Stabilized Aptamers to Platelet Derived Growth Factor and their Use as Oncology Therapeutics
InactiveUS20090053138A1Increase edematous fluid contentIncrease oxygen contentOrganic active ingredientsSugar derivativesVEGF receptorsTumor therapy
Materials and methods are provided for producing and using aptamers as oncology therapeutics capable of binding to PDGF, PDGF isoforms, PDGF receptor, VEGF, and / or VEGF receptor or any combination thereof with affinity and specificity. The compositions of the present invention are particularly useful in solid tumor therapy and can be used alone or in combination with known cytotoxic agents for the treatment of solid tumors. Also disclosed are aptamers having one or more CpG motifs embedded therein or appended thereto.
Owner:ARCHEMIX CORP
Liquid band-aid and preparing method thereof
The invention discloses a liquid band-aid, comprising, active materials, a film-forming material and a solvent. The active materials include cytokines 0.042 to 0.0095%, which are chosen from at least two of EGF (epidermal growth factor), PDGF (platelet derived growth factor), FGF (fibroblast growth factor) and IL-10 (interleukin-10). The film-forming material comprises chitosan hydrochloride 2% to 8%. The solvent is deionized water or distilled water. The invention further discloses a method for preparing the liquid band-aid. By the use of the preparation method and / or the liquid band-aid comprising various biological active materials and prepared by the preparation method, activity of the biological active materials can be retained, and wounds can be efficiently repaired without a scar.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
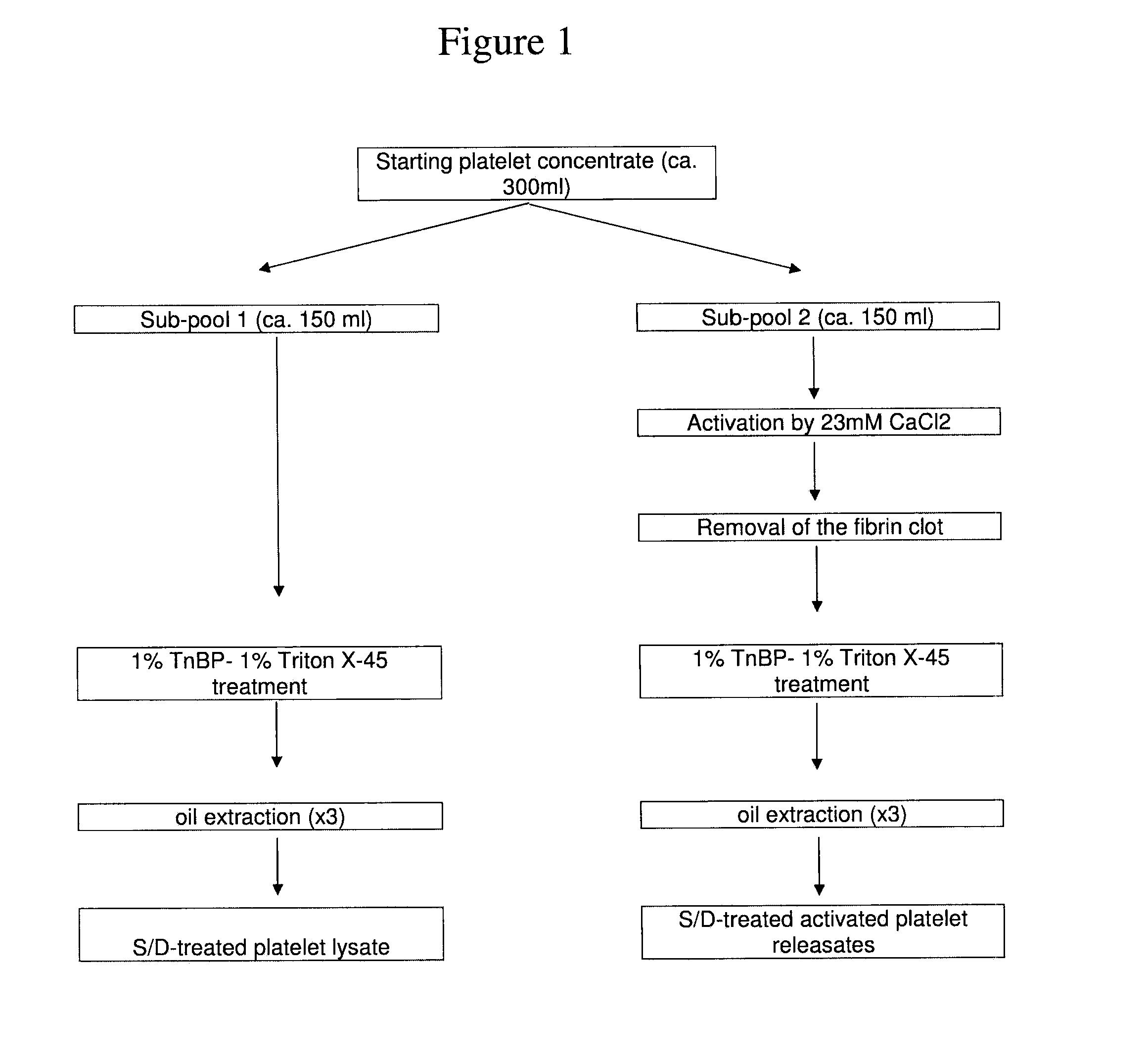
Clottable Concentrate Of Platelet Growth Factors And Preparation Method Thereof
Owner:ZHENG YANG BIOMEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com