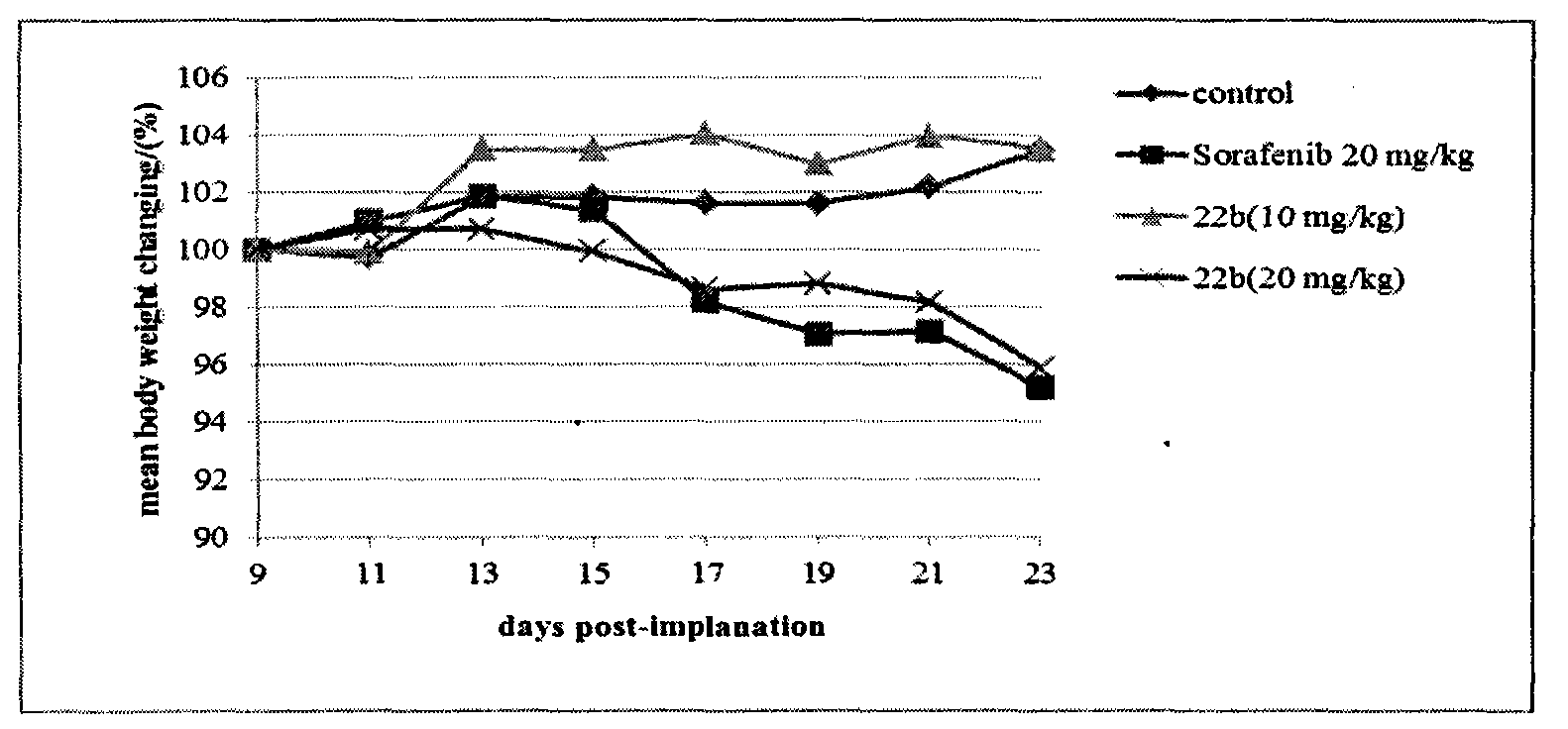
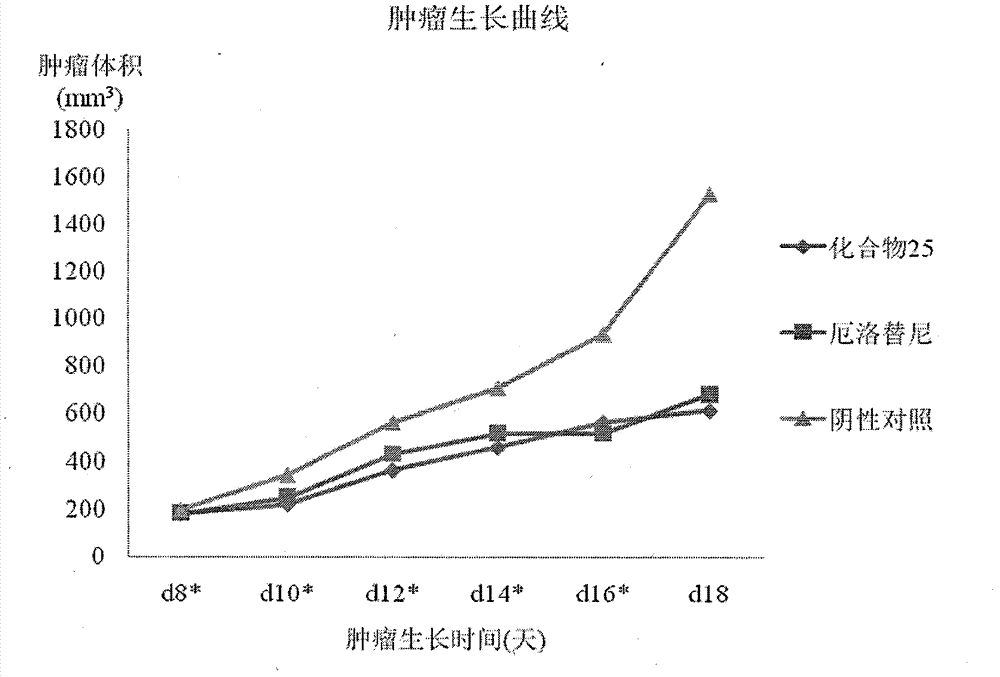
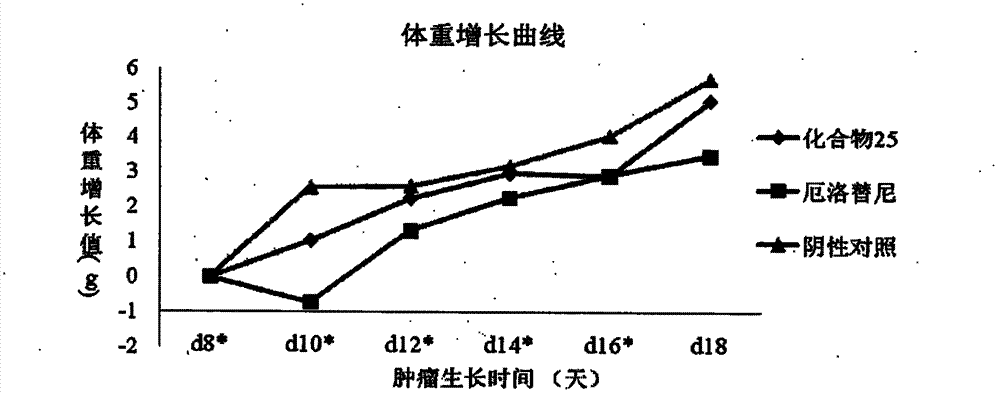
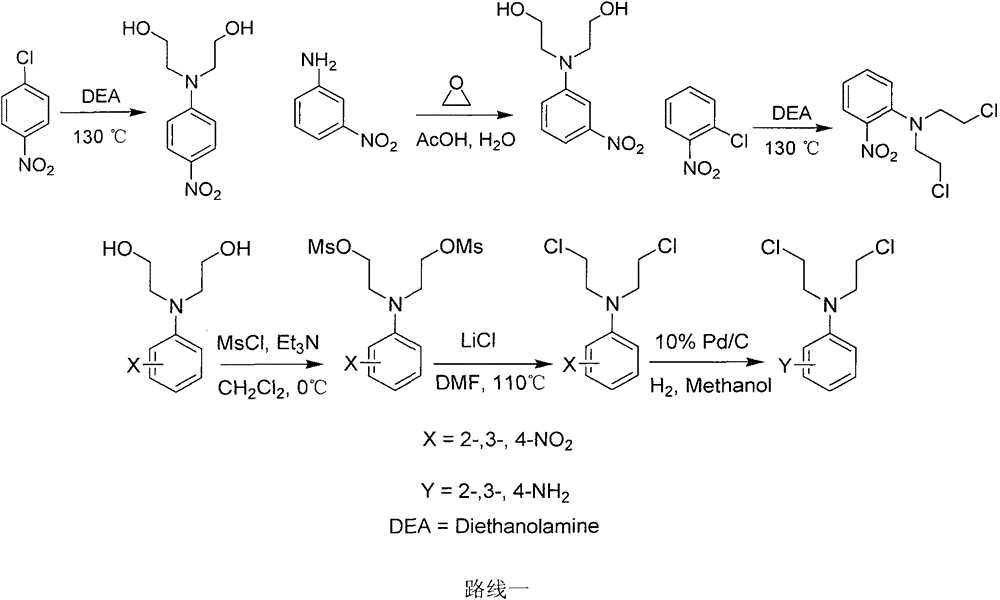
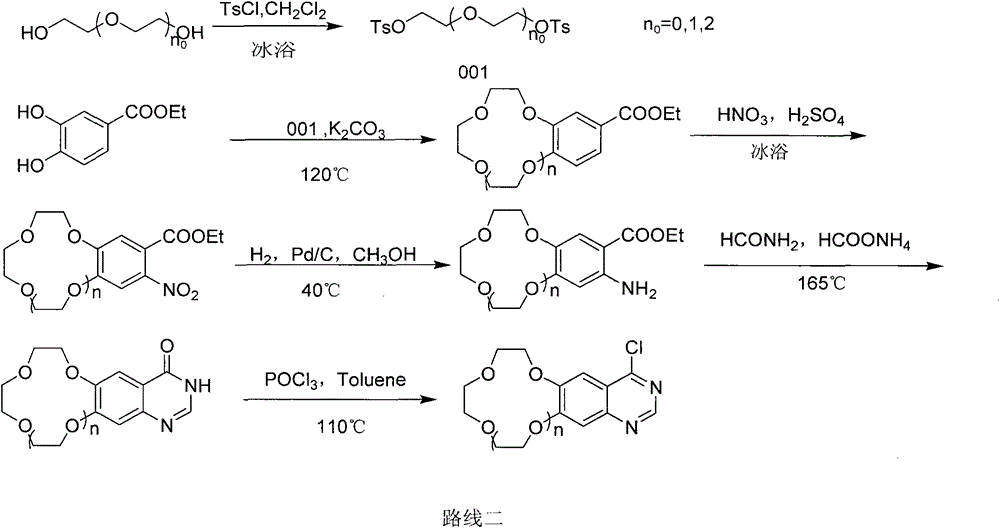
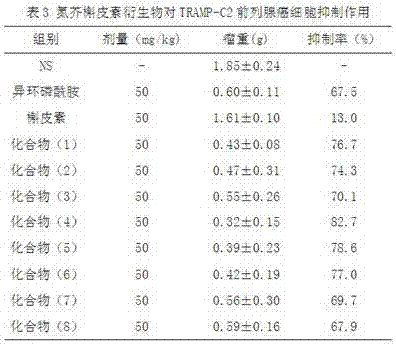
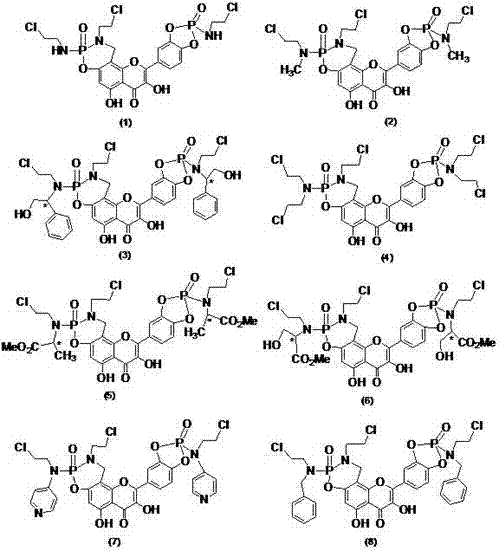
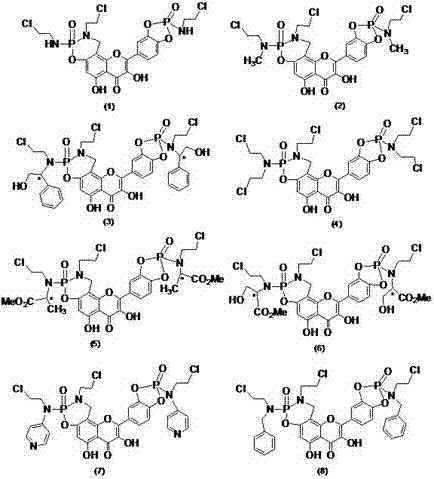
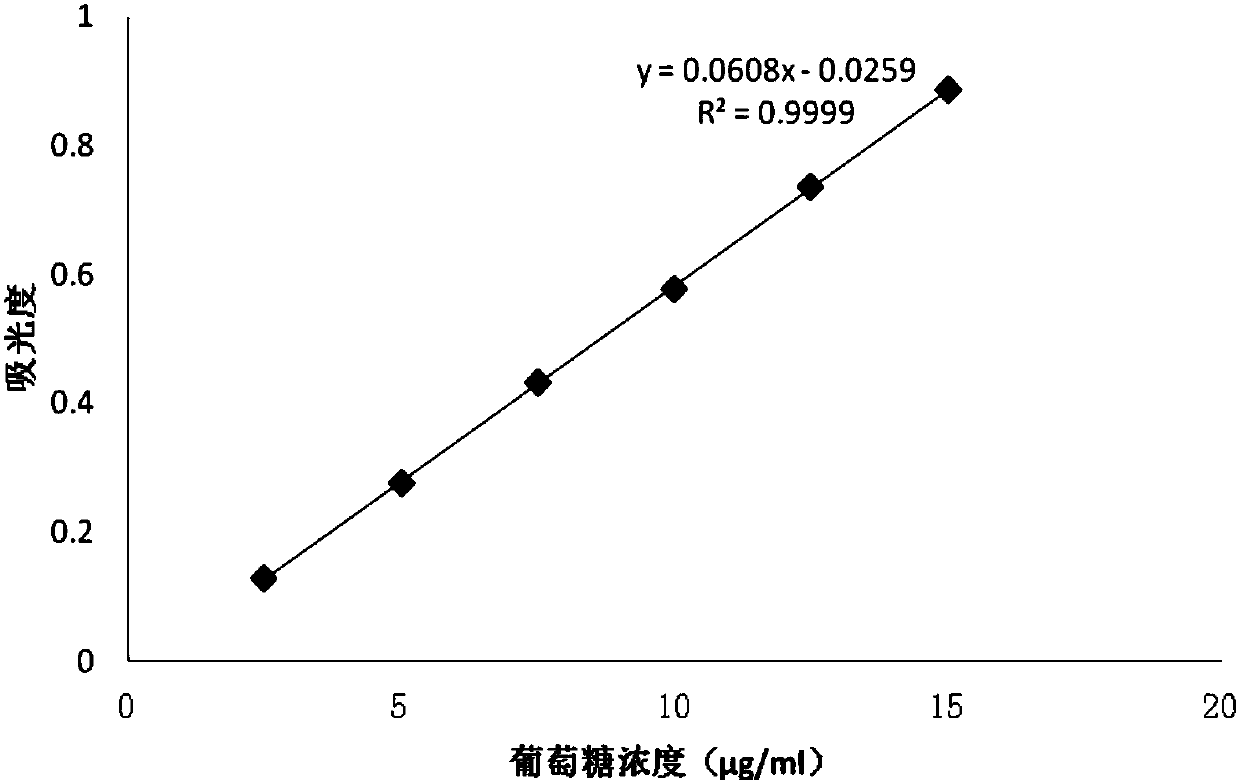
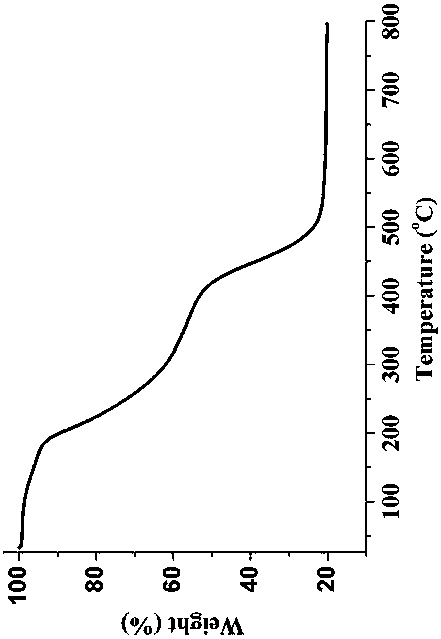
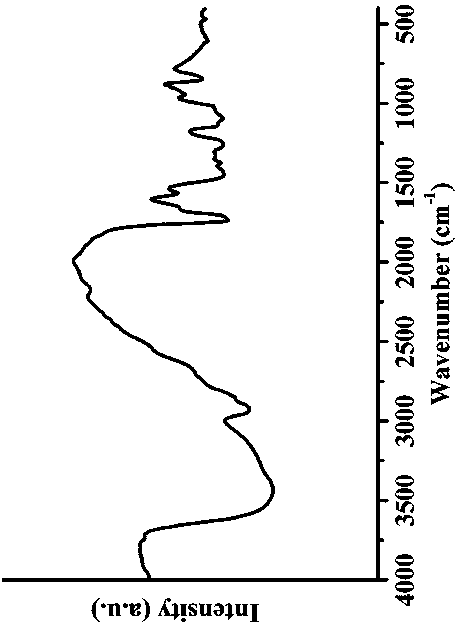
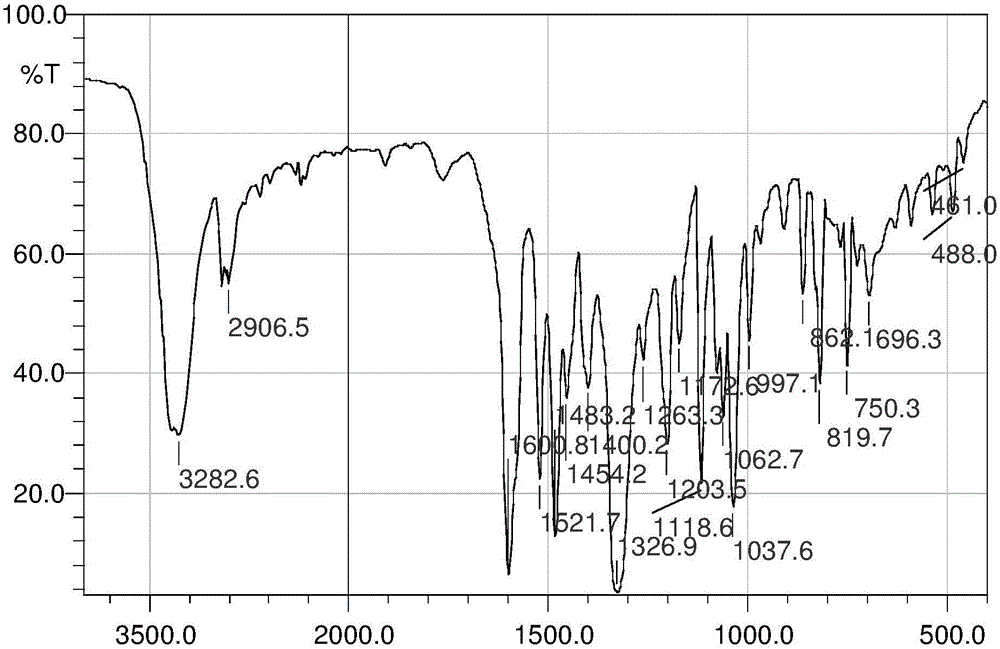
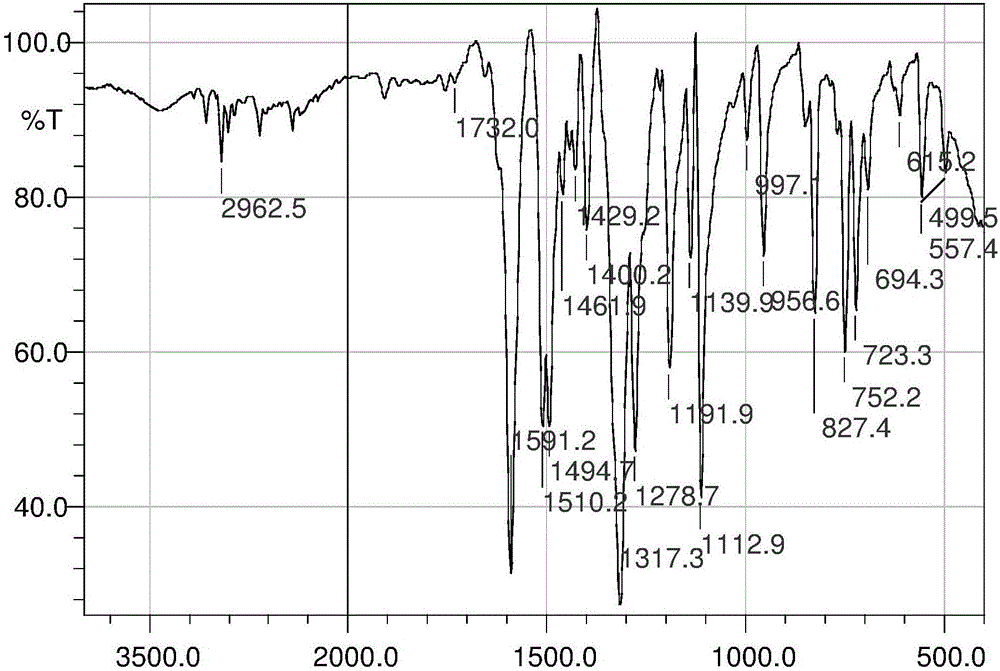
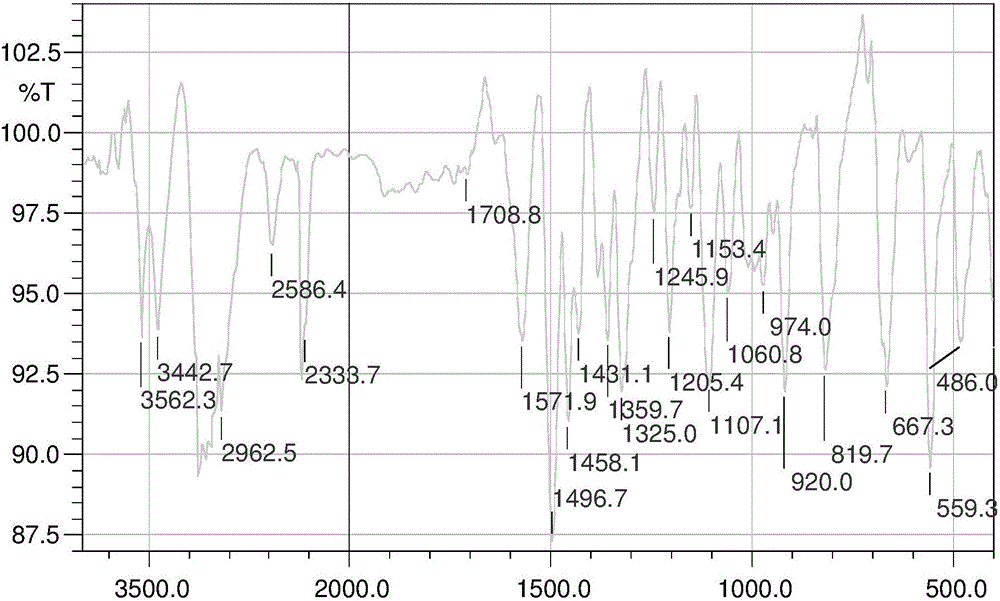
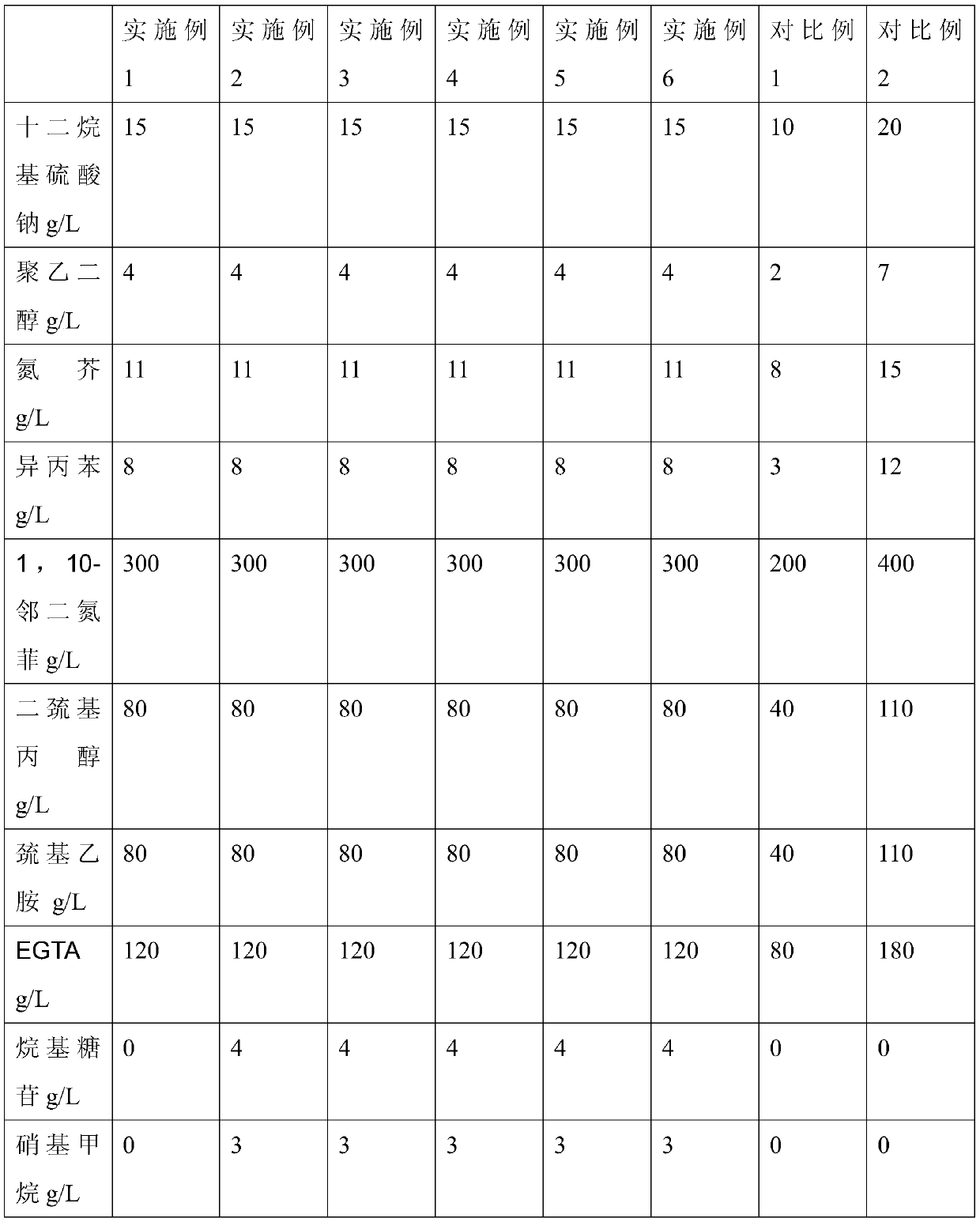
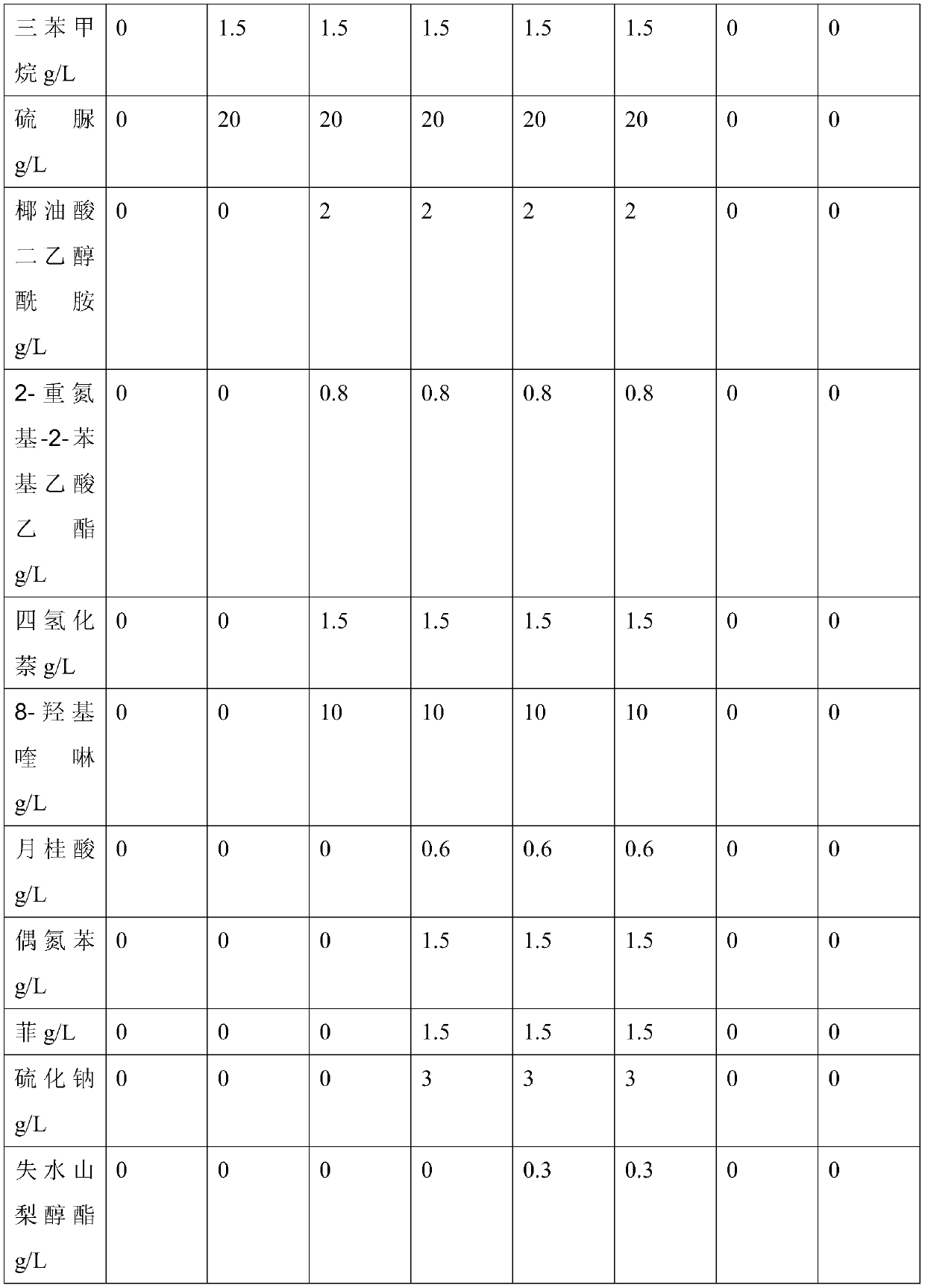
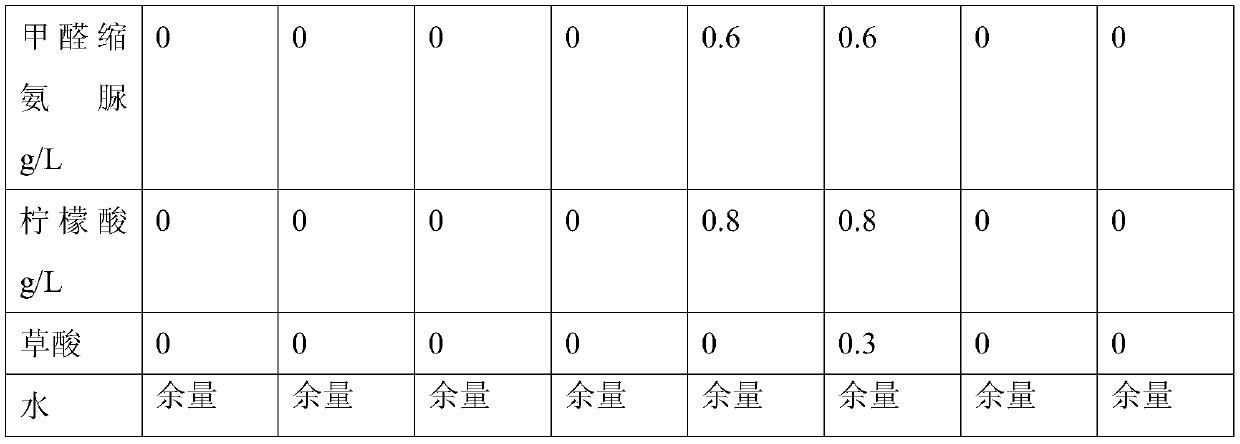
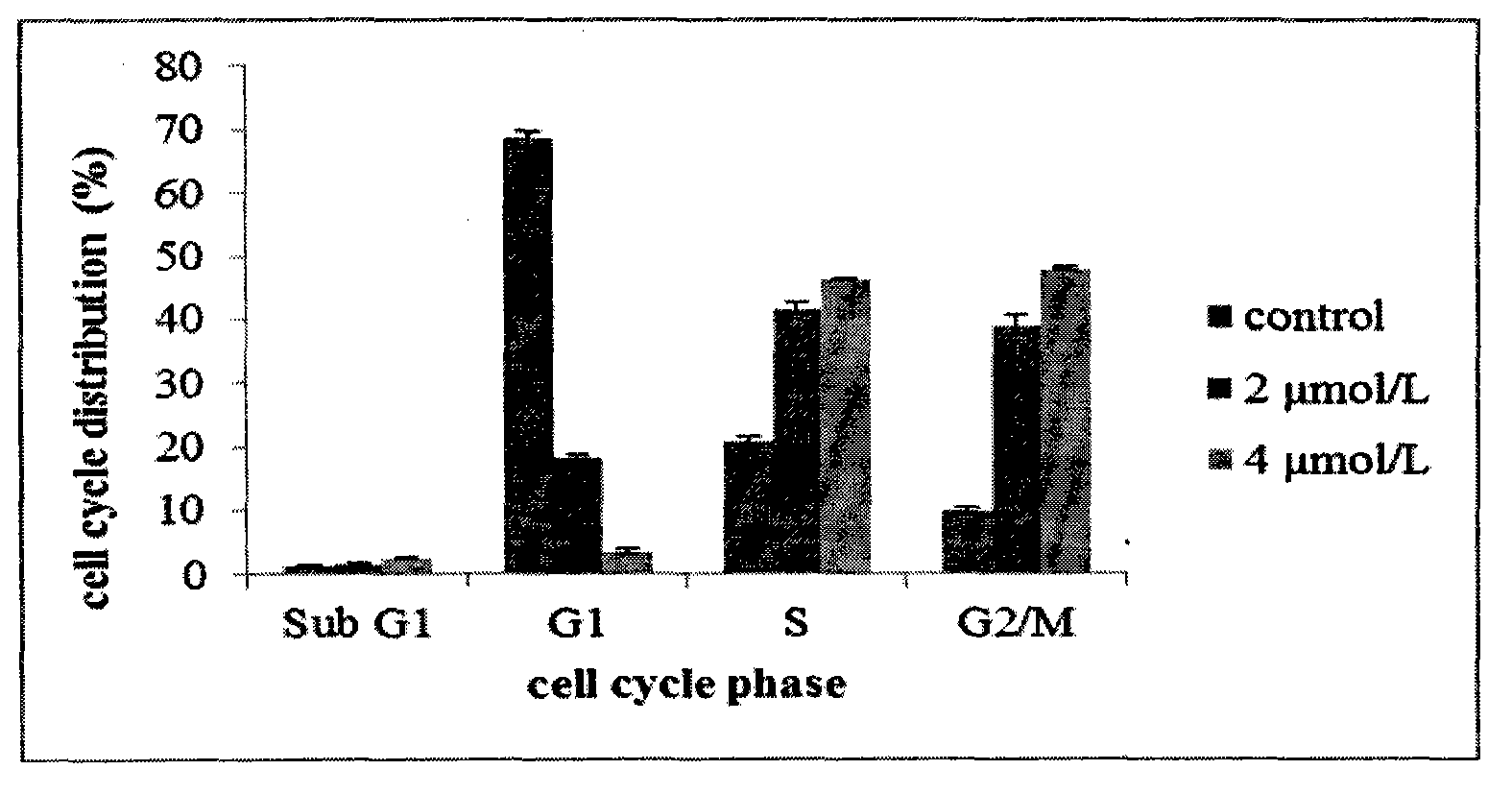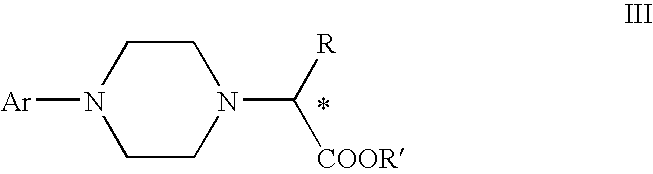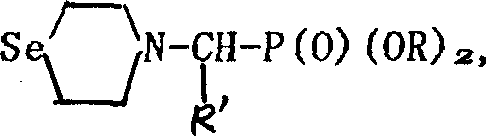Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Nornitrogen mustard" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
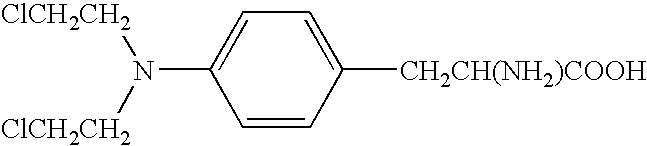
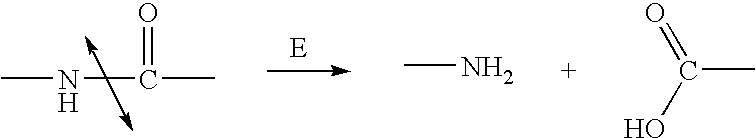
Nitrogen mustards are cytotoxic chemotherapy agents derived from mustard gas. Although their common use is medicinal, in principle these compounds can also be deployed as chemical warfare agents. ... Nitrogen mustards are nonspecific DNA alkylating agents.
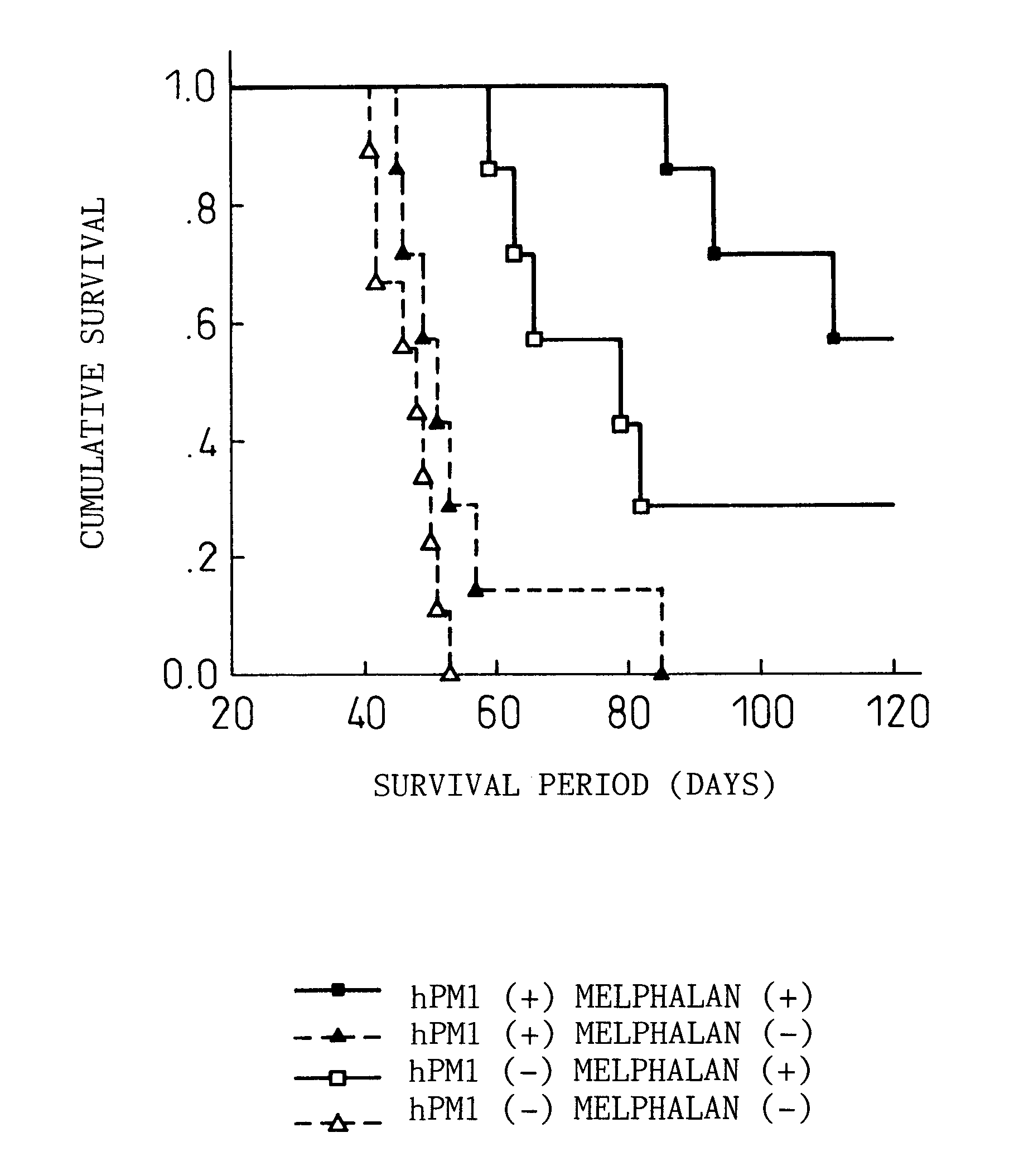
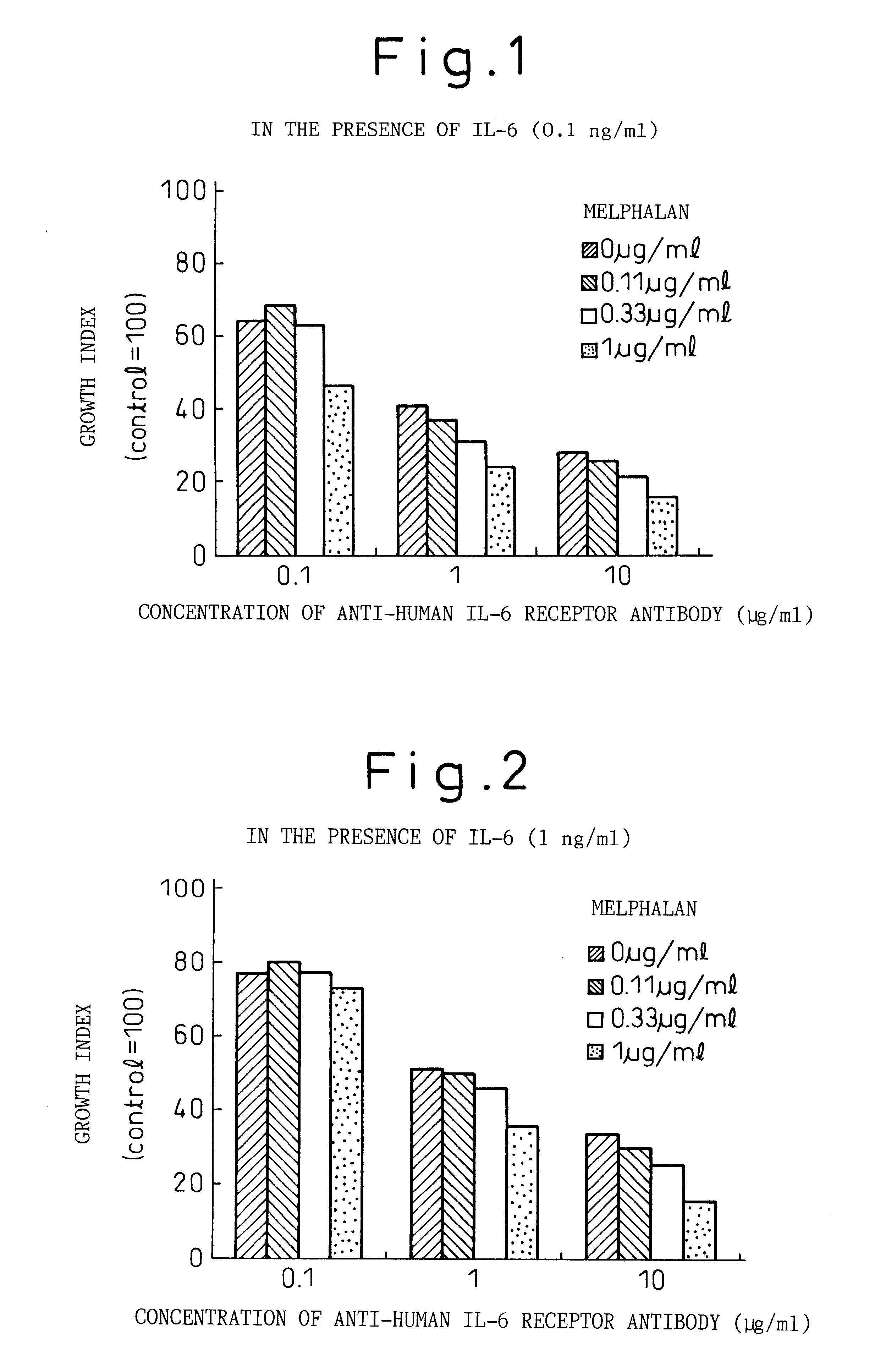
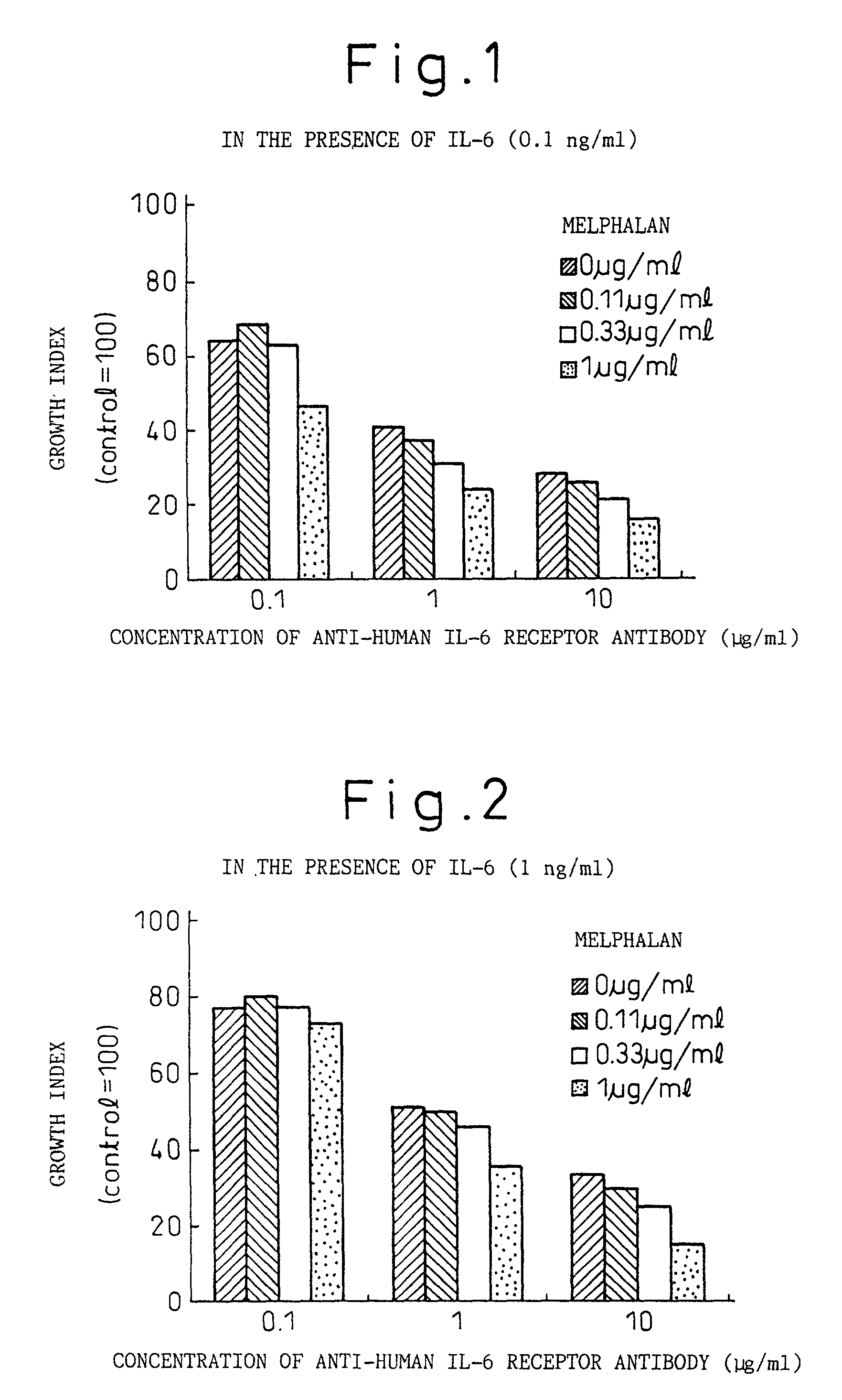
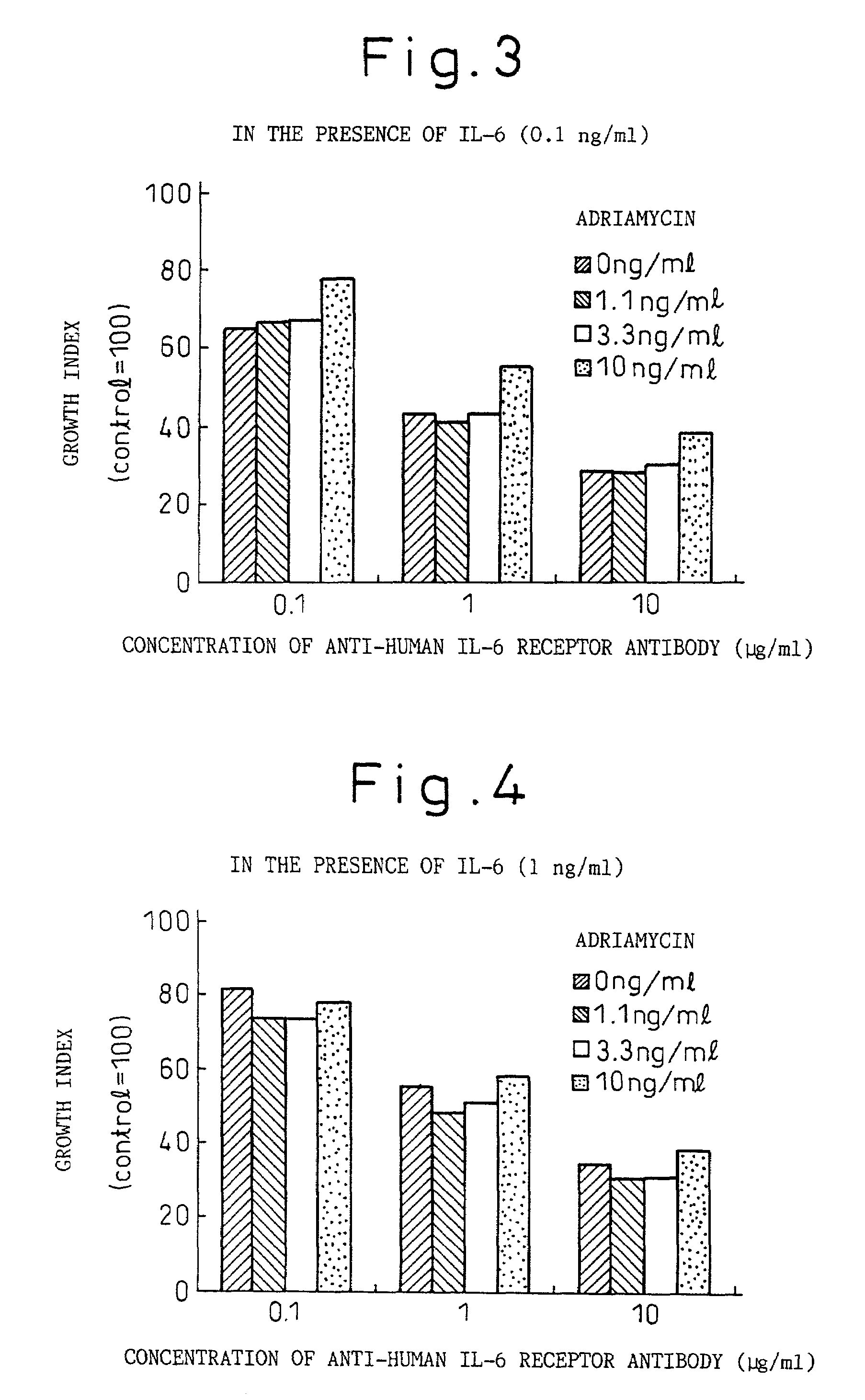
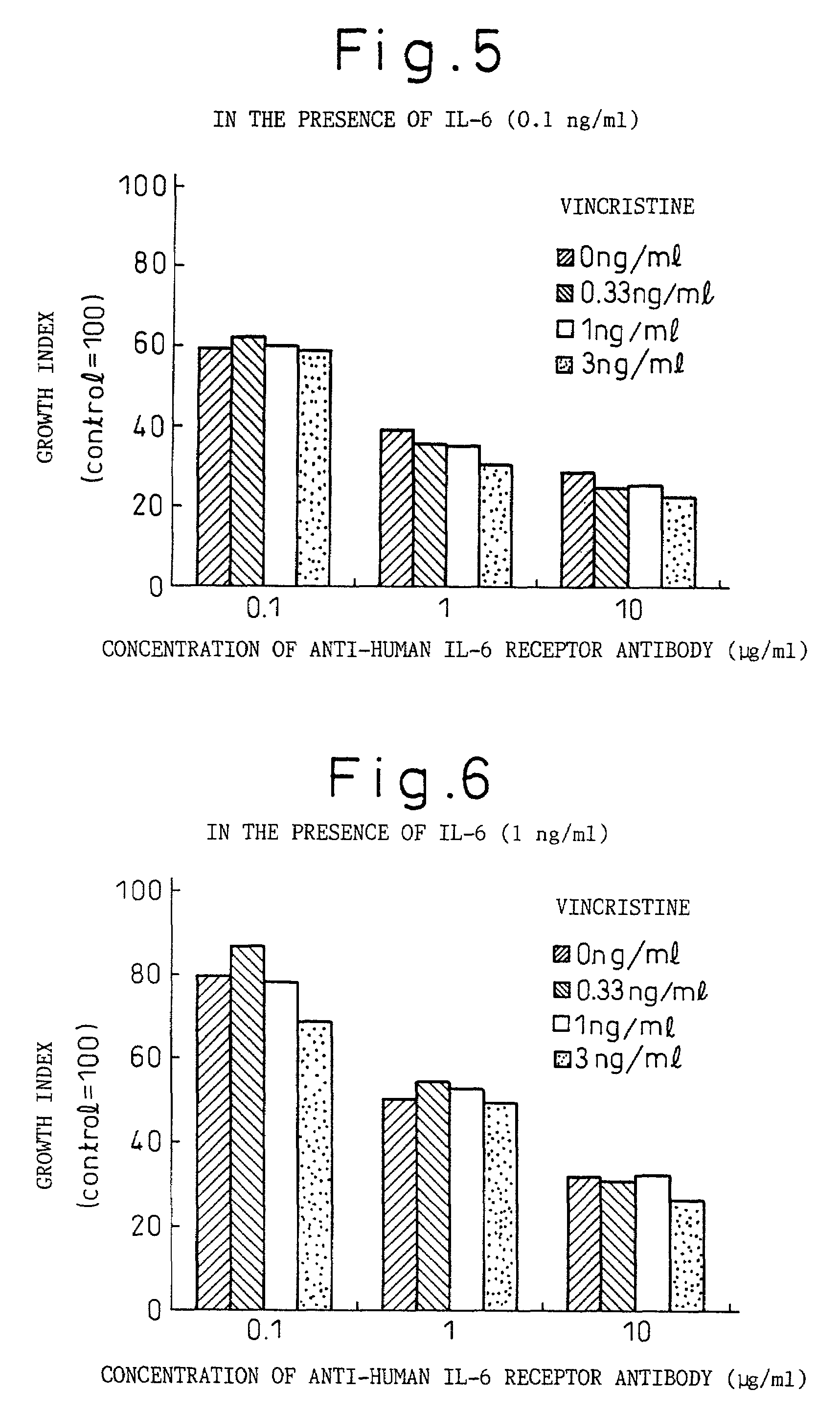
Remedies for myeloma to be used together with nitrogen mustard antitumor agents
A therapeutic agent for myeloma comprising a combined use of a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody. Thus, a therapeutic agent for myeloma comprising anti-IL-6 receptor antibody for use in combination with a nitrogen mustard anticancer agent; a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent for use in combination with anti-IL-6 receptor antibody; and a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody.
Owner:CHUGAI PHARMA CO LTD
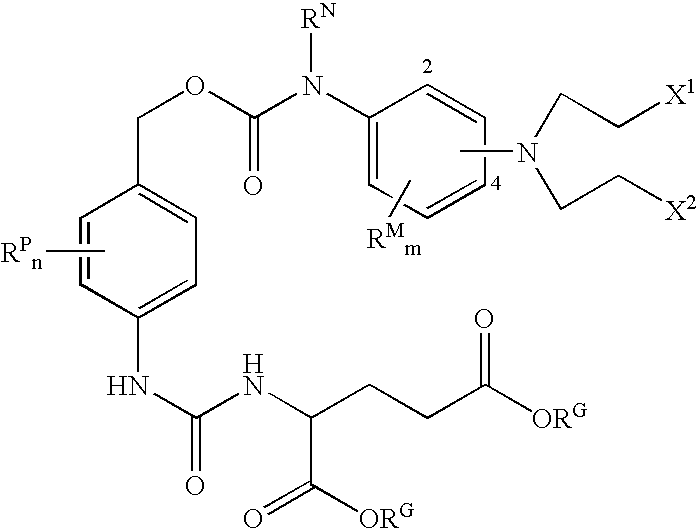
Enzyme activated self-immolative n-substituted nitrogen mustard prodrugs
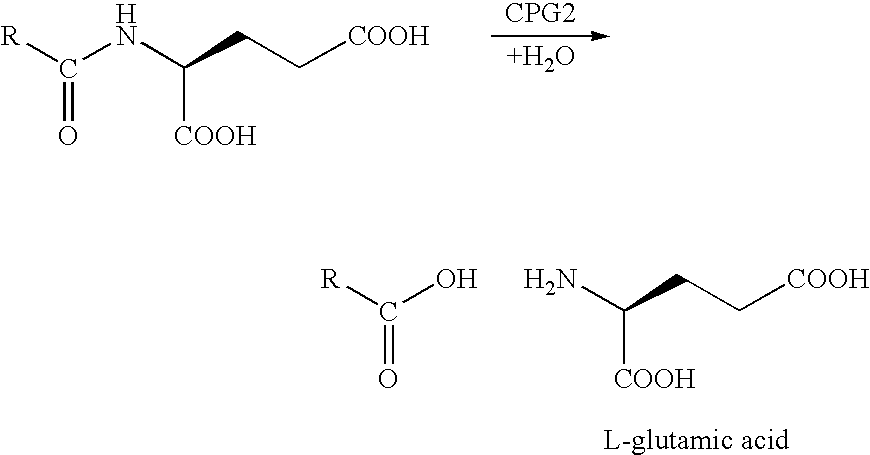
This invention pertains to certain enzyme (CPG2) activated self-immolative nitrogen mustard prodrugs, which are useful in enzyme prodrug therapy (EPT), such as ADEPT and GDEPT, for the treatment of proliferative conditions, such as cancer, and which have the following formula:wherein: RN is independently C1-7alkyl; X1 is independently —I, —Br, or —Cl; X2 is independently —I, —Br, or —Cl; the group —N(CH2CH2X1)(CH2CH2X2) is independently attached at the 2-position or at the 4-position; each RG is independently —H or an ester substituent; n is independently an integer from 0 to 4; each RP, if present, is independently a phenyl substituent; m is independently an integer from 0 to 4; each RM, if present, is independently a mustard substituent; and pharmaceutically acceptable salts, solvates, amides, and esters thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds; such compounds and compositions for use in methods of treatment of the human or animal body by therapy; the use of such compounds and compositions for the manufacture of medicaments for the treatment of proliferative conditions; and the like.
Owner:CANCER RES TECH LTD
Methods for treating melanomas
A therapeutic agent for myeloma comprising a combined use of a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody. Thus, a therapeutic agent for myeloma comprising anti-IL-6 receptor antibody for use in combination with a nitrogen mustard anticancer agent; a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent for use in combination with anti-IL-6 receptor antibody; and a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody.
Owner:CHUGAI PHARMA CO LTD
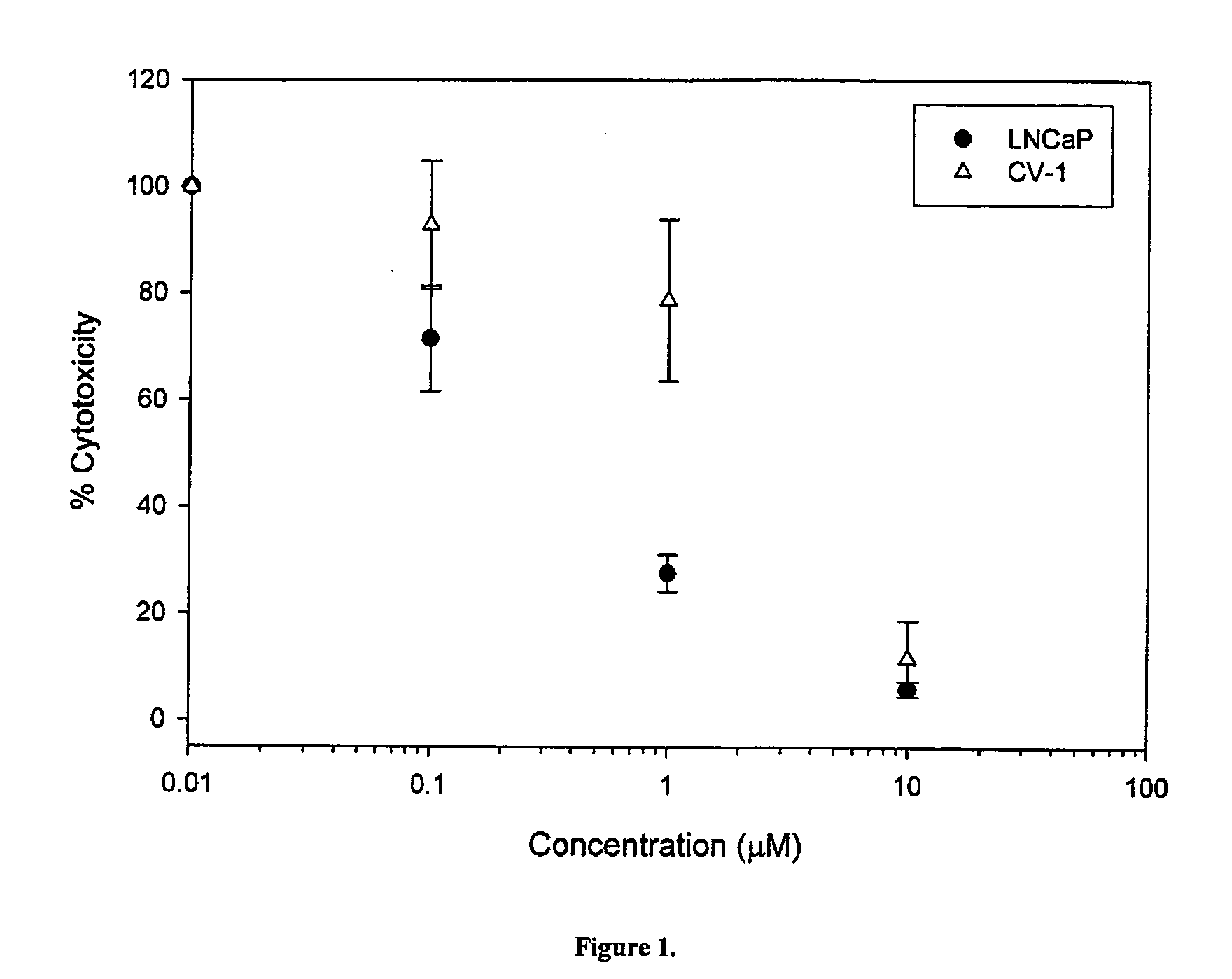
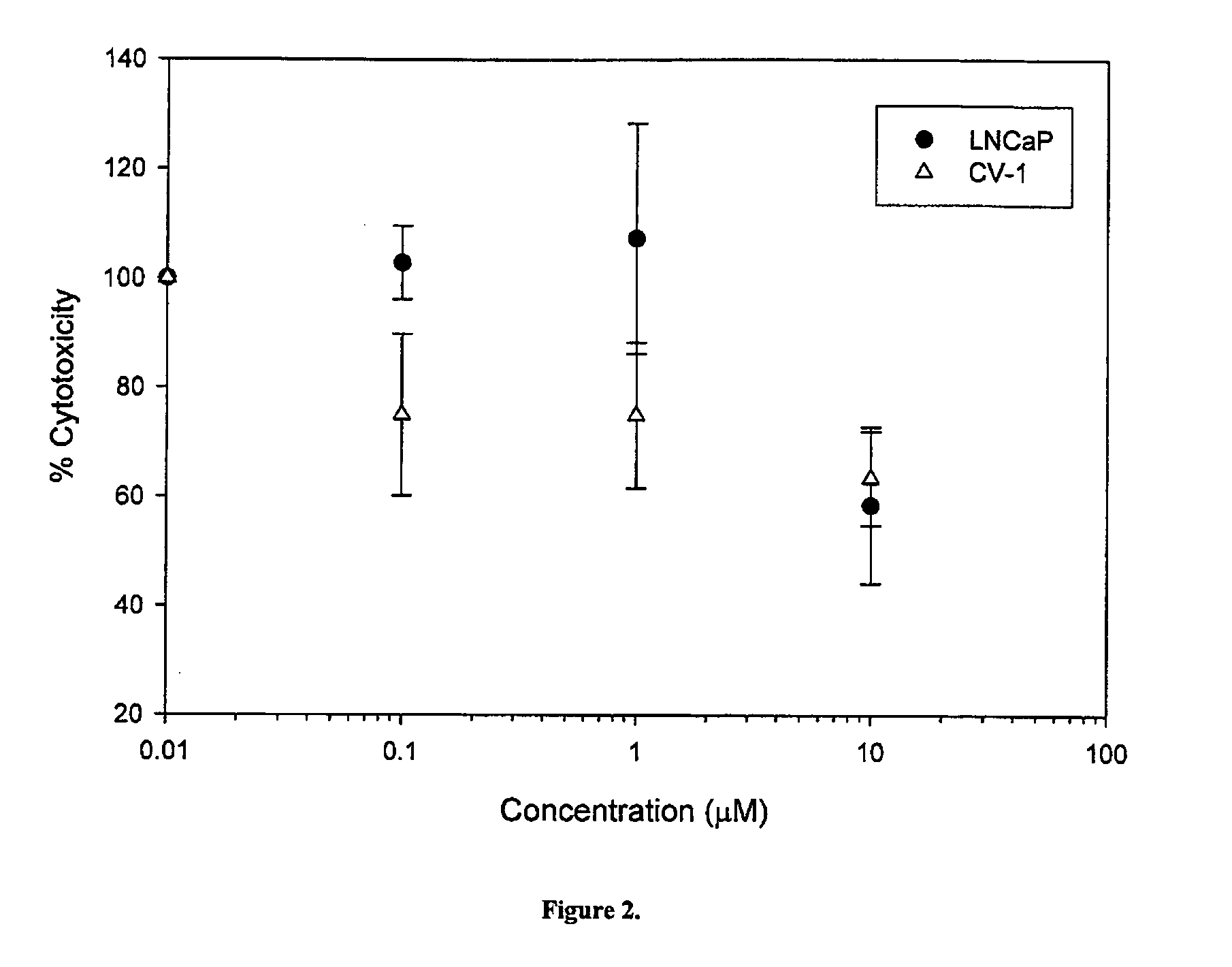
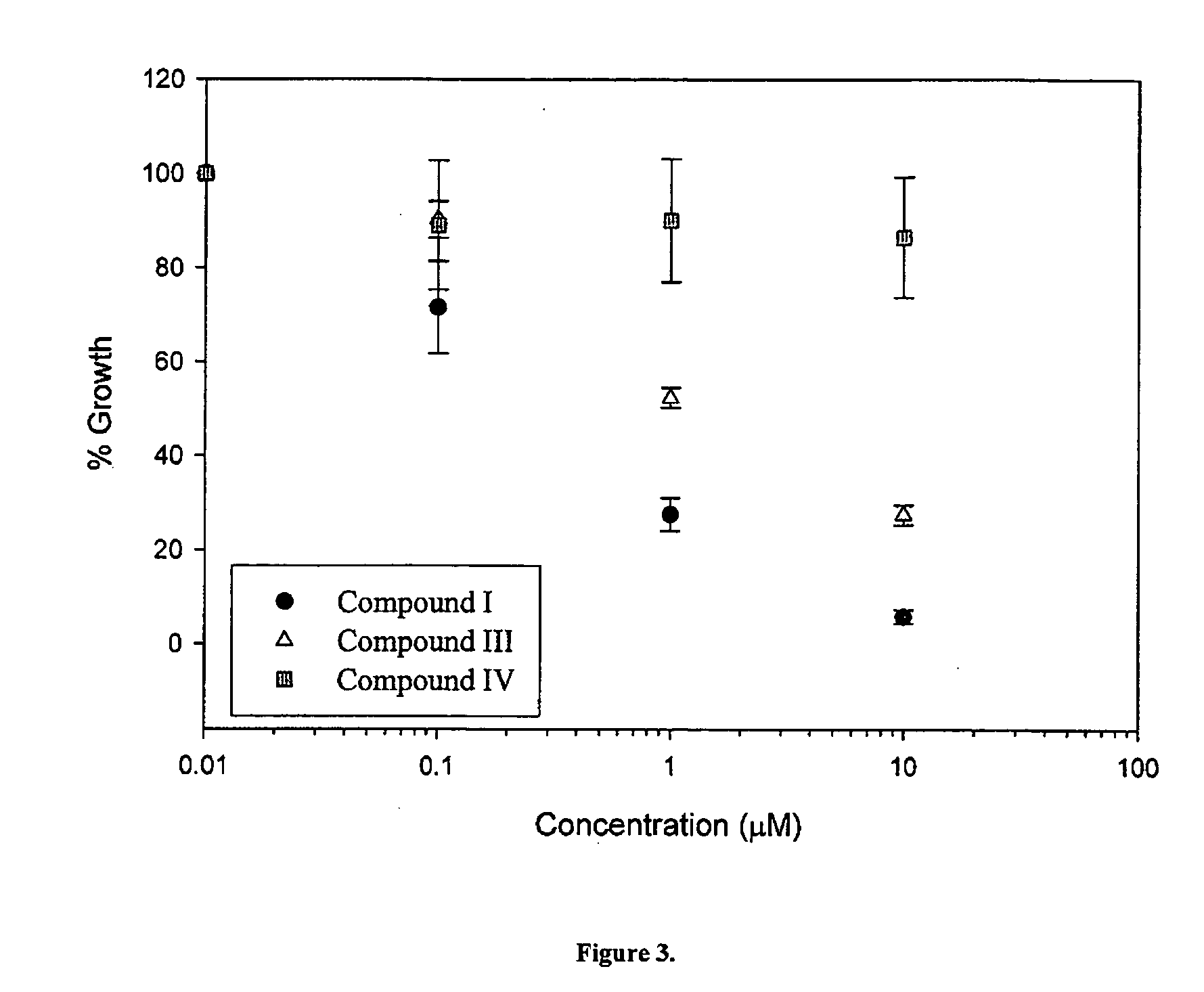
Anti-cancer compounds and methods of use thereof
InactiveUS20050209320A1Shorten the progressDelay the progression of the cancerBiocideCarbamic acid derivatives preparationProstate cancer cellCancer cell
The present invention relates to a novel class of anti-cancer compounds which selectively target androgen receptor (AR)-expressing cancer cells, such as prostate cancer cells and breast cancer cells. These agents comprise an androgen receptor (AR) binding moiety, which selectively targets the compounds to (AR)-expressing cancer cells, and a cytotoxic ablating moiety, such as a nitrogen mustard moiety. The inherent high density expression of the androgen receptor in certain cancers, such as prostate cancer and breast cancer, is thus used as a tool to selectively increase the intracellular concentration of cytotoxic compounds, such as alkylating agents, e.g. DNA alkylating agents, by selectively targeting the agents to the AR-expressing cancer cells. These agents, either alone or in a composition, are thus useful for treating, delaying the progression of, treating the recurrence of, suppressing, inhibiting or reducing the incidence of cancers characterized by the presence of AR-expressing cells, such as prostate cancer. Accordingly, the present invention provides a) methods of selectively killing an (AR)-expressing cancer cell; b) methods of inducing apoptosis in an (AR)-expressing cancer cell; c) methods of treating a cancer characterized by the presence of AR-expressing cells in a subject; d) methods of delaying the progression of a cancer characterized by the presence of AR-expressing cells in a subject; e) methods of treating the recurrence of a cancer characterized by the presence of AR-expressing cells in a subject; f) methods of suppressing, inhibiting or reducing the incidence of a cancer characterized by the presence of AR-expressing cells in a subject; and g) methods of treating metastasis of a cancer characterized by the presence of AR-expressing cells in a subject; by administering to the subject or by contacting the cancer cells with a compound comprising an androgen receptor ligand moiety and an alkylating moiety, such as the novel compounds described herein.
Owner:GTX INCORPORATED
Method for the preparation of reactive hydrogen peroxide in deep eutectic solvents
The subject invention provides a potentially economically viable method for the preparation of hydrogen peroxide (H2O2) in deep eutectic solvents (DES). H2O2 is then used for the destruction of small to large quantities of sulfur and nitrogen mustards and lewisite, their homologous / analogues, and similar chemical warfare agents at ambient conditions in DES without producing any toxic by-products. Furthermore, H2O2 has been used for the destruction of small to large quantities of halogenated hydrocarbons, their homologous / analogues, and similar hazardous chemicals at ambient conditions. H2O2 can be formed by either the electrochemical reduction of oxygen in DES in the presence of water or by dissolving Group 1 (alkali metals) or Group 2 (alkaline earth metals) superoxides, e.g. potassium superoxide, in DES in the presence of water, with / without chemicals used for the enhancement of the solubility of the metal superoxide in the DES, e.g. crown ethers.
Owner:KING SAUD UNIVERSITY
Stabilized Compositions of Volatile Alkylating Agents and Methods of Using Thereof
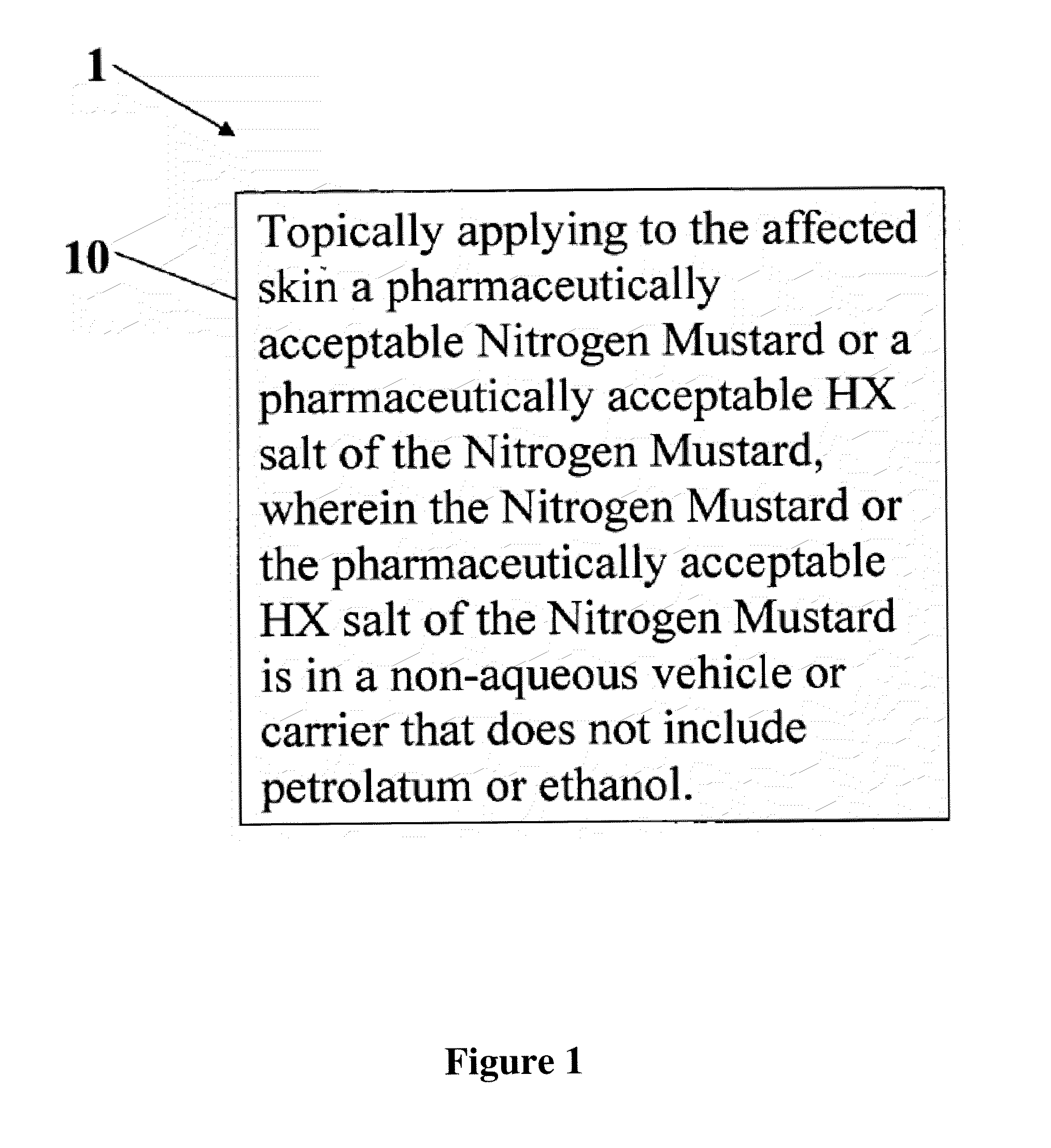
A composition and method for treatment of cancer. The composition for treating a skin disorder, comprising: a Nitrogen Mustard or an HX salt of the Nitrogen Mustard, wherein the Nitrogen Mustard or the HX salt of the Nitrogen Mustard is in a non-aqueous vehicle or carrier that does not include petrolatum or ethanol, wherein the non-aqueous vehicle or carrier that does not include petrolatum or ethanol does not include petrolatum or ethanol. The method comprises topically applying the composition of a Nitrogen Mustard or a HX salt of the Nitrogen Mustard to the affected skin, wherein the Nitrogen Mustard or the HX salt of the Nitrogen Mustard is in a non-aqueous vehicle or carrier that does not include petrolatum or ethanol, wherein the non-aqueous vehicle or carrier does not include petrolatum or ethanol.
Owner:HELSINN BIREX PHARMA
Compositions of alkylating agents and methods of treating skin disorders therewith
InactiveUS20130184243A1BiocidePharmaceutical delivery mechanismAlkylating antineoplastic agentNitrogen mustard
Owner:ACTELION PHARM LTD
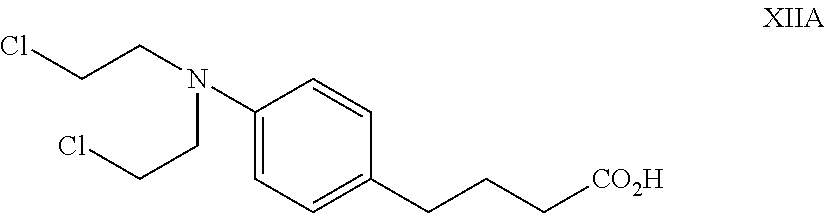
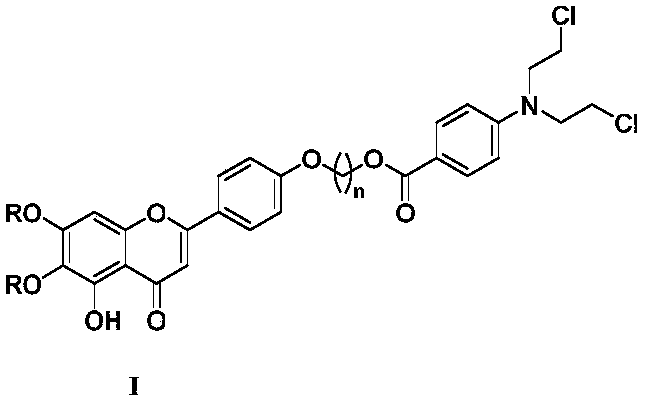
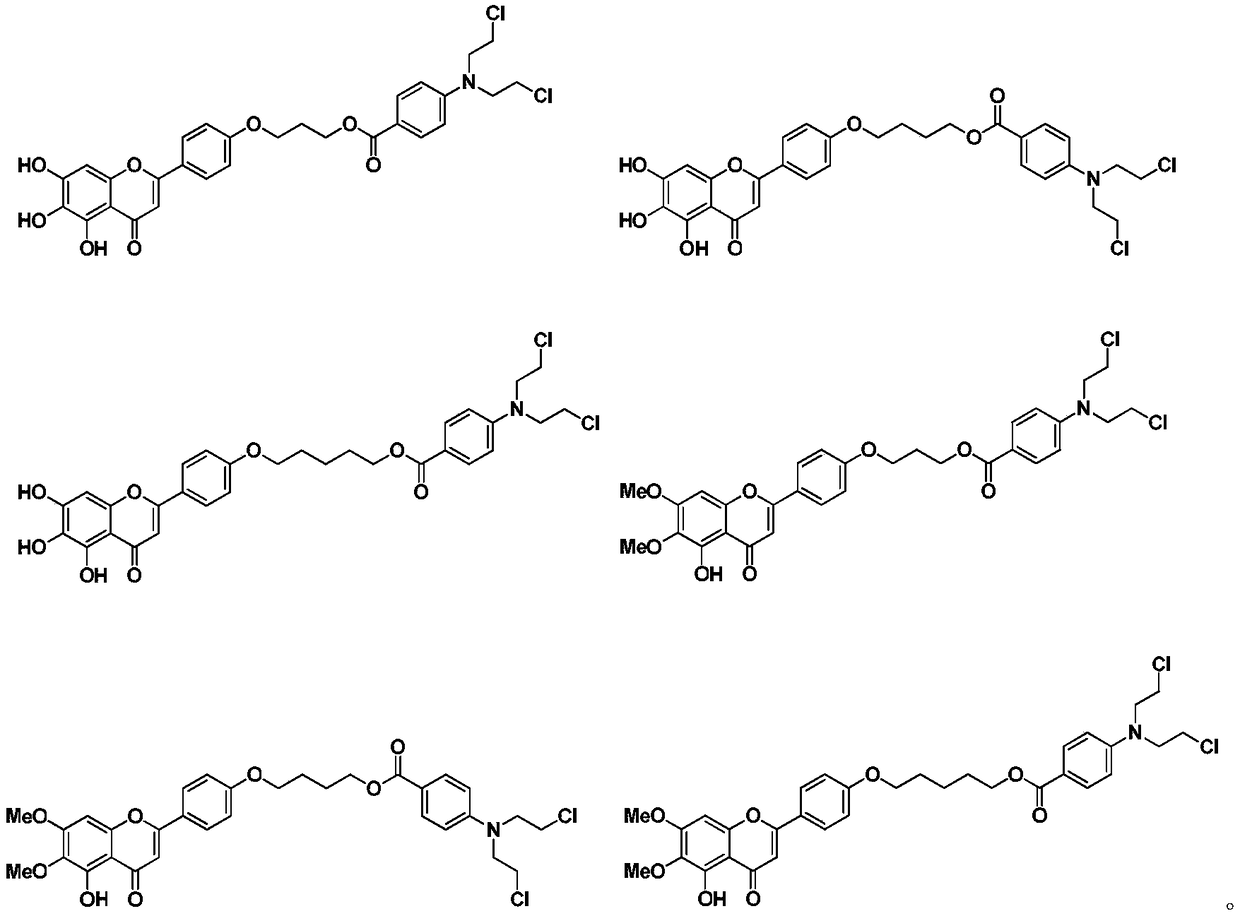
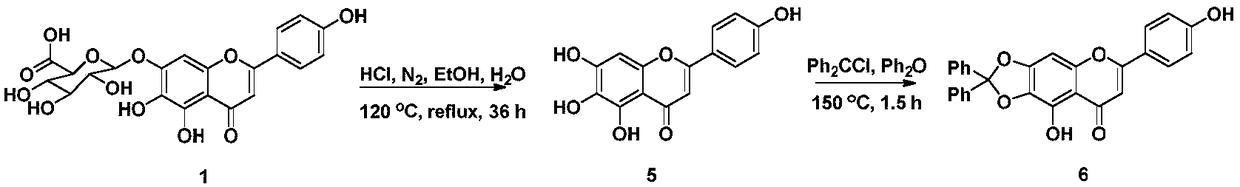
Scutellarin aglycone nitrogen mustard derivative and preparation method and application thereof
ActiveCN108864024AGood antitumor activityGood choiceOrganic active ingredientsOrganic chemistryBenzoic acidAglycone
The invention belongs to the field of natural medicine and medicinal chemistry and relates to a scutellarin aglycone nitrogen mustard derivative and a preparation method and application thereof, in particular to a scutellarin aglycone nitrogen mustard derivative of which 4'-OH is combined with benzoic acid nitrogen mustard and a preparation method and antitumor activity thereof. The structure of the scutellarin aglycone nitrogen mustard derivative and pharmaceutically acceptable salt thereof is as shown in the general formula I, wherein R and n are as described in claims and the specification.The formula I is shown in the description.
Owner:SHENYANG PHARMA UNIVERSITY
Folic acid-benzaldehyde nitrogen mustard-HPMA macromolecule copolymer and preparation and application thereof
InactiveCN103923256AEnhanced inhibitory effectExtended stayOrganic active ingredientsPharmaceutical non-active ingredientsMethacrylamideBenzaldehyde
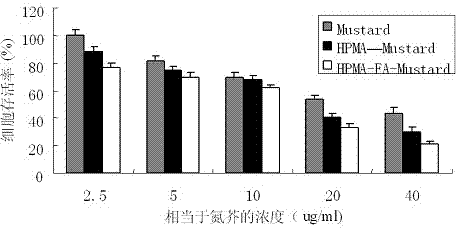
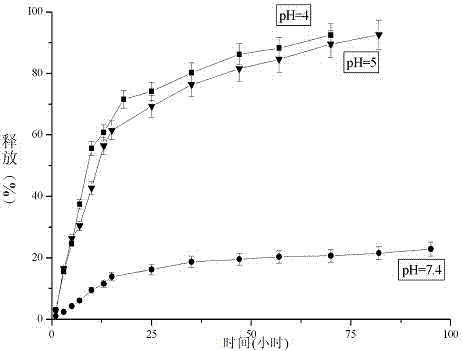
The invention provides a folic acid-benzaldehyde nitrogen mustard-HPMA macromolecule copolymer, which is prepared by bonding folic acid and benzaldehyde nitrogen mustard to N-(2-hydroxypropyl) methacrylate (HPMA) by virtue of covalent bonds and belongs to the field of macromolecular synthesis. According to the folic acid-benzaldehyde nitrogen mustard-HPMA macromolecule copolymer, antineoplastic activity of folic acid, benzaldehyde nitrogen mustard and N-(2-hydroxypropyl) methacrylate are overlapped, so that the inhibiting effect of the polymer on tumor is further improved, and the standing time of anti-cancer medicines on the tumor is greatly prolonged. An experiment proves that the copolymer has a function of targeting intelligent drug release and has the good inhibiting effect on cervical cancer cell HeLa; moreover, because of HPMA, the toxicity of anti-cancer medicines is also reduced, and the high biocompatibility is represented, so that the damage to normal tissues is reduced; therefore, the polymer has good prospect when the polymer used as an HeLa tumor cell inhibiting agent is applied to preparation of anti-tumor medicines.
Owner:NORTHWEST NORMAL UNIVERSITY
Analytic application and method of thiol nucleophilic substitution derivatization reagent
ActiveCN104198603AAchieve separationEasy to detectComponent separationDerivatizationNucleophilic substitution
The invention belongs to the field of analytic chemistry, and relates to an application of a thiol derivatization reagent to detection of mustard gas and related compounds of the mustard gas. The invention also relates to a method for detecting the mustard gas and / or related compounds of the mustard gas. Specifically, the detection method comprises the following steps: using the thiol derivatization reagent or adding the thiol derivatization reagent to a treated or untreated sample to be tested. The application and the method are suitable for the detection of the prototypes of the high-reaction activity mustard gas and the related compounds of the mustard gas, have the advantage of overcoming the abuses that the prototypes cannot be detected and the content detection is incorrect as the mustard gas and nitrogen mustard in the complicated samples undergo rapid prototype or alkylation conversion, have simplicity in operation and good reproducibility and stability, and can be applied to the liquid chromatogram-mass spectrum combined detection of the mustard gas with reaction activity and the related compounds in the environment samples and biological samples and applied to the related virus pharmacological researches.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Aromatic chlorethazine piperazine quaternary ammonium salt derivatives, and preparation and use thereof
InactiveCN101440073AGood water solubilityGood treatment effectOrganic active ingredientsOrganic chemistryNitrogen mustardHydrogen
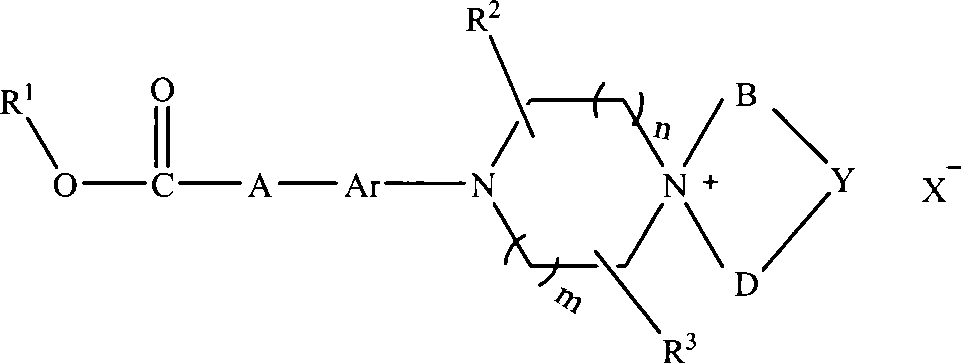
The invention discloses an aryl nitrogen mustard piperazinium derivative with a general formula I or pharmaceutically acceptable salt thereof, wherein R<1> is H, a saturated or unsaturated straight-chain or branched-chain alkyl group, and aryl or substituted aryl, and the alkyl group can be freely substituted by substitutional groups selected from halogens, amino groups, substituted amino groups, hydroxyl groups, cyano groups, nitryl, aryl and substituted aryl; A is saturated or unsaturated straight-chain or branched-chain alkylene, and the alkylene can be substituted by amido; Ar is arylene; R<2> and R<3> is independently hydrogen or methyl; n and m are integers within the range of between 0 and 2, and the n and the m are not zero synchronously; B and D independently represent straight-chain or branched-chain alkyl groups of C1-C3, and straight-chain or branched-chain alkylenes of the C1-C3; Y is -CHR4-, -O-, -S-, -S(O)-, -SO2-, -NR<4>-, and substituted or unsubstituted phenylene, wherein R<4> is H, saturated or unsaturated alkyl groups with 1 to 6 carbon atoms, optionally substituted or unsubstituted aryl, or aromatic heterocyclic-substituted methyl or ethyl; or when the B and the D are alkyl groups, the Y does not exist, that is, the derivative is ring-opened and does not form a spiro ring; and X<-> is pharmaceutically acceptable inorganic or organic anions.
Owner:PEKING UNIV
Two nitrogen mustard derivatives, as well as preparation method and application therefore in tumor treatment
InactiveCN103450096AImprove anti-tumor effectSynthetic steps with high yieldsOrganic active ingredientsOrganic chemistryAlkyl transferIn vivo
The invention discloses novel nitrogen mustard derivatives. The nitrogen mustard derivatives are characterized in that a nitrogen mustard alkylation group is at one end, a 6,7-substituted quinazoline structure is at the other end, the substituent R1 is located at the 4th position of the quinazoline main body and is 2-nitrogen mustard group or 3-nitrogen mustard group, morpholinepropoxy and methoxy are located at the 6th position and the 7th position of the quinazoline main body, and the structural formula is shown in formula A. Experiments prove that the compounds can inhibit the cell cycle at the G2 / M period and are di-functional alkylation agents. The in-vivo anti-tumor activity experiments prove that the compounds are quite good in activity. In addition, the compounds have the superiority to other nitrogen mustard medicines, namely, the compounds are quite low in toxicity. Simultaneously, the compounds are easy to synthesize, and quite high in total yield. All the above advantages prove that the compounds have great potential to function as medicines for treating tumors.
Owner:BEIJING NORMAL UNIVERSITY
Novel quinazoline nitrogen mustard compound, and preparation method and application thereof to treatment of cancer
InactiveCN103193722AHighly innovativeImprove anti-tumor effectOrganic chemistryAntineoplastic agentsEthoxidineMorpholine
A novel quinazoline nitrogen mustard compound is characterized in that: one end is provided with a nitrogen mustard alkylating group; the other end is provided with a 6, 7-substituted quinazoline structure; a substituent R1 is located at a site 4 of a quinazoline matrix, and represents 2-, 3-, 4- nitrogen mustard substituent; and substituents R2, R3 are located at site 6 and 7 of a quinazoline matrix, and represent methoxyethoxy, methoxy, morpholine propoxy, 3-etrahydrofuran oxygen group and hydroxyl group. The compound has a structure shown as a formula A. Experiments show that the compound can cause cross-linking of DNA, and is a bifunctional alkylating agent. In vivo antitumor activity experiment show that the compound has good activity; furthermore, the compound has the advantage of low toxicity, which a nitrogen mustard drug is lack of. At the same time, the compound is easy for synthesis, has high total yield. Advantages of the compound show that it has great potential to become a drug for treatment of cancer.
Owner:BEIJING NORMAL UNIVERSITY
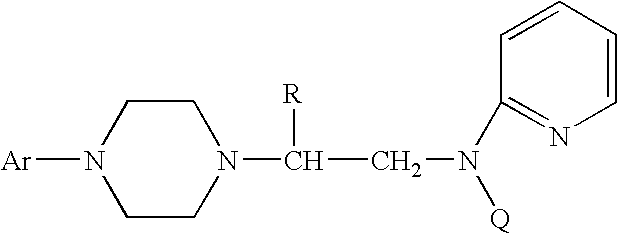
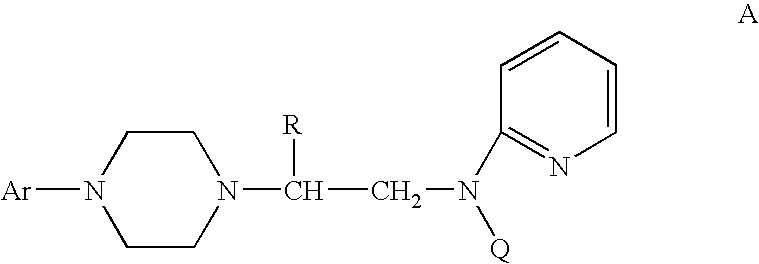
Process for making chiral 1,4-disubstituted piperazines
A process for a stereoselective preparation of novel chiral nitrogen mustard derivatives useful in synthesizing optically active 1,4-disubstituted piperazines of formula: wherein R, Ar, and Q are defined as set forth herein, and intermediate compounds therefor. The 1,4-disubstituted piperazines act as 5HT1A receptor binding agents useful in the treatment of Central Nervous System (CNS) disorders.
Owner:WYETH LLC
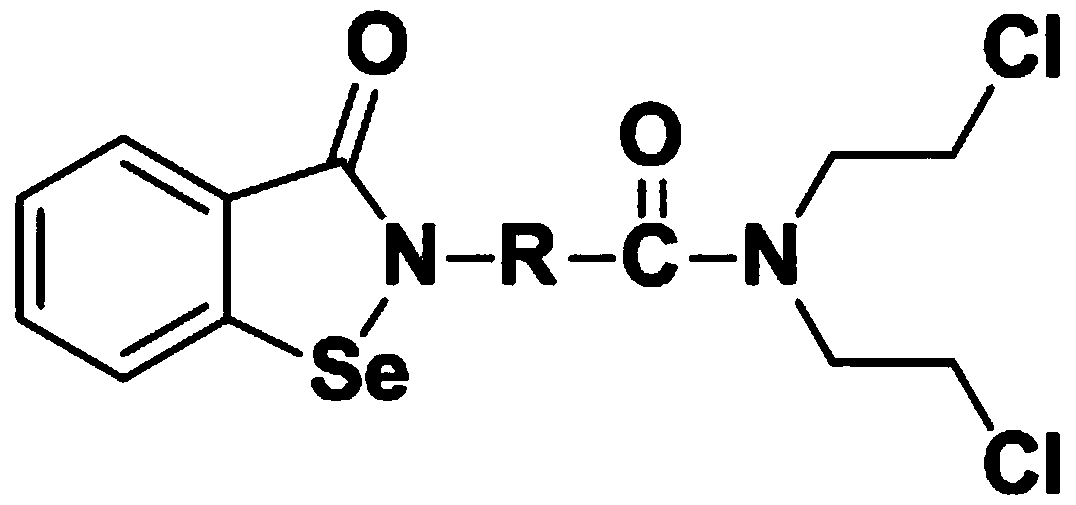
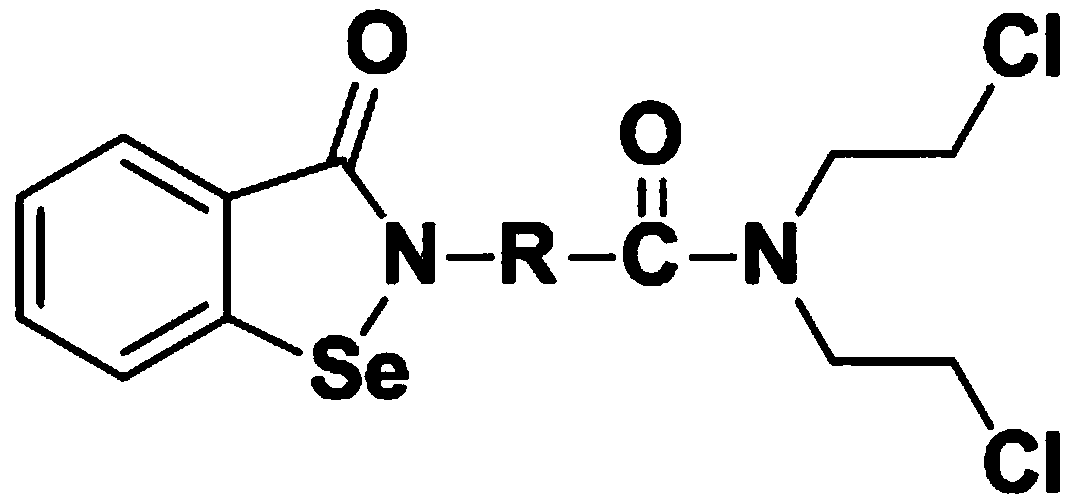
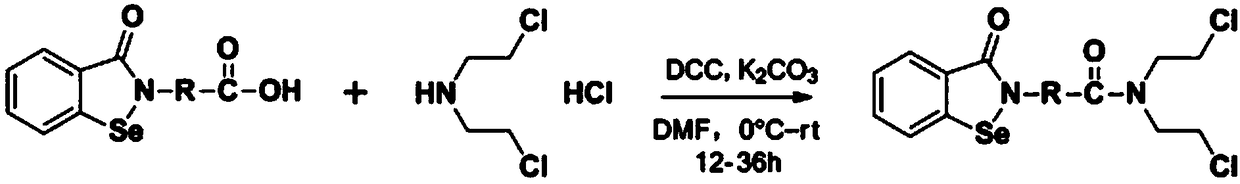
N,N-bis(2-chloroethyl)-2-(benzoisoselenazole-3-one)-amide compound with anti-tumor activity
ActiveCN108503607AGood biocompatibilityLow toxicityOrganic chemistryAntineoplastic agentsChemical synthesisSide effect
The invention relates to an N,N-bis(2-chloroethyl)-2-(benzoisoselenazole-3-one)-amide compound with anti-tumor activity. The amide compound is obtained by taking benzoisoselenazole-3-one-2-amino carboxylic acid and di(2-chloroethyl) amine hydrochloride as raw materials and carrying out condensation reaction in an organic solvent under the actions of a condensation reagent and a catalyst. The amidecompound molecule contains a benzoisoselenazole active group with cancer inhibition, anticancer and attenuation activities and also contains a nitrogen mustard active group with obvious anticancer activity and relatively high biocompatibility, amino acid is taken as a carrier, multiple active groups are bridged and spliced into one chemical molecular structure by virtue of a chemical synthesis means, on the one hand, the combined effect of benzoisoselenazole and a nitrogen mustard anticancer structural unit is played, and toxicity and side effect of a nitrogen mustard bio-alkylating agent arereduced due to introduction of a selenium element; and on the other hand, the aim of efficiently conveying an organic selenium compound to tumor tissues is achieved by virtue of non-selective combining capability of a nitrogen mustard compound.
Owner:济源希健生物医药科技发展有限公司
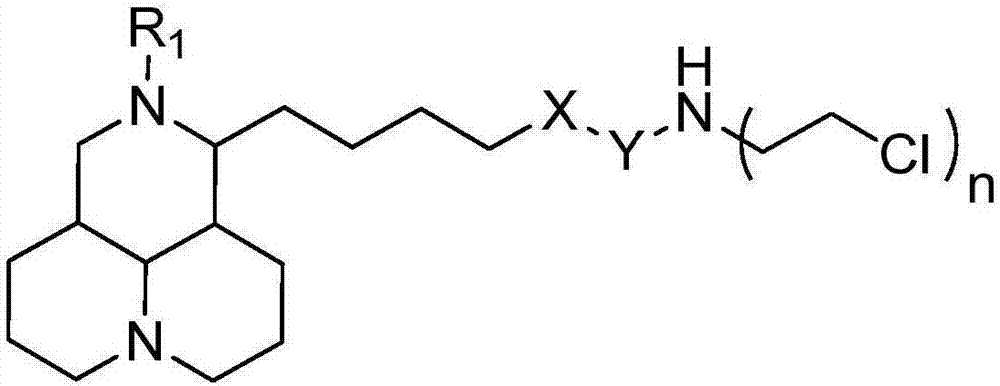
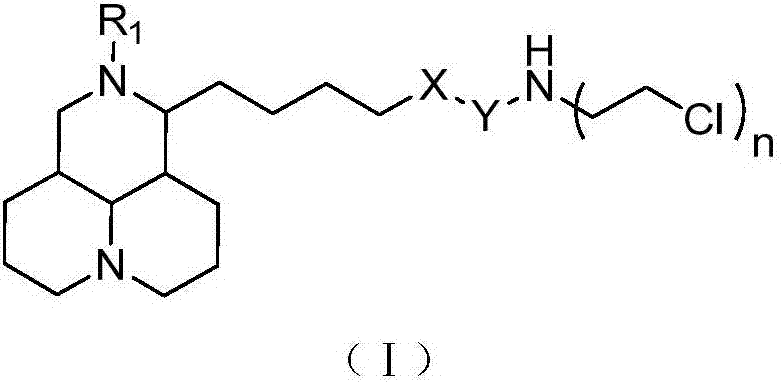
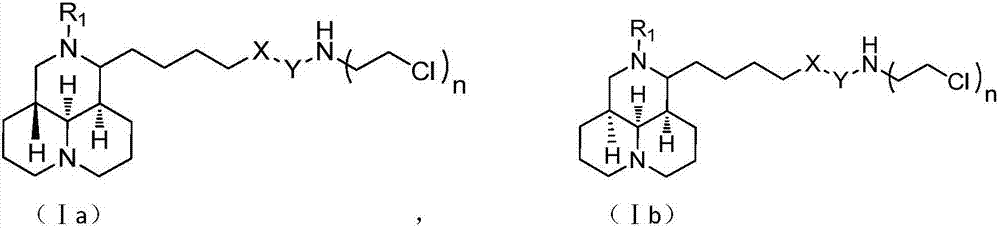
Sophoridine derivative with nitrogen mustard as well as preparation method and application of sophoridine derivative
ActiveCN107129497AGood inhibitory effectEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryCombinatorial chemistryNornitrogen mustard
The invention belongs to the technical field of anti-tumor medicines, and provides a sophoridine derivative with nitrogen mustard as well as a preparation method and application of the sophoridine derivative, and pharmaceutically acceptable salts of the sophoridine derivative, wherein R1, X, Y and n are as shown in the specification. The invention further relates to a preparation method of the compound, and further discloses a medicinal composition of the compound or the pharmaceutically acceptable salts of the compound as active effective components, and application of the components as anti-tumor medicines.
Owner:天津市医药科学研究所
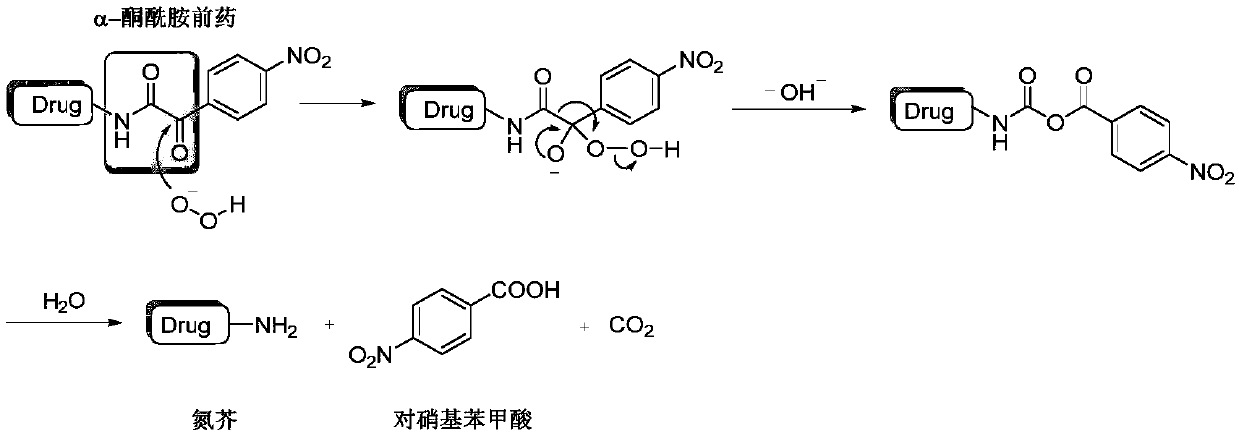
Hydrogen peroxide-responsive nitrogen mustard anti-tumor pro-drug and preparation method thereof
ActiveCN110305036AImprove responsivenessHigh selectivityOrganic active ingredientsOrganic compound preparationHigh cellSide effect
The invention discloses a hydrogen peroxide-responsive nitrogen mustard anti-tumor pro-drug and a preparation method thereof, and belongs to the field of pharmaceutical chemistry. The compound contains an alpha-ketoamide structure and a nitrogen mustard structure, can rapidly respond to H2O2, can be used as the nitrogen mustard anti-tumor pro-drug, has good response effect to H2O2, has high cell selectivity and small toxic and side effects, provides an effective, safe and highly selective anti-tumor drug, enriches the types of nitrogen mustard anti-tumor drugs, and has a good market prospect.
Owner:JIANGNAN UNIV
Application of nitrogen mustard based piperlongumine compound in medicine
The invention provides application of a nitrogen mustard based piperlongumine compound in medicine. On the basis of the research on the nitrogen mustard based piperlongumine compound for inhibiting the activity of malignant cells, it proves that the nitrogen mustard based piperlongumine compound has the good anti-tumor activity, and a new choice is provided for preparing anti-tumor medicine.
Owner:广东永纯医药集团有限责任公司
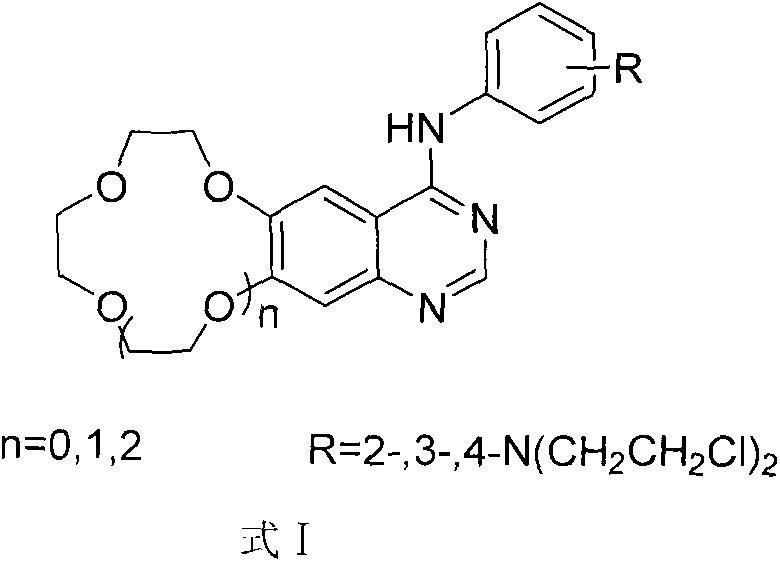
Crown ether ring-shaped quinazoline nitrogen mustard compound, and preparation method and application thereof in tumor treatment
InactiveCN103601730AFew synthetic stepsEasy to synthesizeOrganic active ingredientsOrganic chemistryQuinazolineAntitumor activity
A new crown ether ring-shaped quinazoline nitrogen mustard compound or a pharmaceutically-acceptable salt thereof is characterized in that: one end is provided with a nitrogen mustard alkylated group; the other end is provided with a 6,7-substituted crown ether ring-shaped quinazoline structure; a substituted group R is located at a site 4 of a quinazoline matrix and represents 2-nitrogen mustard group, 3-nitrogen mustard group or 4-nitrogen mustard group; a crown ether ring is located at sites 6 and 7 of the quinazoline matrix and represents 9-crown-3, 12-crown-4 or 15-crown-5; and the compound has a structure represented by a formula I. Experiments show that the compound has better antitumor activity and also has selectivity on different tumor cells. At the same time, the compound is easy to synthesize and simple in operation. Advantages of the compound show that the compound has great potential to become a drug for treatment of tumor.
Owner:BEIJING NORMAL UNIVERSITY
Nitrogen mustard quercetin derivative, and preparation method and application thereof
ActiveCN106854223AHas anticancer activityGroup 5/15 element organic compoundsAntineoplastic agentsNitrogen mustardActive component
The invention relates to a nitrogen mustard quercetin derivative comprising a stereisomer or a tautomer, a preparation method of the nitrogen mustard quercetin derivative, a medicinal composition taking the derivative as an active component, and application of the medicine to cancer treatment. The preparation method of the derivative comprises the following steps: preparing Mannich alkali of quercetin from the quercetin, formaldehyde and chlorethamin, and performing reaction on the Mannich alkali and substituted phosphoramidic dichloride to prepare the nitrogen mustard quercetin derivative. The nitrogen mustard quercetin derivative has an anti-tumor effect.
Owner:西安天一生物技术股份有限公司
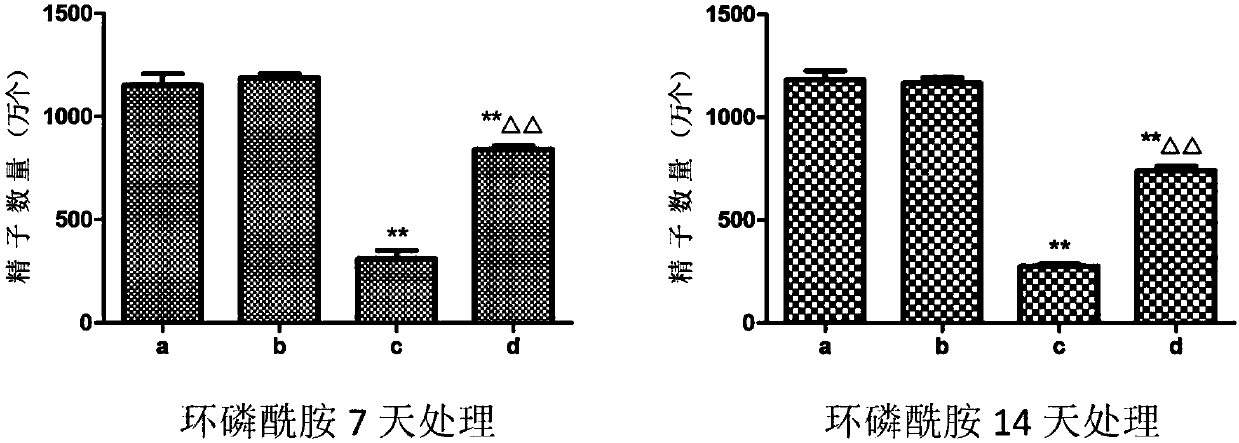
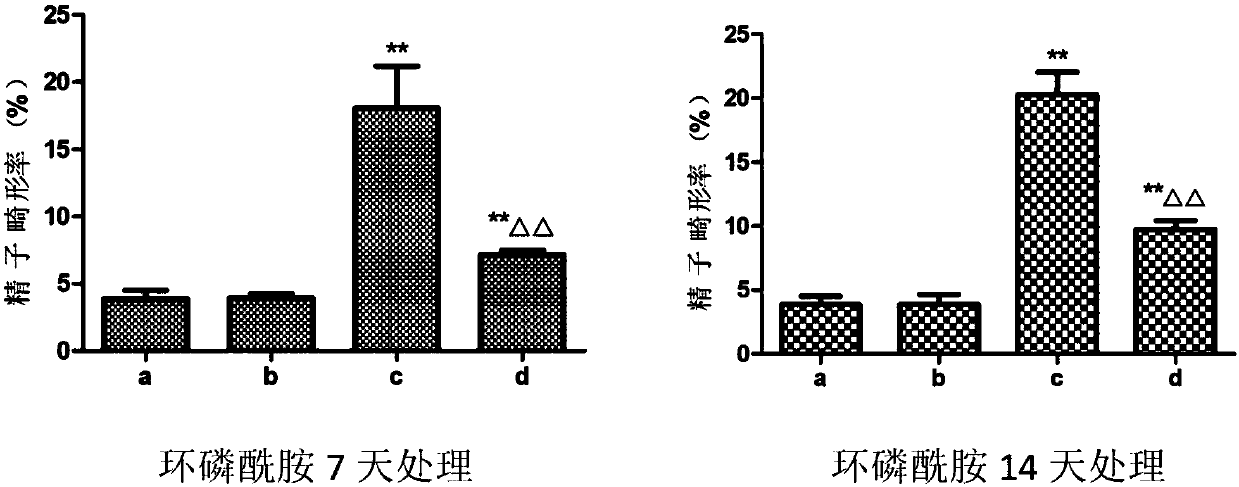
Application of dendrobium polysaccharide in preparation of drugs to prevent or restore reproductive injury after chemotherapy
InactiveCN109985060AReduce and prevent reproductive damageWide variety of sourcesOrganic active ingredientsSexual disorderGynecologySide effect
The invention discloses an application of dendrobium polysaccharide in preparation of drugs to prevent or restore reproductive injury after chemotherapy. The dendrobium polysaccharide applied to prevent and treat male reproductive injury caused by radiotherapy and chemotherapy has obvious therapeutic effect and small side effects, and can effectively reduce the damage caused by nitrogen mustard anticancer drugs to the reproductive system, which not only makes the sperm quantity of mice rise significantly, but also improves the sperm quality, and effectively improves the antioxidant activity ofmouse testicular tissue, and in mouse testicular tissue, the level of SOD, GPx, GSH and CAT is obviously increased while the level of MDA is obviously decreased. The dendrobium polysaccharide with wide source of raw materials is simple in extraction and purification method, high in sugar content and suitable for large-scale production and popularization and has a good prospect in the preparationand / or treatment of reproductive injury after chemotherapy.
Owner:JINAN UNIVERSITY
Azo aryl nitrogen mustard-chloroethylnitrosourea coupled compound, and preparation method and application thereof
ActiveCN107903267AImprove targetingGrowth inhibitionOrganic chemistryAntineoplastic agentsAlkylating antineoplastic agentNitroso
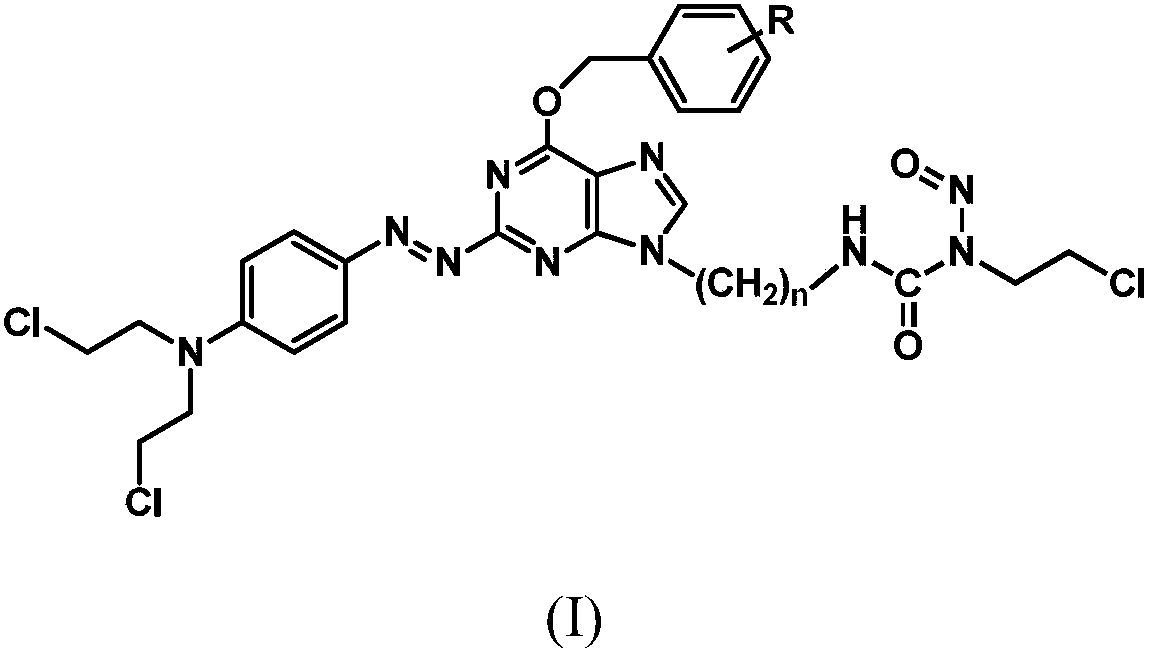
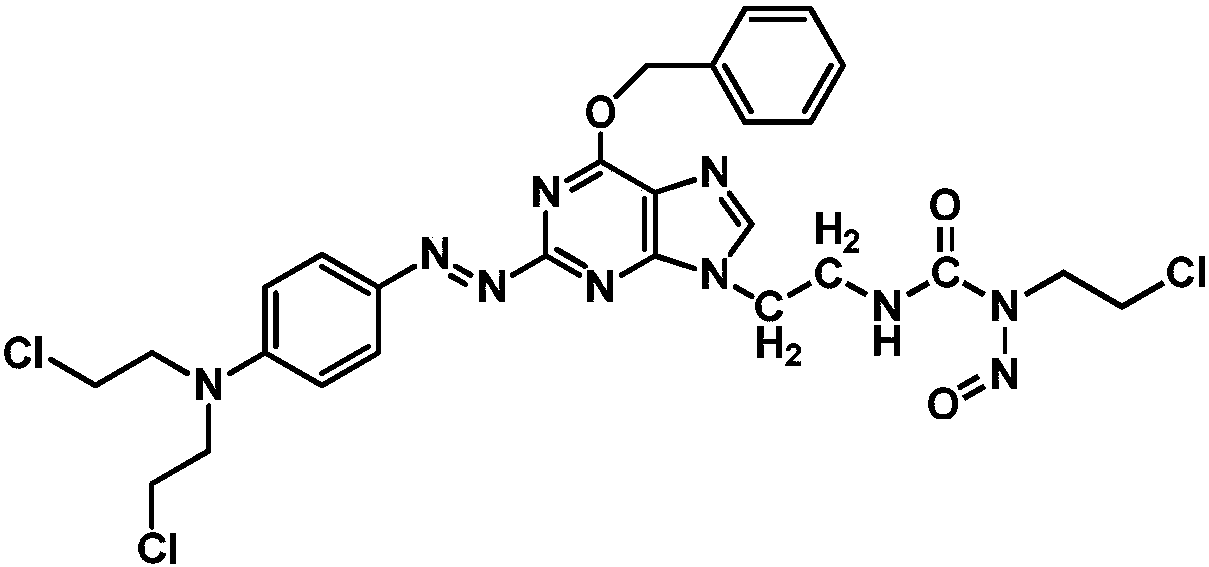
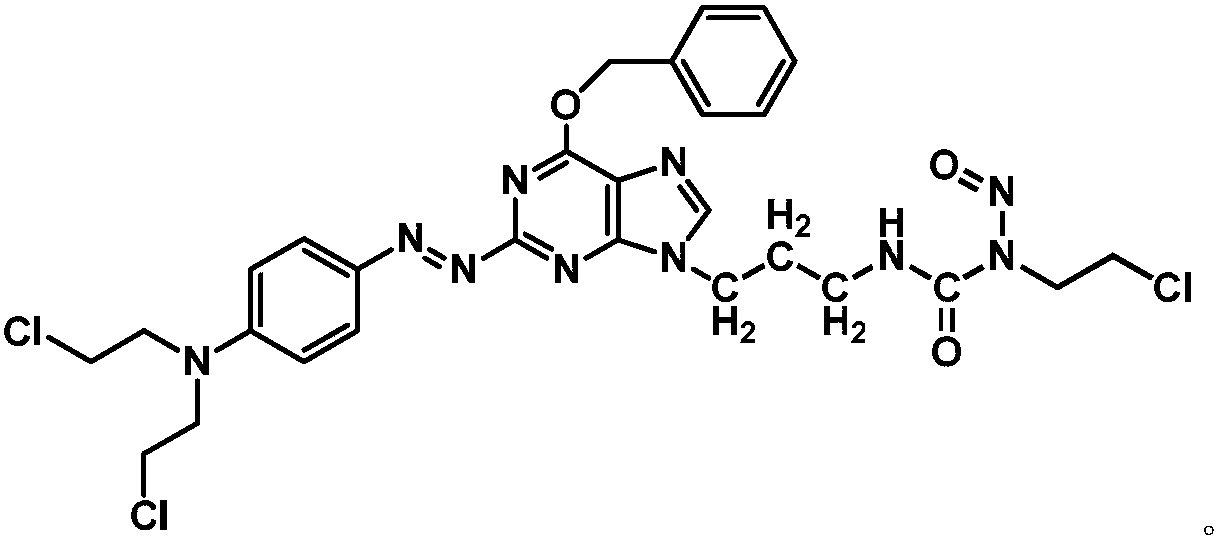
The invention relates to a compound has a structure represented by formula (I), or a pharmaceutically acceptable salt thereof. In the compound, the azo group is a low oxygen-activated pharmacophore, anitrogen-nitrogen double bond in the azo group cleaves and releases aromatic nitrogen mustard and an O6-BG analogue under tumor hypoxic conditions, and the aromatic nitrogen mustard and an O6-BG analogue act as an alkylating agent and an AGT inhibitor in a hypoxic area in a targeting manner to make tumor cells sensitive to the alkylating agent; and the CENUs pharmacophores in the compound can bedecompose to generate chloroethyl carbocations in order to cause cross-linking between DNA strands, so the growth of tumor cells is inhibited.
Owner:BEIJING UNIV OF TECH
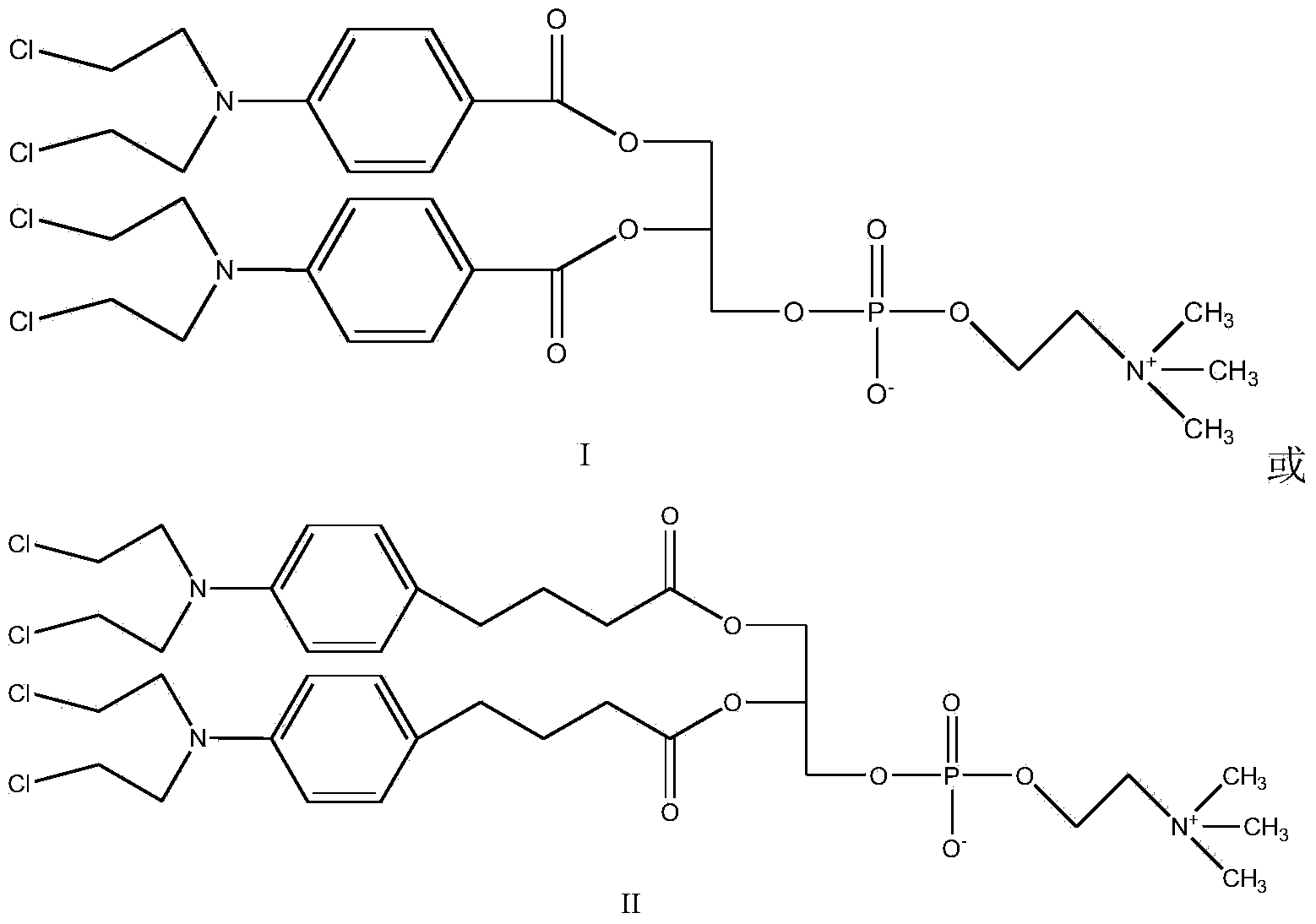
Synthetic method of nitrogen mustard-glycerol phosphatidyl choline compound
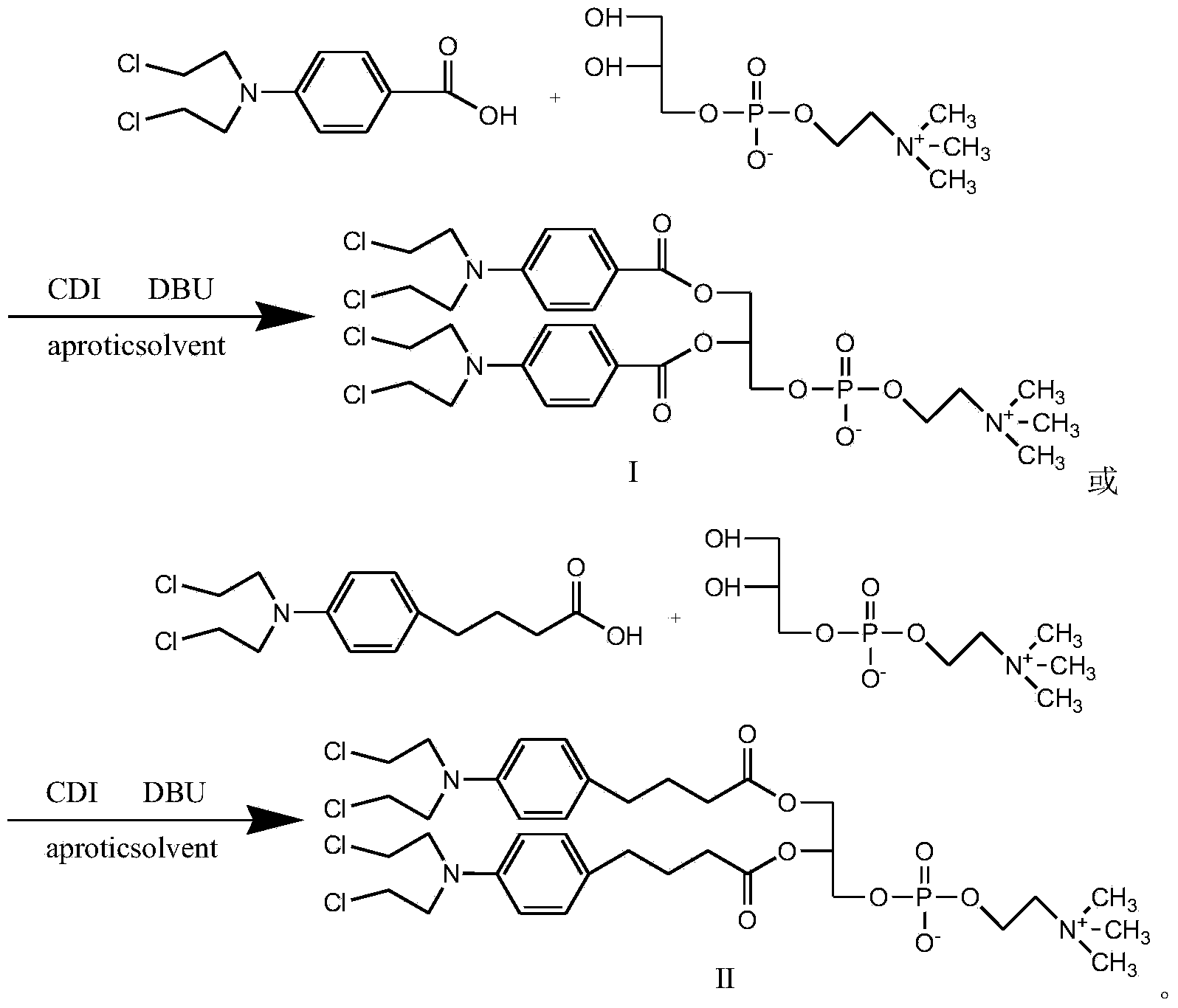
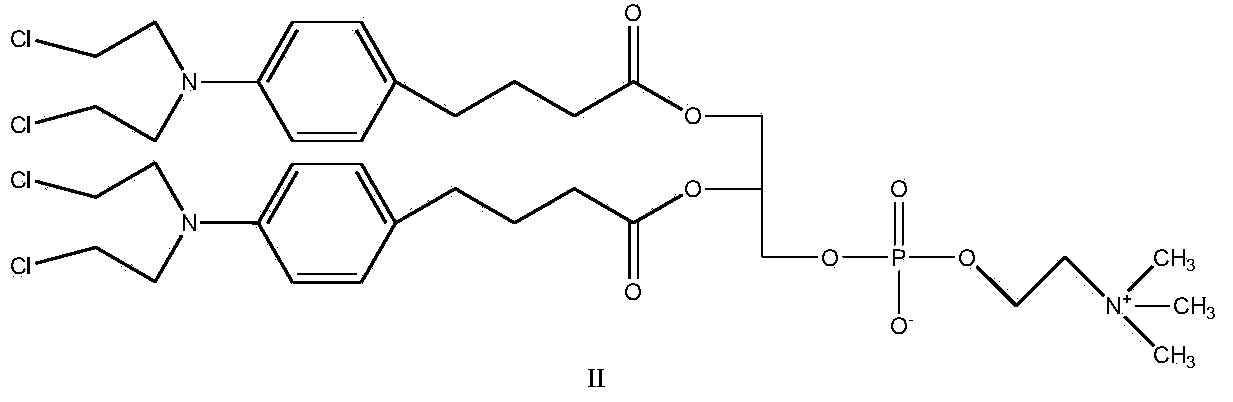
InactiveCN104017019AMild reaction conditionsShort reaction timePhosphatide foodstuff compositionsAntineoplastic agentsGlycerolRoom temperature
The invention discloses a synthetic method of a nitrogen mustard-glycerol phosphatidyl choline compound. The synthetic method comprises the following step of reacting at room temperature by taking a nitrogen mustard-type compound and glycerol phosphatidyl choline as raw materials, taking N, N'-carbonyldimidazole and 1, 8-diazabicyclo C11-7-alkene and taking an aprotic solvent as a reaction solvent, thereby preparing the nitrogen mustard-glycerol phosphatidyl choline compound. The method disclosed by the invention is simple to operate, is mild in reaction condition, and is high in yield.
Owner:SOUTHEAST UNIV
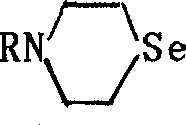
Seleno morpholine, its derivative and their prepn. and use
InactiveCN1336372AIncrease selenium contentOrganic chemistryFertilizer mixturesHalohydrocarbonPotassium borohydride
The present invention relates to preparation of seleno morpholine and its derivative, its general formula is disclosed. The preparation process is as follows: selenium and potassium borohydride react to obtain potassium selenium hydride, then react with nitrogen mustard hydro-chloride, collect seleno morpholine from reaction mixture, the seleno morpholine reacts with halohydrocarbon to obtain alkyl seleno morpholine, selenomorpholine reacts with R'COX to obtain acyl seleno morpholine, selenomorpholine reacts with R'CHO, (RO)2POH to obtain phosphonate ester substituted alkyl morpholine. The invented product can be used as selenium enriching agent for plants, especially for garlic, it can raise selenium content in garlic to more than 10 times.
Owner:WUHAN UNIV
2-methoxyestramustine and derivative thereof, and preparation method and application thereof
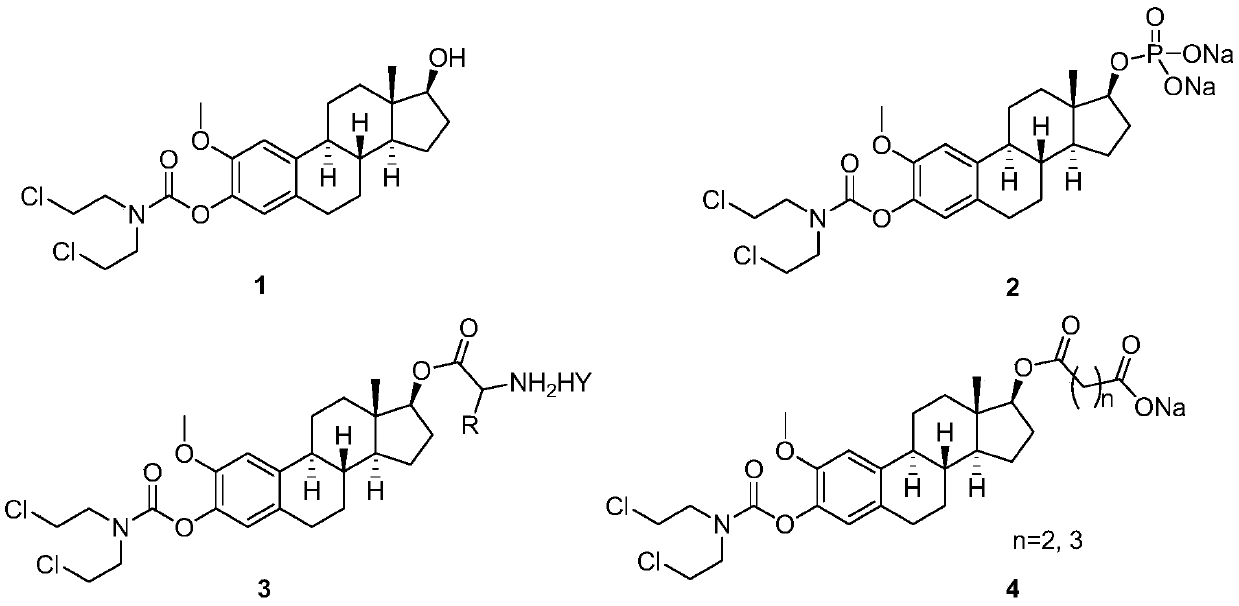
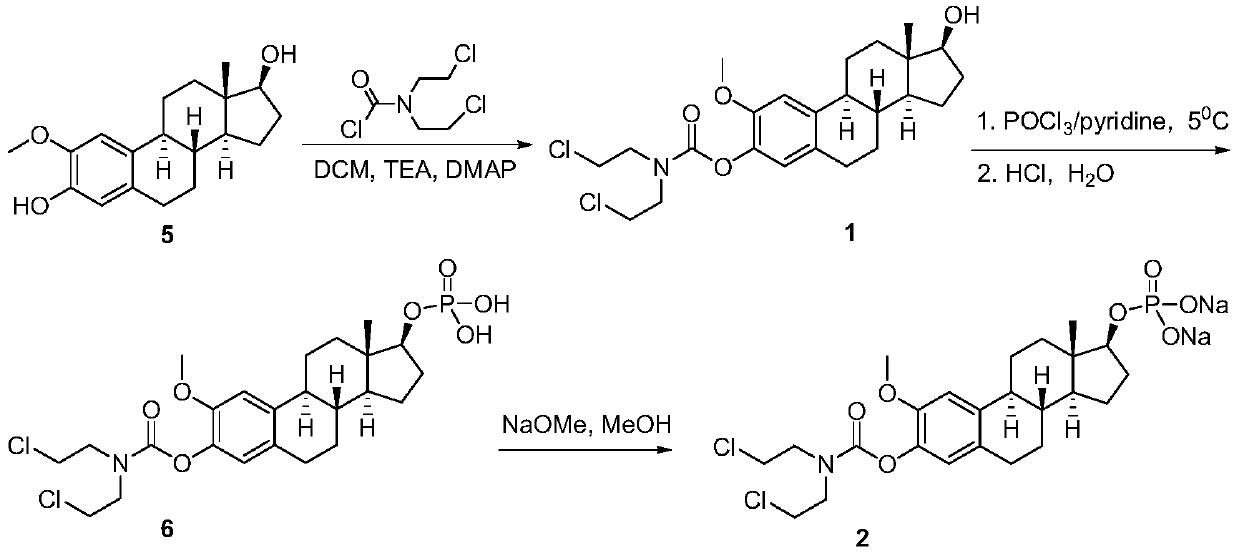
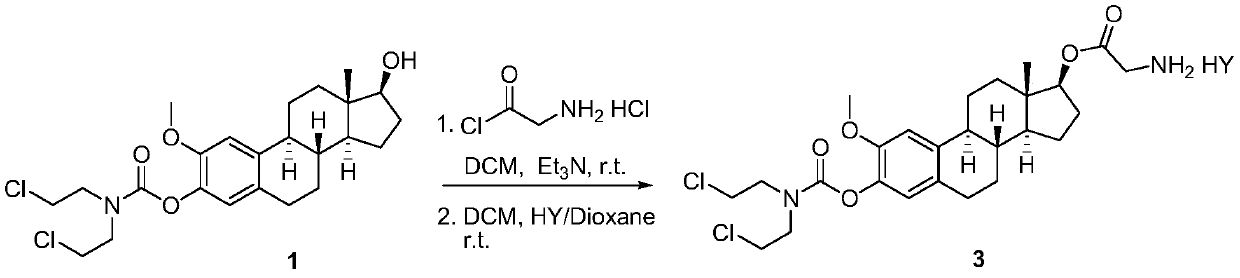
ActiveCN111410677AGood water solubilityLess adverse side effectsOrganic active ingredientsSteroidsAlkylating antineoplastic agentMetabolite
The invention discloses 2-methoxyestramustine and a derivative thereof. The structural formula of the 2-methoxyestramustine is shown as a formula 1-4. According to the 2-methoxyestramustine disclosedby the invention, 2-methoxyestradiol is used as a carrier, an alkylating agent (nitrogen mustard) is connected through carbamate to form a bifunctional drug molecular compound, the whole molecule is used as an anti-mitotic agent, and after carbamate is metabolized and hydrolyzed in vivo, 2-methoxyestradiol released by metabolite mediation can still continue to play an anti-tumor role. The derivative of the 2-methoxyestradiol can be used as the prodrug of the 2-methoxyestradiol. The invention also discloses a preparation method of the 2-methoxyestramustine and the derivative thereof, the preparation method can be used for preparing the 2-methoxyestramustine and the derivative thereof, and the yield is relatively high. The invention further discloses application of the 2-methoxyestramustineand the derivative thereof in a medicine for treating tumors or multiple myeloma.
Owner:周亚耀
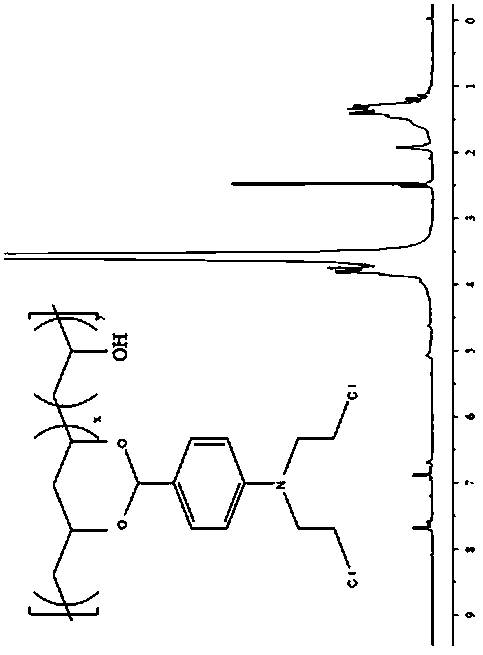
Polyvinyl alcohol-benzaldehyde nitrogen mustard polymer anti-cancer drug, preparation method thereof and application of drug
ActiveCN108785685AExtended stayLow toxicityOrganic active ingredientsPharmaceutical non-active ingredientsBenzaldehydeCancer drugs
The invention relates to a polyvinyl alcohol-benzaldehyde nitrogen mustard polymer anti-cancer drug. The average molecular weight of the anti-cancer drug is 20-40kDa, and the structural formula of theanti-cancer drug is as shown in the specification, wherein x=10-100mol%, and y=0-90mol%. The invention further discloses a preparation method and an application of the anti-cancer drug. The staying time of the anti-cancer drug in a tumor can be prolonged, and harm to a normal tissue is reduced.
Owner:GANSU AGRI UNIV
Nitrogen mustard derivative N,N-di(2-chloroethyl)-N'-hexadecanoyl-1,4-phenylenediamine and preparation method thereof
InactiveCN106631875AImprove therapeutic indexSmall side effectsOrganic compound preparationAntipyreticDichloromethaneChemistry
The invention discloses a structural formula of a nitrogen mustard derivative N,N-di(2-chloroethyl)-N'-hexadecanoyl-1,4-phenylenediamine, and a preparation method thereof. The preparation method comprises the following steps: preparing N,N-di(2-chloroethyl)-1,4-phenylenediamine; putting the reaction raw materials comprising the N,N-di(2-chloroethyl)-1,4-phenylenediamine, dichloromethane and triethylamine into a reactor, cooling in ice-water bath, stirring, dropwise adding a mixed solution of hexadecanoyl chloride and dichloromethane into the reactor, removing the ice-water bath after addition, conducting reaction at room temperature for 12 to 14 hours, sequentially performing washing, drying and normal-pressure distillation on the reaction liquid after the reaction is conducted completely, and purifying the distilled filter cake to obtain the product. The arylamine nitrogen mustard derivative provided by the invention can effectively reduce the toxic and side effects of nitrogen mustard on the premise of enhancing the treatment index of the nitrogen mustard, and has sterilization and inflammation-diminishing curative effects to reduce the risk of complication caused by the fact that the immunity is reduced after a patient is subjected to chemical therapy.
Owner:CHANGAN UNIV
A thiosulfate silver plating additive, its preparation method and its electroplating solution
Owner:NANJING INST OF PROD QUALITY INSPECTION
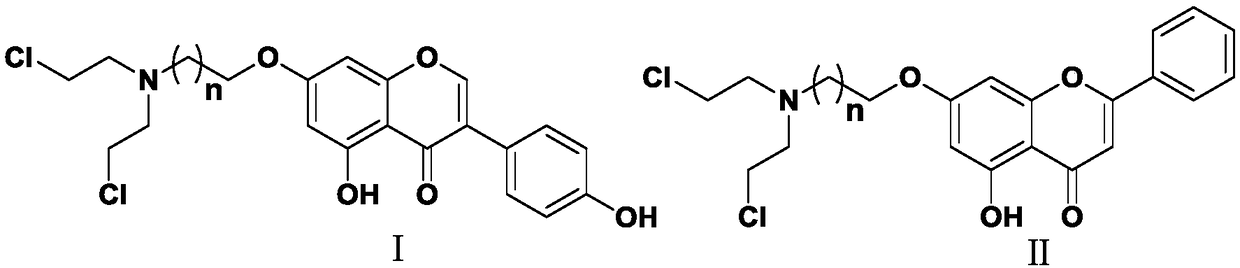
Nitrogen mustard-based flavonoid derivative, preparation method thereof and application in anti-tumor direction
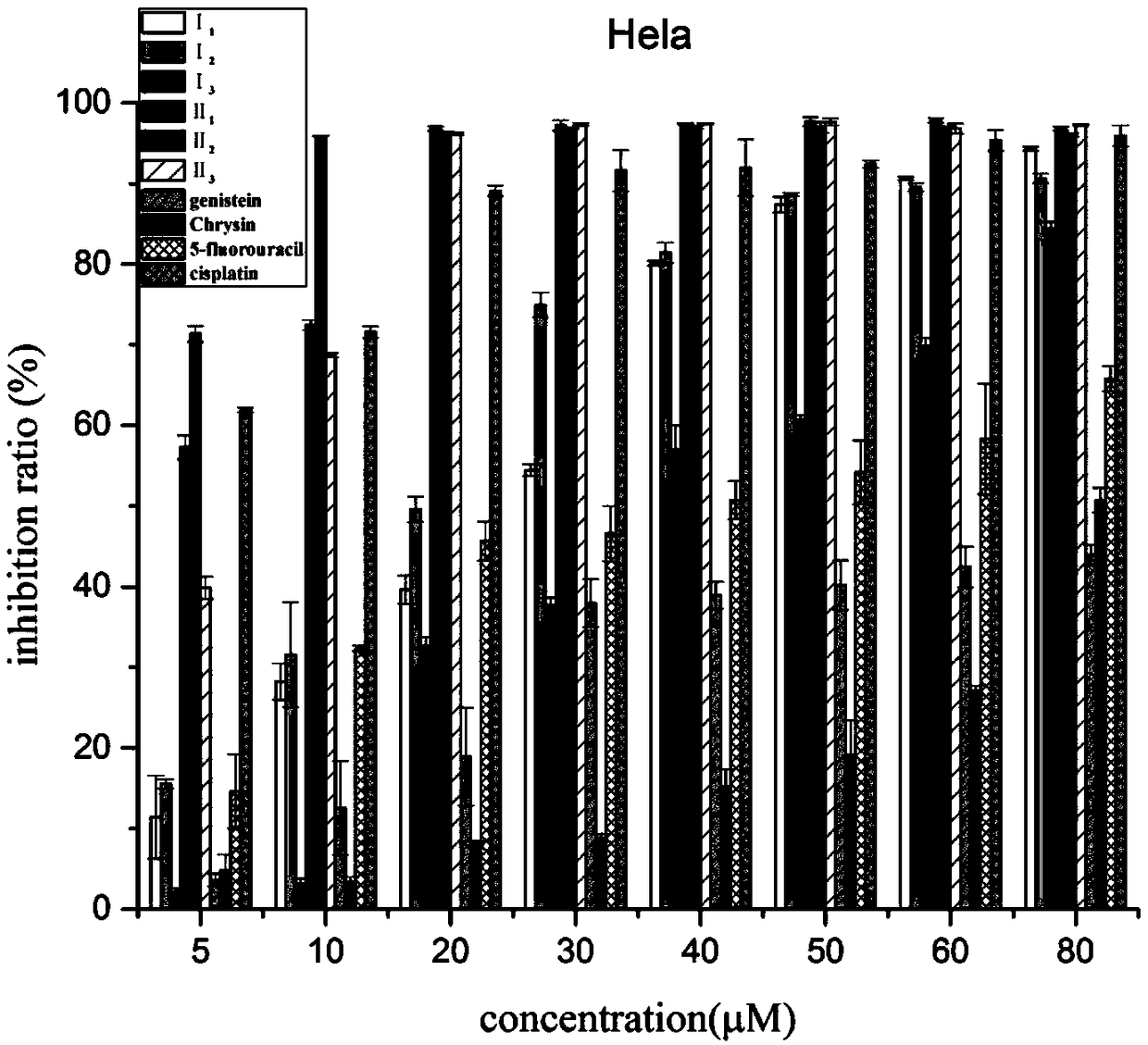
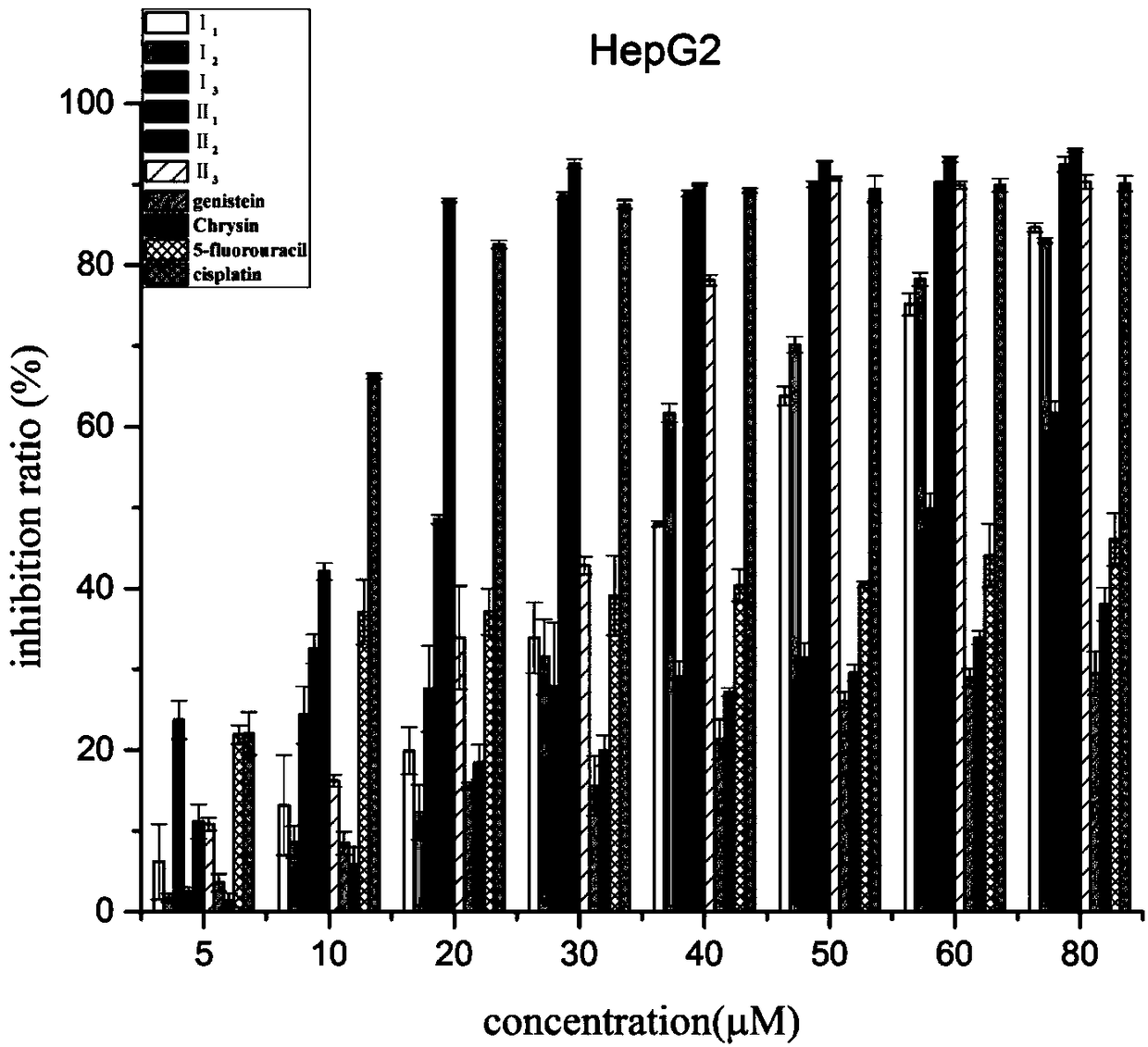
The invention discloses a preparation method of a class of nitrogen mustard-based flavonoid derivatives and application thereof in an anti-tumor activity study. As shown in the formula I and the formula II, n is an integer which is more than or equal to 0. Target compounds are not reported by literatures. The class of compounds can be used for inhibiting the growth activity of various tumor cells.An experiment shows that all the target compounds have better anti-tumor activity for tested seven cancer cells; compared with the existing anti-tumor drug 5-fluorouracil, the target compounds have higher growth inhibition activity for various tumor cells, wherein target compounds II 2 have the best anti-tumor effect, the IC50 value of the target compounds II 2 are all below 10muM for Hela, DU145, PC-3, MCF-7 and SH-SY5Y cells, and compared with an anti-tumor drug cis-platinum, the target compounds II 2 have the same order of magnitude. A further study discovers that the target compounds II 2can be used for inducing cell apoptosis by arresting the cycle of the Hela cells in a G2 / M phase, therefore, the target compounds II 2 are expected to be novel anti-tumor drugs.
Owner:BEIJING NORMAL UNIVERSITY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com